Introduction
Worldwide, prostate cancer (PCa) is the second most
commonly diagnosed type of cancer and sixth leadinwg cause of
cancer-associated mortality among males (1). Mortality associated with PCa results
from distant metastasis, particularly to bone. Specifically, ~80%
of patients with PCa succumb to bone metastasis, and up to 80% of
patients with PCa exhibit bone metastasis at autopsy (2,3). However,
the mechanisms underlying the metastasis of PCa remain to be
elucidated.
In recent years, the epithelial-mesenchymal
transition (EMT) has been established as a regulator of tumor
aggressiveness (4). EMT was
originally identified during embryogenesis, where it was described
as a crucial process involved in differentiation and morphogenesis
(5). EMT has additionally been
attributed to tumor progression and metastasis (6). During EMT, cancer cells lose epithelial
characteristics and acquire mesenchymal properties, including
fibroblastoid morphology, characteristic changes in gene expression
and increased motility. Simultaneously, the cells develop
characteristics of cancer stem cells (7). These changes promote cancer cell
invasiveness, metastasis and resistance to chemotherapy (8–10).
Numerous factors induce EMT, including transforming
growth factor-β (TGF-β), epidermal growth factor (EGF), fibroblast
growth factor (FGF), hepatocyte growth factor (HGF),
platelet-derived growth factor, insulin-like growth factor (IGF)
(11), hypoxia (11,12) and
micro RNA (13). These factors induce
EMT via various signaling pathways, including Wnt, Hedgehog and
Notch (14,15).
In the present study, the association between HGF
and EMT in prostate cancer was investigated. Previous studies have
reported that higher plasma levels of HGF are associated with
advanced stage and poor prognosis in patients with prostate cancer
(16,17). This may be mediated by the promotion
of EMT by HGF in cancer cells. However, the mechanisms by which HGF
induces EMT remain unclear. The present study utilized the PC-3
human prostate cancer cell line as an experimental model. PC-3
cells are negative for EMT (18–21) and
positive for c-Met expression (22).
The present study investigated the effects of HGF on the EMT and
invasive potential of PC-3 cells. Furthermore, the potential
signaling pathways mediating this effect were investigated.
Materials and methods
Cell culture and treatment
PC-3 cells (American Type Culture Collection,
Manassas, VA, USA) were maintained in Dulbecco's modified Eagle's
medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco Life
Technologies) and incubated at 37°C in an atmosphere containing 5%
CO2. Cells were treated with recombinant human HGF
(Sigma-Aldrich, St. Louis, MO, USA) at various concentrations (20,
40 and 60 ng/ml) over varying time-periods (12, 24 and 36 h)
following overnight starvation.
Cell transfection
c-Met small interfering RNA (siRNA) or control siRNA
plasmids (Santa Cruz Biotechnology Inc., Dallas, TX, USA) were
transfected into PC-3 cells using Lipofectamine® 2000 transfection
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). Stable
transfectants were selected in 10 mg/ml puromycin (Life
Technologies, Grand Island, NY USA) 24 h following transfection.
Subsequently, the selection medium was replaced every 3 days.
Following 2 weeks of selection, resistant clones were isolated.
Cells were treated with recombinant human HGF as described
above.
MTT assay
The PC-3 cells (5×103/0.2 ml) were plated
in 96-well plates and stimulated with HGF (60 ng/ml) for 0, 24, 48
or 72 h. The cultures were incubated with 5 mg/ml MTT
(Sigma-Aldrich)for 4 h. The metabolic product was then dissolved in
200 µl buffered dimethyl sulfoxide (Sigma-Aldrich), and the
absorbance at 570 nm was measured with a Bio-Rad microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
Cells were lysed using Extraction and Quantification
ProteoJET Mammalian Cell Lysis Reagent (MBI Fermentas, Ontario,
Canada) with a protease inhibitors (Roche Diagnostics, Basel,
Switzerland). Total protein concentration was estimated using the
BCA method (Pierce Biotechnology Inc., Rockford, IL, USA). A total
of 30 µg clarified protein lysate was electrophoretically resolved
by denaturing 12% SDS-PAGE and electrotransferred onto
nitrocellulose membranes (Bio-Rad Laboratories, Inc.). The
immunoblots were incubated in 3% bovine serum albumin (Sijiqing
Biotech Co. Ltd., Hanzhou, China), 10 mM Tris-hydrochloride (pH
7.5), 1 mM EDTA and 0.1% Tween-20 (Sigma-Aldrich) at room
temperature and probed for 1.5 h with appropriate primary
antibodies, polyclonal rabbit anti-human c-Met (1:200),
phosphorylated-c-Met (p-c-Met; 1:200), anti-human E-cadherin, zinc
finger E-box binding homeobox-1 (Zeb-1; 1:150) and extracellular
signal-related kinase (ERK; 1:200); monoclonal mouse anti-human
vimentin (1:300) and phosphorylated ERK (p-ERK; 1:200; Santa Cruz
Biotechnology, Inc.) at various dilutions. The membranes were then
incubated for 1 h with secondary antibodies, horseradish
peroxidase-conjugated goat anti-rabbit (1:500) and goat anti-mouse
(1:200) immunoglobulin G (Boshide Biotech Co. Ltd., Kaohsiung City,
Taiwan). Monoclonal mouse anti-human GAPDH antibody (1:10,000
dilution; Santa Cruz Biotechnology, Inc.) was used as the internal
control. Blots were imaged using enhanced chemiluminescence
detection system (Pierce Biotechnology, Inc.).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from cells using TRIzol
reagent (Invitrogen Life Technologies). The isolated RNA was
reverse-transcribed into complementary DNA (cDNA) using oligo (dT)
primers and Avian Myeloblastosis Virus Reverse Transcriptase
(Takara Bio, Inc., Shiga, Japan). cDNA (10 µl) was used as a
template for PCR in a final reaction volume of 50 µl. Invitrogen
primers were obtained from Thermofisher Scientific, Inc. (Carlsbad,
CA, USA): The human C-Met primers (sense, 5′-GTTTCCCAATTTCTGACC-3′
and antisense, 5′-TATATCAAAGGTGTTTAC-3′) generated a 516 bp
product. The β-actin primers (sense, 5′-TGGGCATGGGTCAGAAGGAT-3′ and
antisense, 5′-AAGCATTTGCGGTGGACGAT-3′) generated a product of 991
bp. The DNA amplification conditions were as follows: An initial
denaturation step at 95°C for 5 min, 30 cycles at 95°C for 30 sec,
60°C for 30 sec, and 72°C for 40 sec, and a final elongation step
at 72°C for 7 min. The RT-PCR samples were electrophoresed on 1.5%
agarose gel and stained with ethidium bromide (0.5 µg/ml);
Sigma-Aldrich). Images of the gels were then captured using an
ultraviolet transillumination system (Liuyi Biotech Co. Ltd.,
Beijing, China).
In vitro wound-healing assay
Cells were seeded in 6-well plates and grown to
60–70% confluence. Cells were then incubated in Gibco serum-free
medium (Thermofisher Scientifi, Inc.) overnight and treated with
HGF (60 ng/ml). Prior to the addition of HGF, 2-mm scratches were
made in the confluent cell monolayer with a 200-µl pipette tip.
Cell migration into the denuded area was assessed 12 and 24 h
following treatment using a Type CK2 optical microscope (Olympus
Corporation, Tokyo, Japan).
In vitro Transwell invasion assay
Polycarbonate filters (8 µm; EMD Millipore,
Billerica, MA, USA) were coated with 50 µg/cm2
reconstituted Matrigel (Sigma-Aldrich). Cells (5×103)
were seeded into the upper chamber in 300 µl serum-free growth
medium. Cells were incubated under normoxic conditions and allowed
to migrate toward the complete growth medium for 24 and 48 h.
Non-invading cells were removed mechanically using cotton swabs and
cells on the lower surface were subsequently counted
microscopically.
Soft agar assay
Cells were resuspended in 2 ml top agar medium (DMEM
containing 0.4% low-melting agarose and 10% FBS; Sigma-Aldrich) and
then rapidly overlaid on 2 ml bottom agar medium (DMEM containing
0.8% low-melting agarose and 10% FBS) in 6-well culture plates.
Following 2–3 weeks of incubation, colonies >0.1 mm in diameter
were scored as positive. Colony-formation efficiency was evaluated
using a Type CK2 optical microscope (Olympus Corporation).
Statistical analysis
All values are expressed as the mean ± standard
deviation of at least three independent experiments. Statistical
analysis was performed using Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
HGF induces EMT-like changes in PC-3 cells.
Characteristic changes associated with EMT include downregulation
of epithelial markers and upregulation of mesenchymal markers.
These changes are associated with the scattered growth of cancer
cells, enabling cell-cell dissociation, migration and motility
(23). In the present study, western
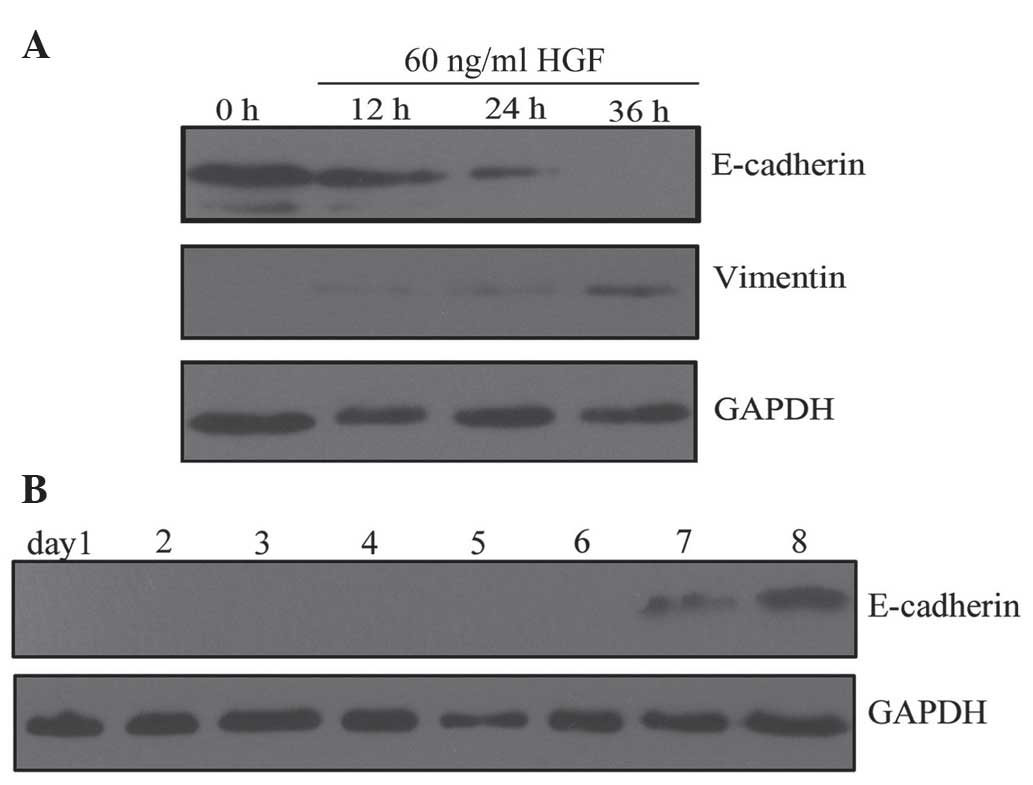
blot analysis revealed that HGF treatment downregulated E-cadherin
expression and upregulated vimentin expression in PC-3 cells in a
time- and dose-dependent manner. PC-3 cells acquired stable,
EMT-like changes following incubation with HGF (60 ng/ml) for 36 h
(Fig. 1A). These EMT-like changes
were not observed at other time-points or HGF concentrations. The
changes lasted for 7 days following withdrawal of HGF (Fig. 1B). These results indicate that HGF
promotes reversible changes in the expression of EMT markers in
PC-3 cells. Based on these results, PC-3 cells were treated with 60
ng/ml HGF for 36 h in all subsequent experiments.
c-Met expression is enhanced following HGF
treatment
To investigate the role of HGF in inducing EMT-like
changes in PC-3 cells, messenger RNA (mRNA) and protein expression
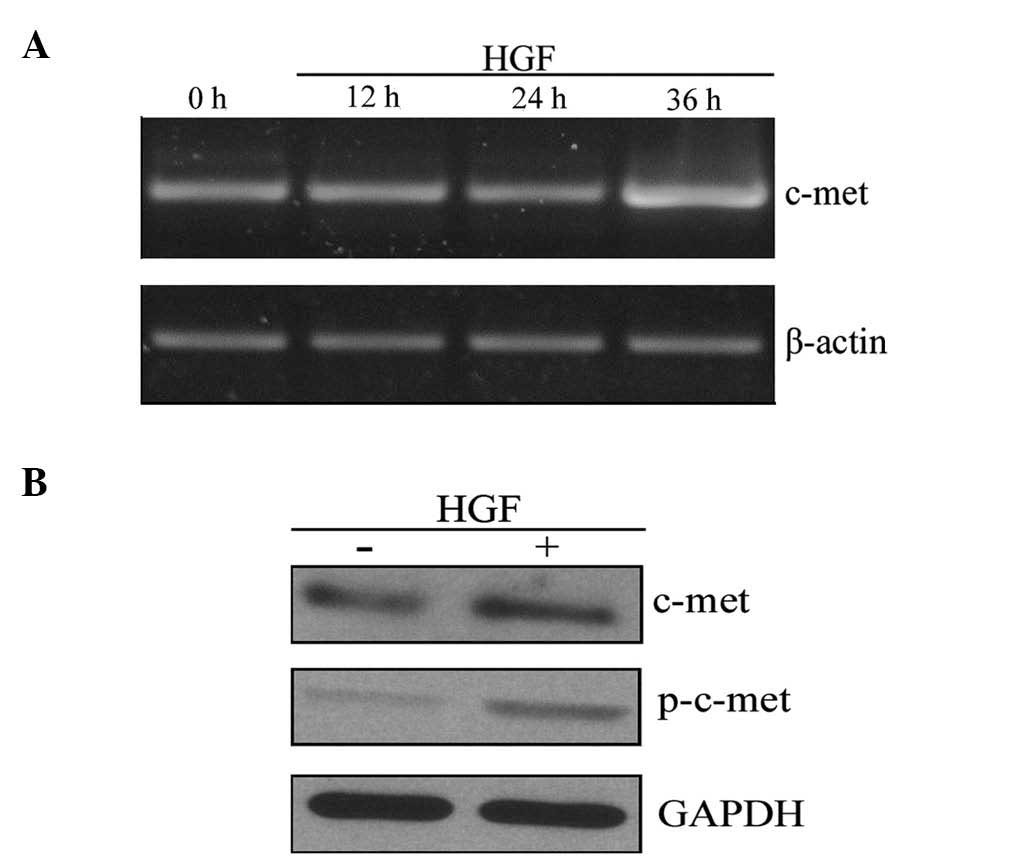
levels of c-Met, the receptor for HGF, were measured. RT-PCR
analysis demonstrated an upregulation of c-Met transcription
following HGF treatment for 36 h (Fig.
2A). Furthermore, c-Met was activated by HGF-mediated
phosphorylation (p-c-Met) and activated c-Met is able to regulate
various downstream target genes. Western blot analysis indicated
that HGF treatment increased the expression of c-Met and p-c-Met
(Fig. 2B). Together, these results
suggest that HGF upregulates c-Met at the mRNA and protein
levels.
HGF treatment increases the invasive
potential of PC-3 cells
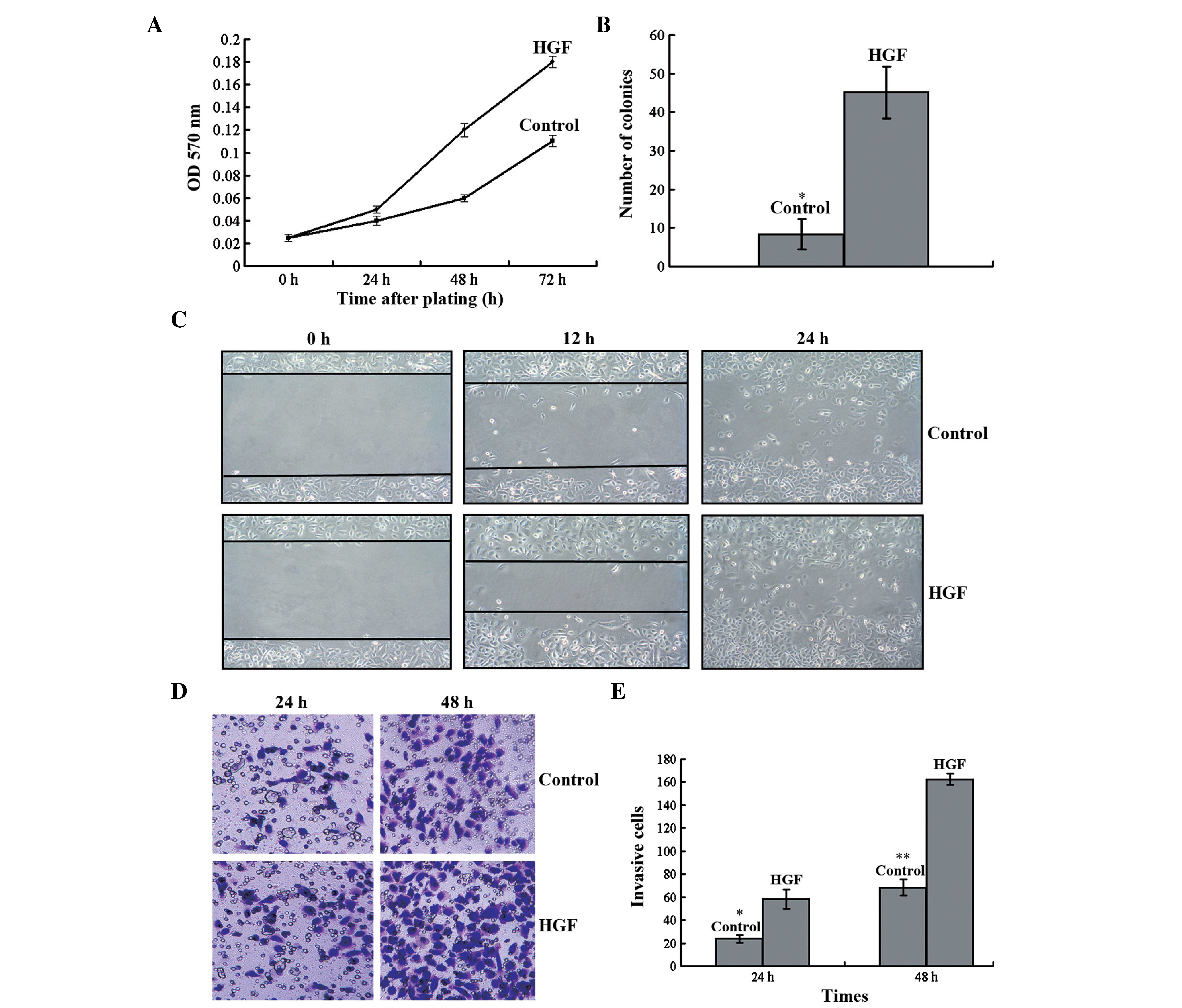
The effect of HGF treatment on the invasive
potential of PC-3 cells was examined. An MTT assay demonstrated
that HGF treatment increased cancer cell proliferation and doubling
time reduced (Fig. 3A). In addition,
HGF treatment increased the number of tumor colonies that developed
in the soft-agar assay (Fig. 3B). In
the wound-healing assay, HGF-treated PC-3 cells demonstrated
increased migratory capacity compared with that of the untreated
cells (Fig. 3C). In the Transwell
assay, HGF-treated cells displayed increased invasion beneath the
insert surface and through the collagen matrix (Fig. 3D and E). Taken together, these results
demonstrate that HGF increased the invasive potential of PC-3
cells.
ERK/mitogen activated protein kinase (MAPK)
signaling is involved in HGF-induced EMT
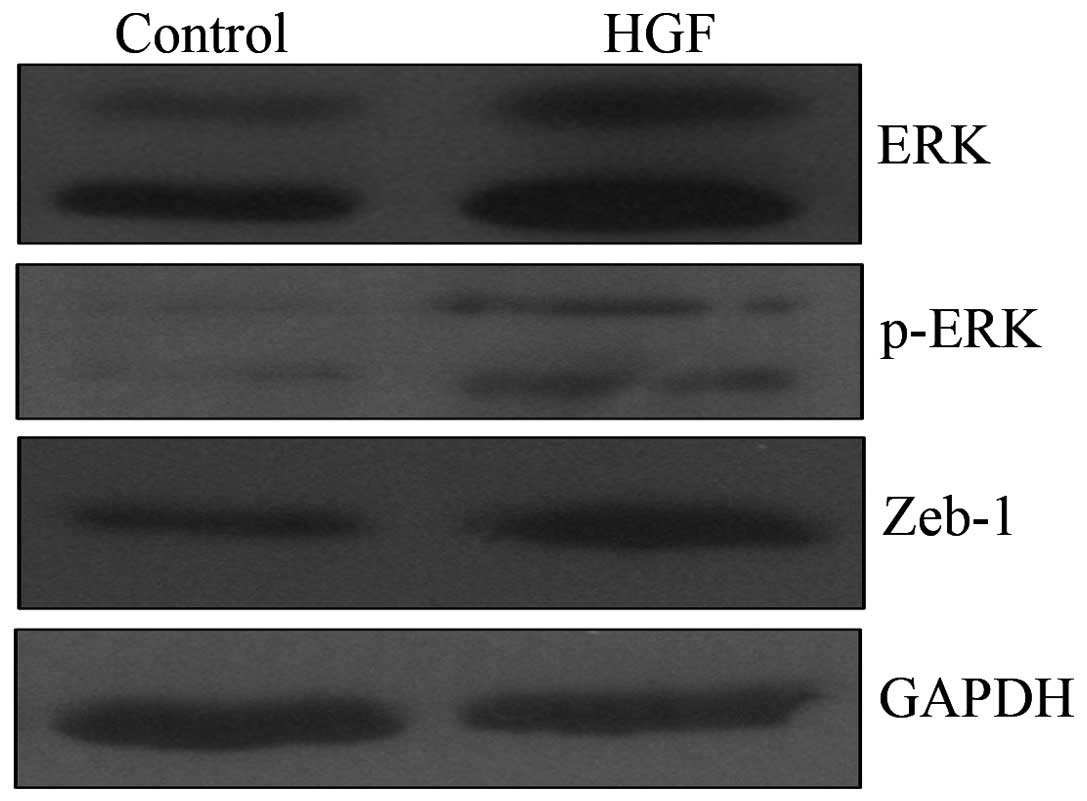
To investigate the molecular mechanism underlying
HGF-induced EMT, the changes in ERK/MAPK expression levels
following HGF incubation were measured. Western blot analysis
indicated that HGF treatment increased the expression levels of ERK
and p-ERK. In addition, HGF treatment increased the expression of
Zeb-1, a direct suppressor of E-cadherin. Thus, the ERK/MAPK
signaling pathway was involved in HGF-induced EMT (Fig. 4).
c-Met siRNA inhibits HGF-induced EMT-like
changes
The effect of c-Met knockdown by siRNA on
HGF-induced EMT-like changes was assessed. Transfection with c-Met
siRNA inhibited c-Met expression in PC-3 cells as compared with
untreated PC-3 cells and cells treated with control siRNA (Fig. 5A). Following incubation with HGF, PC-3
cells and cells treated with control siRNA exhibited downregulation
of E-cadherin and upregulation of vimentin as compared with cells
treated with c-Met siRNA. This demonstrated the role of c-Met in
mediating HGF-induced EMT-like changes (Fig. 5B). There were similar changes observed
in the ERK/MAPK and Zeb-1 signaling pathways. HGF treatment
upregulated ERK, p-ERK and Zeb-1 in PC-3 cells and cells treated
with control siRNA, however, this was not observed in cells treated
with c-Met siRNA (Fig. 5C). Together,
these data suggest that HGF induces EMT-like changes in a
c-Met-dependent manner.
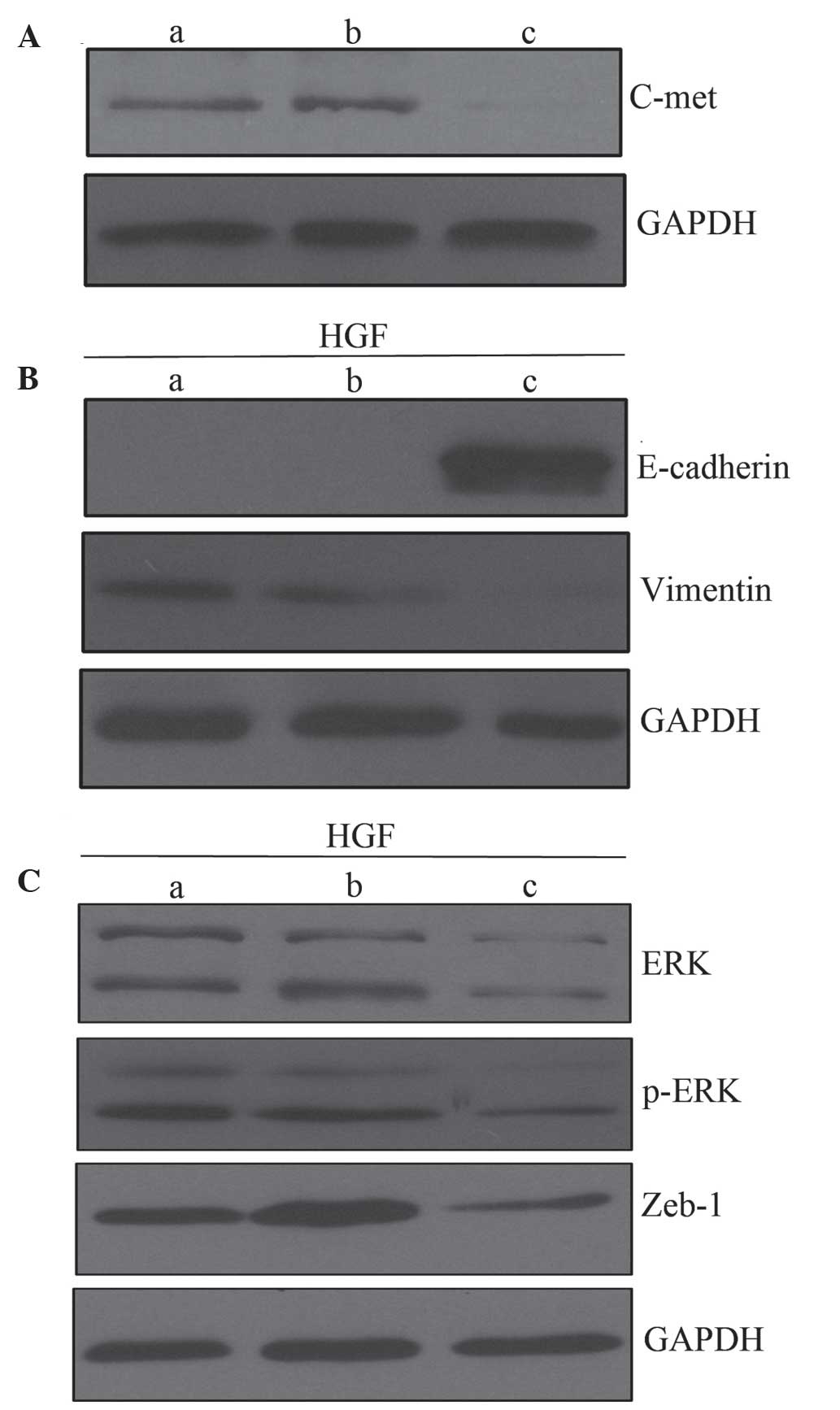 | Figure 5.Role of c-Met in HGF-induced EMT. (A)
Western blot analysis of c-Met expression. (B) c-Met knockdown
inhibits EMT-like changes induced by HGF (60 ng/ml), compared with
untreated PC-3 cells and cells treated with control siRNA. (C) HGF
treatment upregulates ERK, p-ERK and Zeb-1 in PC-3 cells and cells
treated with control siRNA cells, but not in cells treated with
c-Met siRNA. Lanes: a, PC-3 cells; b, cells treated with control
siRNA; and c, cells treated with c-Met siRNA. EMT,
epithelial-mesenchymal transition; siRNA, small interferingRNA;
HGF, hepatocyte growth factor; ERK, extracellular signal-related
kinase; Zeb-1, zinc finger E-box binding homeobox-1; p,
phosphorylated. |
Discussion
HGF binds to its receptor, c-Met, and activates it
through auto-phosphorylation, which induces the transcription of
downstream target genes. Under normal physiological conditions, the
HGF/c-Met signaling pathway regulates tissue and organ
regeneration. Furthermore, HGF is significant in the modulation of
cell morphology, and induction of angiogenesis and
lymphangiogenesis (24).
Previous studies have indicated that HGF stimulates
proliferation, migration and invasion in numerous types of cancer,
including colon, stomach, lung, bladder and prostate cancer
(17). For example, HGF levels are
elevated in the serum of patients with prostate cancer.
Furthermore, elevated HGF levels are associated with metastatic
disease independent of prostate-specific antigen levels or age, and
are associated with a decrease in overall survival rate (25,26). In
addition, Duhon et al (27)
reported that HGF treatment of DU145 prostate tumor cells
stimulated the phosphoinositide 3-kinase (PI3K) and MAPK signaling
pathways, leading to increased cell scattering, motility and
invasion. These effects were prevented by treatment with
epigallocatechin-3-gallate.
Although HGF accelerates the progression of prostate
cancer, the underlying mechanisms remain to be elucidated. The
association between HGF and EMT has been demonstrated in various
cancer models (28,29). However to the best of our knowledge,
no such association has previously been reported in prostate
cancer. One study demonstrated that HGF induced EMT in DU145 cells
(30); however, DU145 cells are
EMT-positive (18,31,32).
Therefore, the present study investigated the effect of HGF on EMT
induction in PC-3 cells.
Typical characteristics of EMT include
downregulation of epithelial markers, for example E-cadherin, and
upregulation of mesenchymal markers, including vimentin, N-cadherin
and α-smooth muscle actin (33,34). In
particular, downregulation of E-cadherin is a key step in the
induction of EMT (35). Intercellular
adhesions are critical for maintaining the epithelial phenotype,
and since E-cadherin is essential for adherent junctions,
downregulation results in the loss of cell polarity and abnormal
differentiation, thus facilitating EMT (9,36).
In the present study, treatment of PC-3 cells with
HGF resulted in EMT-like changes, as indicated by the
downregulation of E-cadherin and upregulation of vimentin. Thus,
HGF induced an EMT-like phenotype in PC-3 cells in a time- and
concentration-dependent manner. Further studies indicated that HGF
stimulation increased the proliferation, migration, invasion and
tumorigenicity of cancer cells. The EMT-like changes were
reversible following withdrawal of HGF for 7 days, which was
similar to the EMT phenotype induced by TGF-β1 (37). These results suggested that growth
factors are required to maintain the EMT phenotype. Numerous growth
factors, including FGF, IGF, TGF-β and HGF, are secreted from
stromal cells (38). Under continued
stimulation from these growth factors, cancer cells acquire a
stable EMT phenotype. Therefore, the results of the present study
demonstrate the bidirectional interaction and co-evolution of
tumors and their stroma in cancer progression.
The effect of HGF on the expression of its receptor
c-Met, at the mRNA and protein levels, was investigated. c-Met
overexpression has been identified in the majority of human cancers
(39,40). In the present study, c-Met expression
was promoted by HGF-dependent transcriptional upregulation. This
result is consistent with the findings of Boccaccio and Comoglio
(41) regarding prostate cancer.
Notably, in the present study, there was a marked elevation in
p-c-Met following HGF treatment, demonstrating that HGF activates
c-Met in prostate cancer cells. Knockdown of c-Met by siRNA
prevented HGF-induced EMT-like changes. These results demonstrate
that HGF induced EMT in a c-Met-dependent manner in PC-3 cells.
Various oncogenic effects of HGF and c-Met are
mediated by a complex downstream signaling network, most
prominently the MAPK and PI3K/Akt signaling pathways (42). In the present study, ERK was
phosphorylated by HGF, and PC-3 cells expressed high basal levels
of p-ERK and ERK. These changes were blocked by c-Met knockdown
using siRNA. These data suggested that the functional expression of
ERK is significant in HGF-induced EMT in PC-3 cells. A comparable
effect was observed in HGF-induced EMT in hepatocellular cancer
(43).
The present study demonstrated that HGF upregulated
Zeb-1 in PC-3 cells. As with other zinc finger transcription
factors, including SNAIL and SLUG, Zeb-1 has been linked to
E-cadherin repression (44).
Repression of E-cadherin enhances the ability of cancer cells to
migrate to distant sites (45). HGF
interacts with early growth response factor-1 through the MAPK
signaling pathway, which binds to the Snail promoter, leading to
rapid induction and execution of EMT (46). Another study revealed that the Zeb
gene was activated upon activation of SNAIL (47). SW480 colorectal cancer cells possess a
mesenchyme-like phenotype, which is characterized by loosely
attached cells that lack membranous E-cadherin. Silencing of Zeb-1
by siRNA resulted in a cellular phenotype resembling the
mesenchymal-epithelial transition (48). The results of the present study are
consistent with the above-mentioned studies, and demonstrate the
role of Zeb-1 in EMT in prostate cancer.
In conclusion, the results of the present study
revealed that HGF directly promotes EMT and carcinogenic properties
in prostate cancer via the ERK signaling pathway. Specific
molecular targeting of this signaling pathway may provide
therapeutic benefit in patients exhibiting prostate cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 30700968 and
81341066).
References
|
1
|
Jin JK, Dayyani F and Gallick GE: Steps in
prostate cancer progression that lead to bone metastasis. Int J
Cancer. 128:2545–2561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jacobs SC: Spread of prostatic cancer to
bone. Urology. 21:337–344. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah RB, Mehra R, Chinnaiyan AM, Shen R,
Ghosh D, Zhou M, Macvicar GR, Varambally S, Harwood J, Bismar TA,
et al: Androgen-independent prostate cancer is a heterogeneous
group of diseases: Lessons from a rapid autopsy program. Cancer
Res. 64:9209–9216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scheel C and Weinberg RA: Cancer stem
cells and epithelial-mesenchymal transition: Concepts and molecular
links. Semin Cancer Biol. 22:396–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morel AP, Lievre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y,
Kato Y, Shinto O, Noda S, Kashiwagi S, Aomatsu N, Hirakawa T, et
al: Hypoxia stimulates the EMT of gastric cancer cells through
autocrine TGFβ signaling. PLoS One. 8:e623102013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo Y, He DL, Ning L, Shen SL, Li L, Li X,
Zhau HE and Chung LW: Over-expression of hypoxia-inducible
factor-1alpha increases the invasive potency of LNCaP cells in
vitro. BJU Int. 98:1315–1319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lamouille S, Subramanyam D, Blelloch R and
Derynck R: Regulation of epithelial-mesenchymal and
mesenchymal-epithelial transitions by microRNAs. Curr Opin Cell
Biol. 25:200–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chung LW, Huang WC, Sung SY, Wu D,
Odero-Marah V, Nomura T, Shigemura K, Miyagi T, Seo S, Shi C, et
al: Stromal-epithelial interaction in prostate cancer progression.
Clin Genitourin Cancer. 5:162–170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hashem M and Essam T: Hepatocyte growth
factor as a tumor marker in the serum of patients with prostate
cancer. J Egypt Natl Canc Inst. 17:114–120. 2005.PubMed/NCBI
|
|
17
|
Yasuda K, Nagakawa O, Akashi T, Fujiuchi
Y, Koizumi K, Komiya A, Saiki I and Fuse H: Serum active hepatocyte
growth factor (AHGF) in benign prostatic disease and prostate
cancer. Prostate. 69:346–351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo Y, He DL and Ning L: Expression of
‘epithelial-mesenchymal transition’ associated proteins in prostate
cancer cell lines with different metastatic potentials and its
significance. Zhonghua Nan Ke Xue. 12:696–700. 2006.(In Chinese).
PubMed/NCBI
|
|
19
|
Gu X, Zerbini LF, Otu HH, Bhasin M, Yang
Q, Joseph MG, Grall F, Onatunde T, Correa RG and Libermann TA:
Reduced PDEF expression increases invasion and expression of
mesenchymal genes in prostate cancer cells. Cancer Res.
67:4219–4226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Veveris-Lowe TL, Lawrence MG, Collard RL,
Bui L, Herington AC, Nicol DL and Clements JA: Kallikrein 4 (hK4)
and prostate-specific antigen (PSA) are associated with the loss of
E-cadherin and an epithelial-mesenchymal transition (EMT)-like
effect in prostate cancer cells. Endocr Relat Cancer. 12:631–643.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Whitbread AK, Veveris-Lowe TL, Lawrence
MG, Nicol DL and Clements JA: The role of kallikrein-related
peptidases in prostate cancer: Potential involvement in an
epithelial to mesenchymal transition. Biol Chem. 387:707–714. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Yue D, Li K, Liu YL, Ren CS and
Wang P: The role of TRPC6 in HGF-induced cell proliferation of
human prostate cancer DU145 and PC3 cells. Asian J Androl.
12:841–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J and Weinberg RA:
Epithelial-Mesenchymal Transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin TA, Mason MD and Jiang WG:
Hepatocyte growth factor signaling in cancer metastasis. Curr
Signal Transduct Ther. 6:180–190. 2011. View Article : Google Scholar
|
|
25
|
Naughton M, Picus J, Zhu X, Catalona WJ,
Vollmer RT and Humphrey PA: Scatter factor-hepatocyte growth factor
elevation in the serum of patients with prostate cancer. J Urol.
165:1325–1328. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Humphrey PA, Halabi S, Picus J, Sanford B,
Vogelzang NJ, Small EJ and Kantoff PW: Prognostic significance of
plasma scatter factor/hepatocyte growth factor levels in patients
with metastatic hormone-refractory prostate cancer: Results from
cancer and leukemia group B 150005/9480. Clin Genitourin Cancer.
4:269–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duhon D, Bigelow RL, Coleman DT, Steffan
JJ, Yu C, Langston W, Kevil CG and Cardelli JA: The polyphenol
epigallocatechin-3-gallate affects lipid rafts to block activation
of the c-Met receptor in prostate cancer cells. Mol Carcinog.
49:739–749. 2010.PubMed/NCBI
|
|
28
|
Previdi S, Maroni P, Matteucci E, Broggini
M, Bendinelli P and Desiderio MA: Interaction between human-breast
cancer metastasis and bone microenvironment through activated
hepatocyte growth factor/Met and b-catenin/Wnt pathways. Eur J
Cancer. 46:1679–1691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ogunwobi OO and Liu C: Hepatocyte growth
factor upregulation promotes carcinogenesis and
epithelial-mesenchymal transition in hepatocellularcarcinoma via
Akt and COX-2 pathways. Clin Exp Metastasis. 28:721–731. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fram ST, Wells CM and Jones GE:
HGF-induced DU145 cell scatter assay. Methods Mol Biol. 769:31–40.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baritaki S, Huerta-Yepez S, Sahakyan A,
Karagiannides I, Bakirtzi K, Jazirehi A and Bonavida B: Mechanisms
of nitric oxide-mediated inhibition of EMT in cancer: Inhibition of
the metastasis-inducer Snail and induction of the
metastasis-suppressor RKIP. Cell Cycle. 9:4931–4940. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yates CC, Shepard CR, Stolz DB and Wells
A: Co-culturing human prostate carcinoma cells with hepatocytes
leads to increased expression of E-cadherin. Br J Cancer.
96:1246–1252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cano A, Perez-Moreno MA, Rodrigo I,
Locasioa A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimada S, Mimata A, Sekine M, Mogushi K,
Akiyama Y, Fukamachi H, Jonkers J, Tanaka H, Eishi Y and Yuasa Y:
Synergistic tumour suppressor activity of E-cadherin and p53 in a
conditional mouse model for metastatic diffuse-type gastric cancer.
Gut. 61:344–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Odero-Marah VA, Wang R, Chu G, Zayzafoon
M, Xu J, Shi C, Marshall FF, Zhau HE and Chung LW: Receptor
activator of NF-kappaB Ligand (RANKL) expression is associated with
epithelial to mesenchymal transition in human prostate cancer
cells. Cell Rese. 18:858–870. 2008. View Article : Google Scholar
|
|
38
|
Chung LW, Baseman A, Assikis V and Zhau
HE: Molecular insights into prostate cancer progression: The
missing link of tumor microenvironment. J Urol. 173:10–20. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang WG, Davies G, Martin TA, Parr C,
Watkins G, Mason MD, Mokbel K and Mansel RE: Targeting matrilysin
and its impact on tumor growth in vivo: The potential
implications in breast cancer therapy. Clin Cancer Res.
11:6012–6019. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
You WK and McDonald DM: The hepatocyte
growth factor/c-Met signaling pathway as a therapeutic target to
inhibit angiogenesis. BMB Rep. 41:833–839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boccaccio C and Comoglio PM: Invasive
growth: A MET-driven genetic programme for cancer and stem cells.
Nat Rev Cancer. 6:637–645. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Migliore C and Giordano S: Molecular
cancer therapy: Can our expectation be MET? Eur J Cancer.
44:641–651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ogunwobi OO and Liu C: Hepatocyte growth
factor upregulation promotes carcinogenesis and
epithelial-mesenchymal transition in hepatocellular carcinoma via
Akt and COX-2 pathways. Clin Exp Metastasis. 28:721–731. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nieto MA and Cano A: The epithelial -
mesenchymal transition under control: Global programs to regulate
epithelial plasticity. Semin Cancer Biol. 22:361–368. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C,
Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, et al: Upregulation
of TWIST in prostate cancer and its implication as a therapeutic
target. Cancer Res. 65:5153–5162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Grotegut S, von Schweinitz D, Christofori
G and Lehembre F: Hepatocyte growth factor induces cell scattering
through MAPK/Egr-1-mediated upregulation of Snail. EMBO J.
25:3534–3545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peña C, García JM, García V, Silva J,
Domínguez G, Rodríguez R, Maximiano C, de Herreros García A, Muñoz
A and Bonilla F: The expression levels of the transcriptional
regulators p300 and CtBP modulate the correlations between SNAIL,
ZEB1, E-cadherin and vitamin D receptor in human colon carcinomas.
Int J Cancer. 119:2098–2104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Spaderna S, Schmalhofer O, Hlubek F, Berx
G, Eger A, Merkel S, Jung A, Kirchner T and Brabletz T: A
transient, EMT-linked loss of basement membranes indicates
metastasis and poor survival in colorectal cancer.
Gastroenterology. 131:830–840. 2006. View Article : Google Scholar : PubMed/NCBI
|