Introduction
Hepatocellular carcinoma (HCC) is a common
malignancy and the third most common cause of cancer-associated
mortality worldwide (1). The
diagnosis of early-stage HCC is complex due to its non-specific
symptoms and atypical features. The majority of patients are
therefore diagnosed with late-stage disease or distant metastasis,
with <20% of patients eligible for surgical treatment at the
point of diagnosis (2). Over the
previous 30 years, percutaneous ethanol injection (PEI) has become
an important method for the treatment of patients exhibiting HCC.
Among its advantages are its minimal invasiveness, simplicity, low
cost and relatively low occurrence of complications (3,4). However,
PEI may give rise to serious adverse effects such as serious
alcohol allergy, liver failure and abdominal bleeding (5). The present case report describes a
patient exhibiting acquired amegakaryocytic thrombocytopenic
purpura (AATP) caused by PEI with a reduction or complete absence
of megakaryocytes in the bone marrow and resulting in reduced
platelet counts in the peripheral blood.
Case report
A 49-year-old woman diagnosed with HCC and cirrhosis
associated with hepatitis B virus (HBV) infection was admitted to
Beijing 302nd Hospital on October 11, 2013. The patient's complete
blood count test upon hospital admission showed the following:
Leukocytes, 4.08×109/l (normal range,
4×109/l); erythrocytes, 2.84×1012/l (normal
range, 3.68–5.13×1012/l); hemoglobin, 106 g/l (normal
range, 113–151 g/l); and platelets, 56.00×109/l (normal
range, 100–300×109/l). The patient's coagulation
function tests demonstrated a prothrombin time (PT)/prothrombin
time activity (PTA) of 13.1 sec/79% (normal range, 11–14.3
sec/65–130%). The patient was positive for hepatitis B surface
antigen, with an HBV DNA quantity of <40 U/ml (normal range,
<40 U/ml). Liver function tests showed the following: Alanine
aminotransferase (ALT), 34 U/l (normal range, 35–55 U/l); aspartate
aminotransferase (AST), 23 U/l (normal range, 8–40 U/l); albumin,
35 g/l; total bilirubin (normal range, 35–55 g/l), 18 µmol/l
(normal range, 3.4–20.5 µmol/l); and α-fetoprotein, 1,210 ng/ml
(normal range, 0–10 ng/ml). An electrocardiogram and a thoracic
computed tomography scan did not reveal any abnormalities. Enhanced
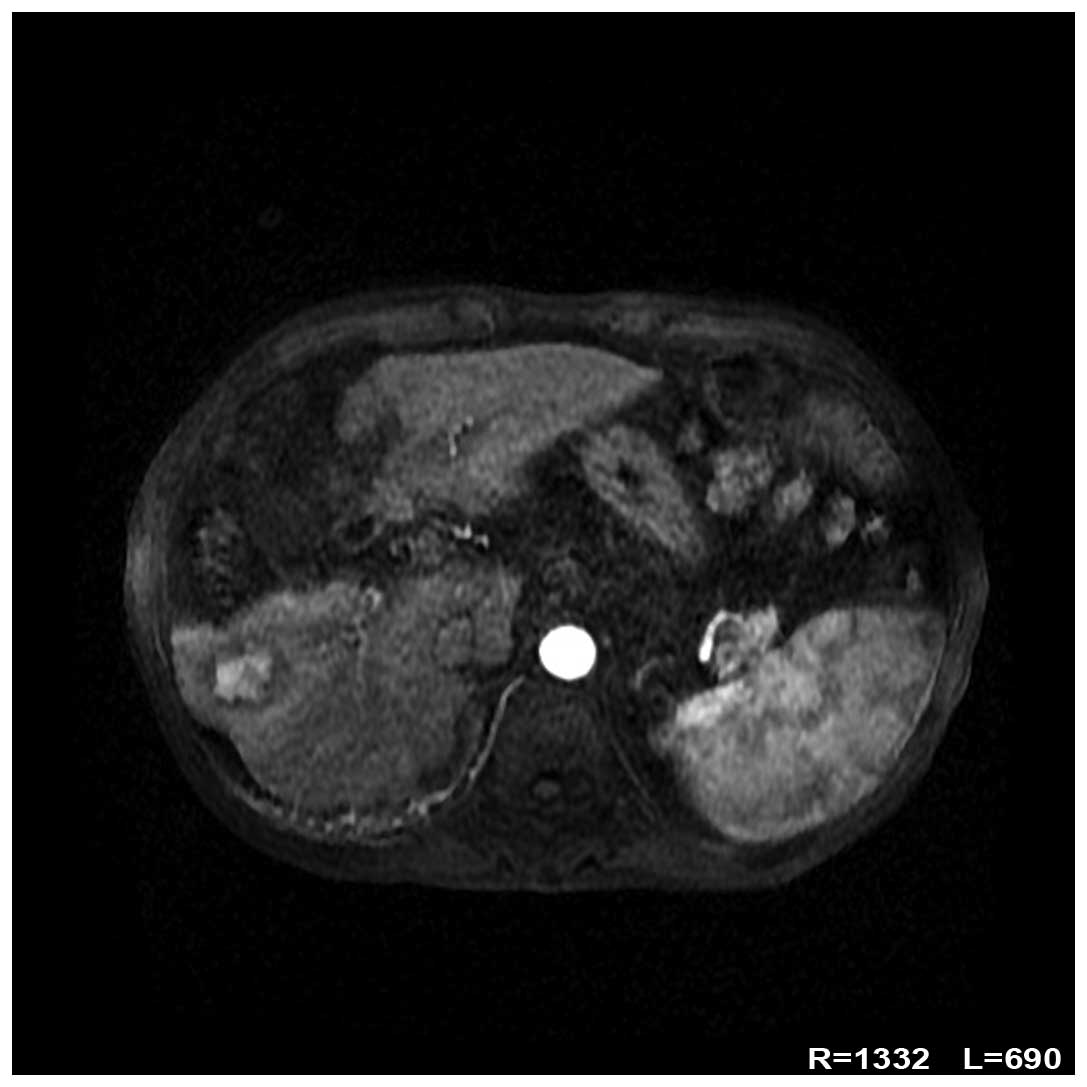
magnetic resonance imaging (Fig. 1)
revealed a round focus, measuring ~2.9×2.1 cm, in the anterior
portion of the right lobe of the liver. The focus was significantly
enhanced in the arterial phase, while the contrast agent faded in
the portal and delayed phases. These findings were consistent with
a diagnosis of HCC.
The patient refused surgical resection and
transarterial chemoembolization (TACE). Radiofrequency ablation
(RFA) was not a suitable treatment as the tumor nodule was adjacent
to the intestinal canal. The patient was treated with PEI, with the
focus receiving a total dose of 5 ml anhydrous ethanol. The morning
subsequent to treatment, the patient discovered multiple petechiae
on all four limbs. The patient did not experience gum bleeding,
hemoptysis, hematemesis, melena, palpitations, chest discomfort,
abdominal pain or cervical rigidity. An emergency complete blood
count showed the following: Leukocytes, 5.99×109/l;
erythrocytes, 2.90×1012/l; hemoglobin, 107 g/l; and
platelets, 2.00×109/l. Coagulation function tests
revealed a PT/PTA of 13.1 sec/77.6%. Liver function tests indicated
the following: ALT, 45 U/l; AST, 56 U/l; albumin, 36 g/l; and total
bilirubin, 22 µmol/l. Upon platelet transfusion and injection of
recombinant human interleukin (IL)-11, the patient's platelet count
rose to 30.00×109/l. Two bone marrow punctures were
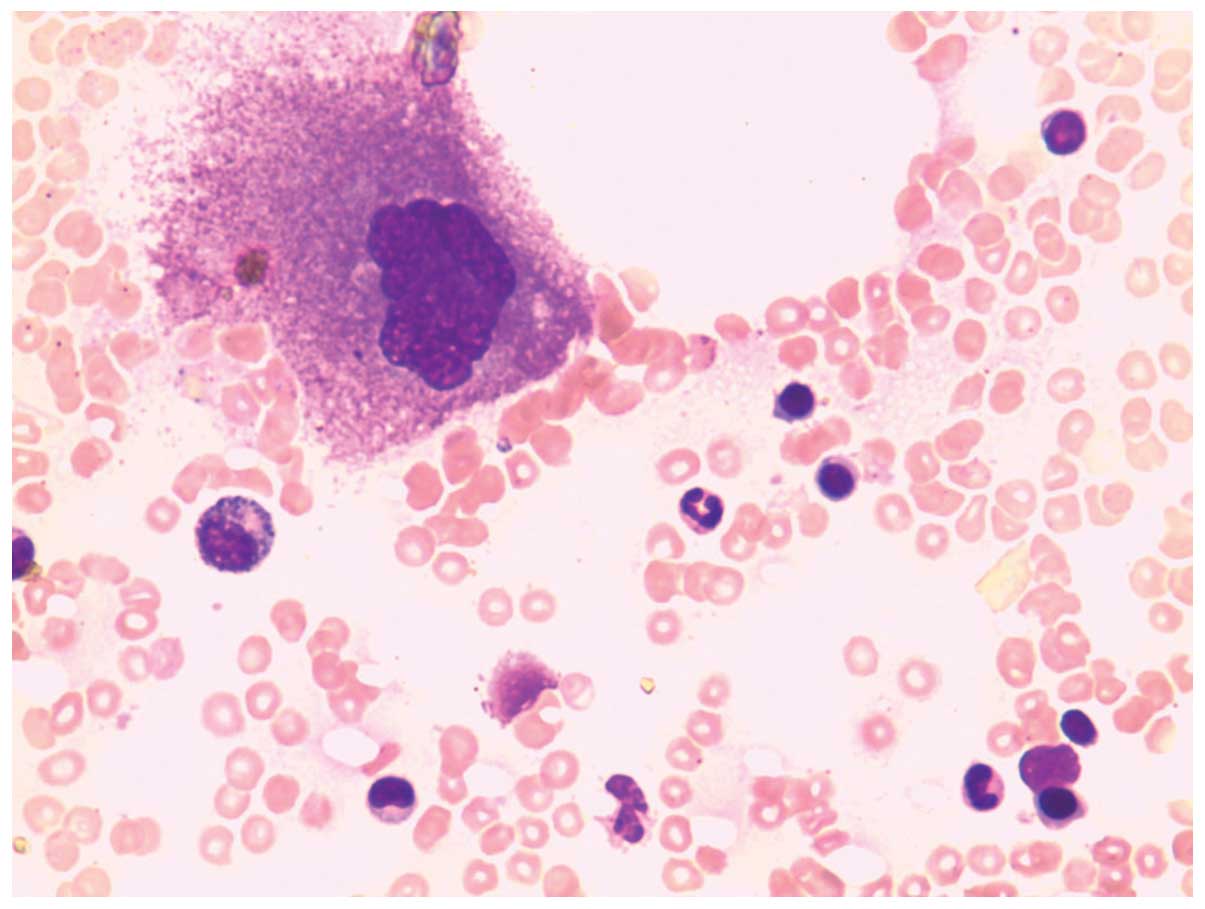
performed to confirm the diagnosis. A cytological study (Fig. 2) revealed active proliferation of
granulocytes and erythrocytes. These granulocytes and erythrocytes,
as well as lymphoid cells, demonstrated a typical morphology.
Megakaryocytes, however, showed a marked decrease in number and a
maturation defect. The patient was diagnosed with AATP, and
received a second platelet transfusion and injection of recombinant
human IL-11, which increased the platelet count to
50.00×109/l. A physical examination revealed the
disappearance of petechiae from all four limbs. A complete blood
count 3 months after treatment showed the following: Leukocytes,
4.66×109/l; erythrocytes, 3.13×1012/l;
haemoglobin, 113 g/l; and platelets, 34.00×109/l.
The patient was discharged when all the symptoms had
disappeared, and regular reviews were undertaken in the local
hospital. The regular follow-up phone calls revealed that the
patient remained stable.
Discussion
Treatment options for patients exhibiting inoperable
HCC include TACE, RFA, argon-helium cryoablation and PEI. PEI has
been utilized to treat HCC lesions <3 cm in diameter (6). The 1-, 3- and 5-year overall survival
rates in patients treated with PEI for HCCs <5 cm in diameter
have been reported as 95, 75 and 59%, respectively (7). The most common complications of PEI
treatment for HCC are abdominal pain and a self-limiting fever
(8). In a study of 746 patients with
HCC who underwent PEI, only 13 (1.7%) exhibited serious adverse
reactions, including hemorrhage in 5 patients, pleuritis in 2,
hemobilia in 2 and a hepatic abscess in 1 patient (9). Similarly, in a study of 2,147 HCC
patients who received PEI, only 45 (2.1%) experienced serious
adverse reactions (4). Serious
hypotension has additionally been reported in two patients
following PEI treatment (10). Thus,
compared with alternative treatment regimens, PEI treatment is
effective, cheap and safe, leading to fewer adverse effects in
patients with HCC.
AATP is a clinically rare bleeding disease,
characterized by a decrease or complete absence of megakaryocytes
in the bone marrow and resulting in decreased platelet counts in
the peripheral blood (11). By
contrast, the remaining hematopoietic lineages are normal (12,13). AATP
is not associated with patient age, however, it is more common in
women, particularly young women, compared with men, whilst being
rarely observed in children. The typical clinical presentation of
AATP is a marked decrease in platelet count in the peripheral blood
(14). The etiology of AATP remains
to be elucidated, and it may be associated with drugs, infections,
autoimmune diseases and radiation exposure (12,13).
Certain drugs have been observed to selectively inhibit
megakaryocytes, including thiazide diuretics, estrogen,
acetaminophen, alcohol and others (13,15).
In certain alcoholic patients, thrombocytopenia is
primarily a result of direct marrow suppression of platelet
production following alcohol consumption (16,17).
Suppression of platelet production that is sufficient enough to
produce thrombocytopenia requires consumption of large quantities
of ethanol over several days (18).
However, a study in which guinea pigs were allowed to ingest
ethanol ad libitum showed that, although blood ethanol did
not reach measurable levels, the average platelet count declined by
16% during the 4-week study period (19). Thrombocytopenia induced by alcohol
ingestion is accompanied by a decrease in the number of marrow
megakaryocytes. Vacuolated proerythroblasts and granulocyte
precursors may be observed, as well as multinuclear erythroblasts
and megaloblasts. Vacuolization of the periphery of mature
megakaryocytes has been observed (17). Thrombocytopenia typically resolves in
5–21 days following cessation of ethanol ingestion, occasionally
with transient rebound thrombocytosis (16).
Prior to the onset of thrombocytopenia, the current
patient received PEI, which was the probable cause of the AATP.
Thus, we speculate that this adverse reaction may be due to the
selective inhibition of megakaryocyte maturation by ethanol,
leading to thrombocytopenia. Multiple blood tests for the patient
demonstrated a significant reduction in platelet count, excluding
the possibility of pseudothrombocytopenia. Cytological examination
of the patient's bone marrow suggested normal or hyperproliferation
of all hematopoietic lineages, with the exception of
megakaryocytes, in which a defect in maturation was observed.
Following the diagnosis of AATP, the patient received platelet
transfusions and injections of recombinant human IL-11. The
platelet count rose to 50.00×109/l, and the patient was
subsequently discharged.
IL-11 was approved by the US Food and Drug
Administration for the treatment of bone marrow failure diseases
and thrombocytopenia following chemotherapy in November 1997
(20). IL-11 augments the growth of
megakaryocytic progenitors in the presence of IL-3, and acts to
promote megakaryocyte maturation rather than proliferation
(16). IL-11 is a multifunctional
regulator of hematopoiesis in bone marrow stromal cells, and has a
significant regulatory role in bone marrow megakaryocytes (21,22). Due
to the megakaryocyte maturation inhibition observed in the current
patient, IL-11 treatment was administered and exerted a significant
effect.
Although PEI for the treatment of HCC is relatively
safe and effective, with few adverse effects, complications may
occur due to individual variations. To manage such cases as the
present case of post-PEI AATP, we recommend the routine monitoring
of the platelet count of all patients undergoing PEI, which will
enable the early discovery and timely management of this
complication.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Said A and Wells J: Management of
hepatocellular carcinoma. Minerva Med. 100:51–68. 2009.PubMed/NCBI
|
|
3
|
Livraghi T, Benedini V, Lazzaroni S,
Meloni F, Torzilli G and Vettori C: Long term results of single
session percutaneous ethanol injection in patients with large
hepatocellular carcinoma. Cancer. 83:48–57. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shiina S, Tateishi R, Imamura M, Teratani
T, Koike Y, Sato S, Obi S, Kanai F, Kato N, Yoshida H, et al:
Percutaneous ethanol injection for hepatocellular carcinoma:
20-year outcome and prognostic factors. Liver Int. 32:1434–1442.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weis S, Franke A, Berg T, Mössner J, Fleig
WE and Schoppmeyer K: Percutaneous ethanol injection or
percutaneous acetic acid injection for early hepatocellular
carcinoma. Cochrane Database Syst Rev. 1:CD0067452015.PubMed/NCBI
|
|
6
|
Ebara M, Kita K, Yoshikawa M and Ohto M:
Non-vascular interventional radiology - percutaneous ethanol
injection (PEI) in hepatocellular carcinoma smaller than 3 cm in
diameter. Gan To Kagaku Ryoho. 16:3311–3318. 1989.(In Japanese).
PubMed/NCBI
|
|
7
|
Morimoto M, Numata K, Sugimori K, Shirato
K, Kokawa A, Oka H, Hirasawa K, Koh R, Nihommatsu H and Tanaka K:
Successful initial ablation therapy contributes to survival in
patients with hepatocellular carcinoma. World J Gastroenterol.
13:1003–1009. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castroagudín JF, Delgado M, Villanueva A,
Bustamante M, Martínez J, Otero E, Tomé S, Martínez SM, Segade FR,
Conde R, et al: Safety of percutaneous ethanol injection as
neoadjuvant therapy for hepatocellular carcinoma in waiting list
liver transplant candidates. Transplant Proc. 37:3871–3873. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livraghi T, Giorgio A, Marin G, Salmi A,
de Sio I, Bolondi L, Pompili M, Brunello F, Lazzaroni S and
Torzilli G: Hepatocellular carcinoma and cirrhosis in 746 patients:
Long-term results of percutaneous ethanol injection. Radiology.
197:101–108. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gelczer RK, Charboneau JW, Hussain S and
Brown DL: Complications of percutaneous ethanol ablation. J
Ultrasound Med. 17:531–533. 1998.PubMed/NCBI
|
|
11
|
Lai DW, Loughran TP Jr, Maciejewski JP,
Sasu S, Song SX, Epling-Burnette PK and Paquette RL: Acquired
amegakaryocytic thrombocytopenia and pure red cell aplasia
associated with an occult large granular lymphocyte leukemia. Leuk
Res. 32:823–827. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quintás-Cardama A: Acquired
amegakaryocytic thrombocytopenic purpura successfully treated with
limited cyclosporin A therapy. Eur J Haematol. 69:185–186. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tristano AG: Acquired amegakaryocytic
thrombocytopenic purpura: Review of a not very well-defined
disorder. Eur J Intern Med. 16:477–481. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirsh EH, Vogler WR, McDonald TP and Stein
SF: Acquired hypomegakaryocytic thrombocytopenic purpura.
Occurrence in a patient with absent thrombopoietic stimulating
factor. Arch Intern Med. 140:721–723. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gewirtz AM and Hoffman R: Transitory
hypomegakaryocytic thrombocytopenia: Aetiological association with
ethanol abuse and implications regarding regulation of human
megakaryocytopoiesis. Br J Haematol. 62:333–344. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaushansky K, Lichtman MA, Beutler E,
Kipps TJ, Seligsohn U and Prchal J: Williams Hematology (8th). New
York, NY: McGraw-Hill. 2010.PubMed/NCBI
|
|
17
|
Latvala J, Parkkila S and Niemelä O:
Excess alcohol consumption is common in patients with cytopenia:
Studies in blood and bone marrow cells. Alcohol Clin Exp Res.
28:619–624. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tristano AG: Acquired amegakaryocytic
thrombocytopenic purpura: Review of a not very well-defined
disorder. Eur J Int Med. 16:477–481. 2005. View Article : Google Scholar
|
|
19
|
Smith CM 2nd, Tobin JD Jr, Burris SM and
White JG: Alcohol consumption in the guinea pig is associated with
reduced megakaryocyte deformability and platelet size. J Lab Clin
Med. 120:699–706. 1992.PubMed/NCBI
|
|
20
|
Kantarjian H, Giles F, List A, Lyons R,
Sekeres MA, Pierce S, Deuson R and Leveque J: The incidence and
impact of thrombocytopenia in myelodysplastic syndromes. Cancer.
109:1705–1714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weich NS, Wang A, Fitzgerald M, Neben TY,
Donaldson D, Giannotti J, Yetz-Aldape J, Leven RM and Turner KJ:
Recombinant human interleukin-11 directly promotes
megakaryocytopoiesis in vitro. Blood. 90:3893–3902.
1997.PubMed/NCBI
|
|
22
|
Cantor SB, Elting LS, Hudson DV Jr and
Rubenstein EB: Pharmacoeconomic analysis of oprelvekin (recombinant
human interleukin-11) for secondary prophylaxis of thrombocytopenia
in solid tumor patients receiving chemotherapy. Cancer.
97:3099–3106. 2003. View Article : Google Scholar : PubMed/NCBI
|