Introduction
Worldwide, liver cancer is the third most common
cause of cancer-associated mortality (1). There are various approaches to the
treatment of liver cancer, including chemotherapy, intervening
therapy, liver transplantation and resection, which remains the
most effective and the primary choice for the majority of patients
(2). The prognosis for liver cancer
patients following curative resection is improved compared to the
prognosis without resection (3), and
the 5-year survival rate increases between <10% and 30–50%
(4). Sorafenib, a mitogen-activated
protein kinase inhibitor (5), is the
most effective systemic chemotherapy agent for patients with
advanced hepatocellular carcinoma (6). However, alternative treatment strategies
for aggressive liver cancer are urgently required.
Natural compounds have been revealed to be
beneficial when used alone or in combination with conventional
therapies for the prevention of tumor progression or treatment of
human malignancies (7). Since the
1940s, 48.6% of all cancer therapeutic agents approved by the US
Food and Drug Administration and similar organizations are natural
products or direct derivatives (8).
The Rabdosia genus is a rich source of
diterpenoids, particularly ent-kaurene diterpenoids
(9), which exhibit significant
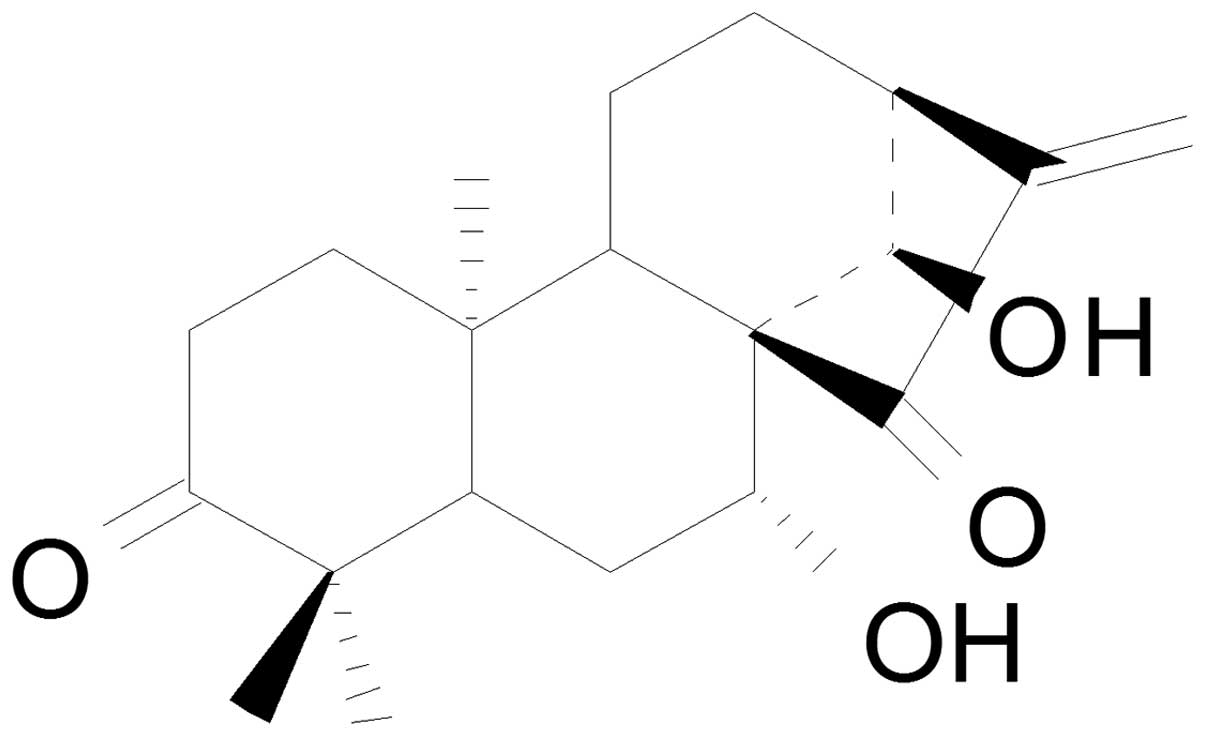
cytotoxicity and antitumor activities (10). Glaucocalyxin A (GLA; Fig. 1), also termed leukamenin F or
(5β,7α,9β,10α)-7,14-dihydroxykaur-16-ene-3,15-dione, is a chemical
form of ent-kaurene diterpenoids that demonstrates a wide
range of biological activities. GLA has been reported to attenuate
lipopolysaccharide-stimulated neuroinflammation (11), inhibit protein kinase B
phosphorylation in human-derived malignant glioma U87MG cells
(12), cause a marked increase of
platelet cyclic adenosine monophosphate levels (13), inhibit
H2O2-induced H9c2 cardiomyocyte injury
(14), and induce apoptosis in a
radical oxygen species-dependent mitochondrial dysfunction pathway
(15). Furthermore, the intracellular
glutathione-redox system is important in regulating the GLA-induced
cytotoxicity on HL-60 cells (16).
In liver cancer, GLA has suppressed liver
fibrogenensis, inhibited the proliferation of hepatic stellate
cells (17) and has demonstrated
cytotoxicity towards HepG2 cells (18), although the sensitivity of GLA to
various types of liver cancer cells varied. The present study
investigated the effect of GLA on liver cancer cells, revealing
that GLA significantly inhibits the growth of the human liver
cancer Focus and SMMC-772 cells.
Materials and methods
Chemicals and antibodies
GLA was isolated from the leaves of Rabdosia
umbrosa according to previously published protocols (19). GLA was prepared as a 50 mmol/l stock
solution in dimethyl sulfoxide (DMSO), and stored at 4°C. The
purity of the stock solution was >98%. 5-fluorouracil (5-FU) was
purchased from Sigma-Aldrich (St. Louis, MO, USA). The primary
antibodies used in western blotting were: Monoclonal mouse
anti-human β-actin monoclonal antibody (cat. no. A5316; 1:5,000;
Sigma-Aldrich, St. Louis, MO, USA); polyclonal rabbit anti-human
poly(adenosine diphosphate-ribose) polymerase (PARP) antibody (cat.
no. 9542; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA); and polyclonal rabbit anti-human caspase 3 antibody (cat. no.
9662; 1:500; Cell Signaling Technology, Inc.). The secondary
antibodies were horseradish peroxidase-conjugated anti-rabbit (cat.
no. 7074; 1:2,000) and anti-mouse immunoglobulin G (cat. no. 7076;
1:5,000) (Cell Signaling Technology, Inc.).
Cell lines and cell culture
The human liver cancer SMMC-7721, epithelial HeLa,
liver cancer SK-HEP1 and liver cancer HepG2 cell lines were
obtained from American Type Culture Collection (Manassas, VA, USA).
The human liver cancer Focus, pancreatic cancer PANC-1, leukemia
K562, stomach cancer HGC-27, adenocarcinoma A549 and liver cancer
QGY-7703 cell lines were purchased from the Chinese Academy of
Sciences (Beijing, China). The SMMC-7721, HeLa, Focus and HepG2
cells were cultured in Dulbecco's Modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific), while
the K562, A549, SK-HEP1, QGY-7703, PANC-1 and HGC-27 cells were
cultured in RPMI-1640 (Invitrogen; Thermo Fisher Scientific) with
10% FBS. All cells were cultured at 37°C in a humidified incubator
with 5% CO2.
Cell viability assay
Cell viability was determined using a modified cell
counting kit-8 (CCK-8) cellular proliferation assay (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). Cells were plated
in 96-well plates, and incubated with 0.00, 3.13, 6.25, 12.50,
25.00 and 50.00 µmol/l GLA or 5-FU for 48 h. CCK-8 was administered
for 2 h, followed by absorbance measurement at 450 nm using a
microplate reader (Model 550; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Sub-G1 analysis
Focus cells were plated in 6-well plates and
incubated in DMEM with various concentrations of GLA for 24 h. DMEM
with 0.1% DMSO) was set as a control. The cells were harvested and
washed in phosphate buffered saline (PBS) and resuspended in PBS
containing 0.03% Triton X-100 (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). The cells were then stained with a solution of 50
µg/ml propidium iodide (PI) for 15 min prior to analysis by flow
cytometry (FCM; FACStar™ Plus; BD Biosciences, Franklin Lakes, NJ,
USA). The sub-G1 cell subset was observed to reflect the percentage
of apoptotic cells. The cycle distribution of cells was calculated
by ModFit LT™ version 2.0 (Verity Software House, Inc., Toronto,
ON, Canada).
Cell-cycle analysis
The Focus and SMMC-7721 cells were plated in 12-well
plates and incubated in DMEM with various concentrations of GLA for
24 h. DMEM with 0.1% DMSO was set as a control. The cells were
collected and washed in PBS and resuspended in PBS containing 0.03%
Triton X-100 and 200 mg/ml RNase A (Sigma-Aldrich). The cells were
stained in a solution of 50 µg/ml PI for 15 min prior to analysis
by FCM (FACStar Plus). The cycle distribution of cells was
calculated by ModFit LT.
Western blot analysis
The cells were lysed in ice-cold cell lysis buffer
(Cell Signaling Technology, Inc.) containing 25 mmol/l Tris-HCl (pH
7.5), 150 mmol/l NaCl, 1 mmol/l Na3VO4, 1%
Triton X-100 and protease cocktails. Equal amounts of lysates were
resolved with 10% SDS-PAGE and transferred to a nitrocellulose
membrane (GE Healthcare Life Sciences, Chalfont, UK). The membranes
were stained with Ponceau S (Sigma-Aldrich) and probed with the
β-actin antibody to confirm equivalent loading and protein
transfer. Peroxidase-conjugated secondary antibodies were detected
using enhanced chemiluminescence (GE Healthcare Life Sciences).
Statistical analysis
Data were expressed as the mean ± standard deviation
from ≥3 sets of independent experiments. The Student's t-test was
used to determine the significance of statistical differences.
P<0.05 was considered to indicate a statistically significant
difference.
Results
GLA inhibits the growth of human
cancer cells
A CCK-8 assay was used to investigate the cytotoxic
activity of GLA on human cancer cells at various concentrations
(0.0, 5.0 and 50.0 µmol/l) for 48 h. GLA exhibited cytotoxicity
against cancer cells from different tissues, including the PANC-1,
K562, HGC-27, A549, SMMC-7721, HeLa, QGY-7703 and HepG2 cell lines
(Fig. 2A).
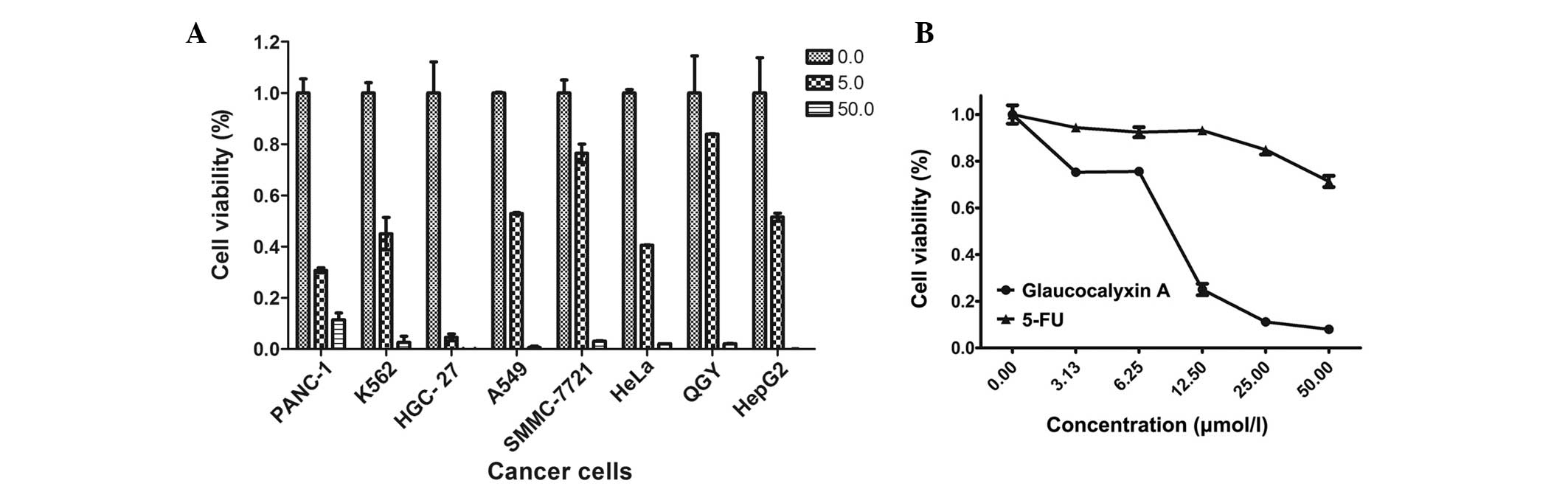 | Figure 2.GLA inhibits the growth of cancer
cells. Cancer cells were seeded in a 96-well plate and treated with
various concentrations of GLA. Cell viability was measured by a
cell counting kit-8 assay, which was administered for 2 h,
following analysis using an absorbance measurement at 450 nm. (A)
The PANC-1, K562, HGC-27, A549, SMMC-7721, HeLa, QGY-7703 and HepG2
cells were treated with 50.00 or 5.00 µmol/l GLA for 48 h. (B)
SMMC-7721 cells were treated with 0.00, 3.13, 6.25, 12.50, 25.00
and 50.00 µmol/l GLA or 5-FU for 48 h. Cells incubated with 0.1%
dimethyl sulfoxide were presented as 0.00 µmol/l and set as a
control. The data are expressed as the mean ± standard deviation of
results from 3 independent experiments. GLA, glaucocalyxin A; 5-FU,
fluorouracil. |
To compare the effects of GLA with currently
available chemotherapies, the same quantities of 5-FU and GLA were
added into the DMSO medium used for SMMC-7721 cell culture. The
synthesized chemotherapy drug 5-FU inhibits the growth of cancer
cells by inhibiting thymidylate synthase, an enzyme which is
essential for DNA synthesis (19).
The administration of 3.13, 6.15, 12.25, 25.00 and 50.00 µmol/l GLA
to SMMC-7721 cells led to cell viabilities of 0.752±0.015,
0.756±0.015, 0.250±0.025, 0.112±0.08 and 0.08±0.004 µmol/l,
respectively. The administration of the same concentrations of 5-FU
to SMMC-7721 cells led to cell viabilities of 0.944±0.012,
0.924±0.021, 0.932±0.016, 0.849±0.020 and 0.714±0.024 µmol/l,
respectively. Therefore, GLA decreased the cell viability of
SMMC-7721 cells more rapidly than 5-FU. The difference between the
cell viabilities induced by GLA and 5-FU administration became more
significant when the concentration of the agents was increased
(Fig. 2B).
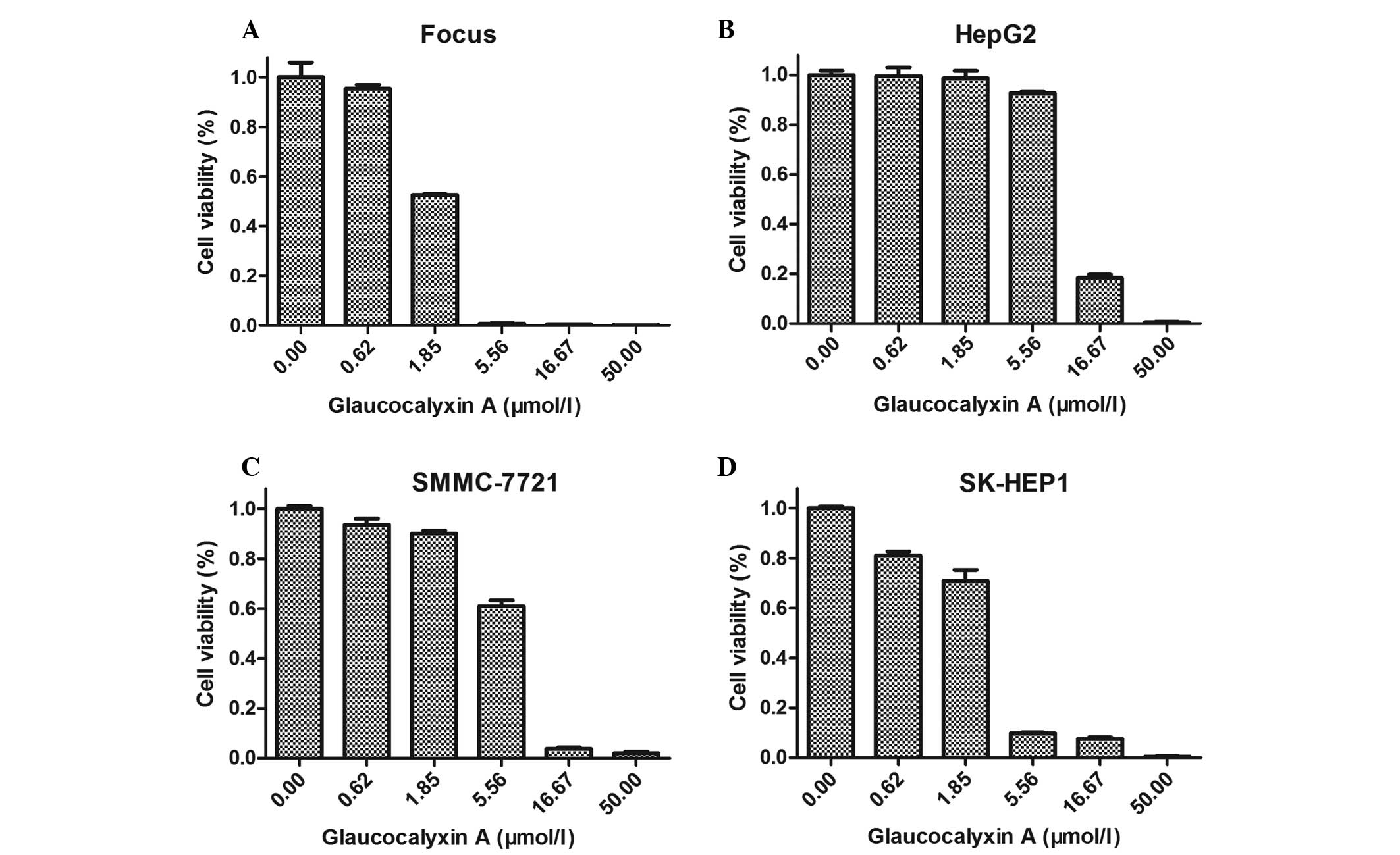
GLA inhibits the growth of liver
cancer cells
For additional observation of the effect of GLA
treatment, the liver cancer cells were plated in 96-well plates and
incubated with DMEM and various concentrations of GLA (0, 0.62,
1.85, 5.56, 16.67 and 50.00 µmol/l) for 48 h. DMEM with the same
volume of DMSO was set as a control. With increased concentrations
of GLA (0, 0.62, 1.85, 5.56, 16.67 and 50.00 µmol/l), the liver
cancer cells became round, floated and underwent cell death. The
decrease of viable cell numbers with various concentrations of GLA
is demonstrated in Fig. 3. The half
maximal inhibitory concentration of GLA in Focus, SMMC-7721, HepG2
and SK-HEP1 cells was 2.70±0.09, 5.58±0.20, 8.22±0.56 and 2.87±0.36
µmol/l, respectively. Therefore, GLA inhibits the growth of liver
cancer cells, particularly Focus and SK-HEP1 cells.
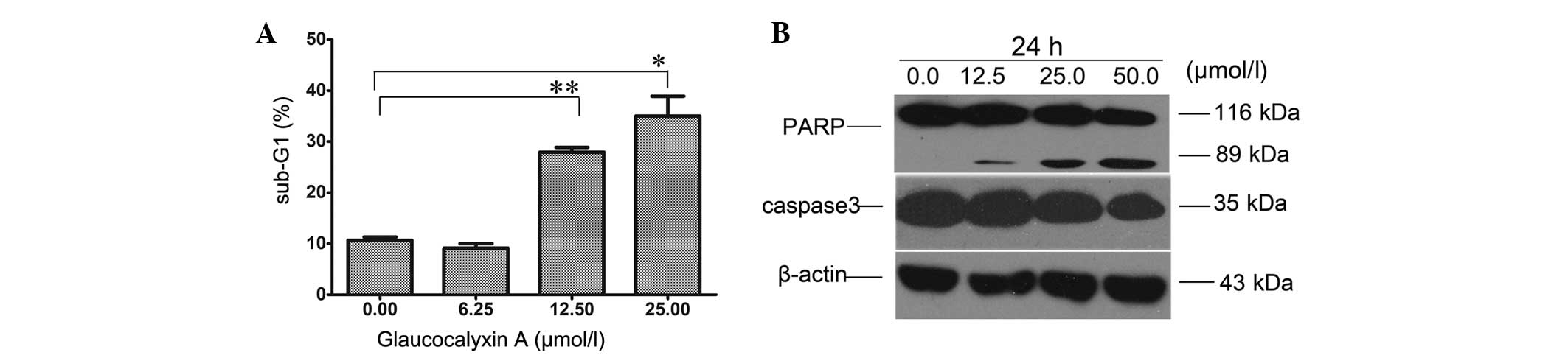
GLA induces apoptosis in liver cancer
cells
Inhibition of proliferation and induction of
apoptosis in cancer cells are each effective methods to clear
tumors. To investigate whether the cell death of liver cancer cells
induced by GLA was due to apoptosis, the present study analyzed
Focus cells treated with GLA by FCM. The sub-G1 cell subset was
observed to reflect the percentage of apoptotic cells. Focus cells
incubated with GLA demonstrated a significant trend of
dose-dependent apoptosis (Fig. 4A).
When the cells were incubated with GLA for 48 h, the apoptotic rate
increased slowly when the GLA concentration was <6.25 µmol/l,
and then rose rapidly when the concentration increased to 12.50
µmol/l. Western blotting revealed that cleaved-PARP was observed
when the cells were treated with 12.50 µmol/l of GLA. There was a
decrease in the expression of caspase 3 and full length PARP (116
kDa), and a dose-dependent increase in cleaved PARP (89 kDa)
(Fig. 4B). These findings indicate
that GLA treatment leads to the apoptosis of liver cancer
cells.
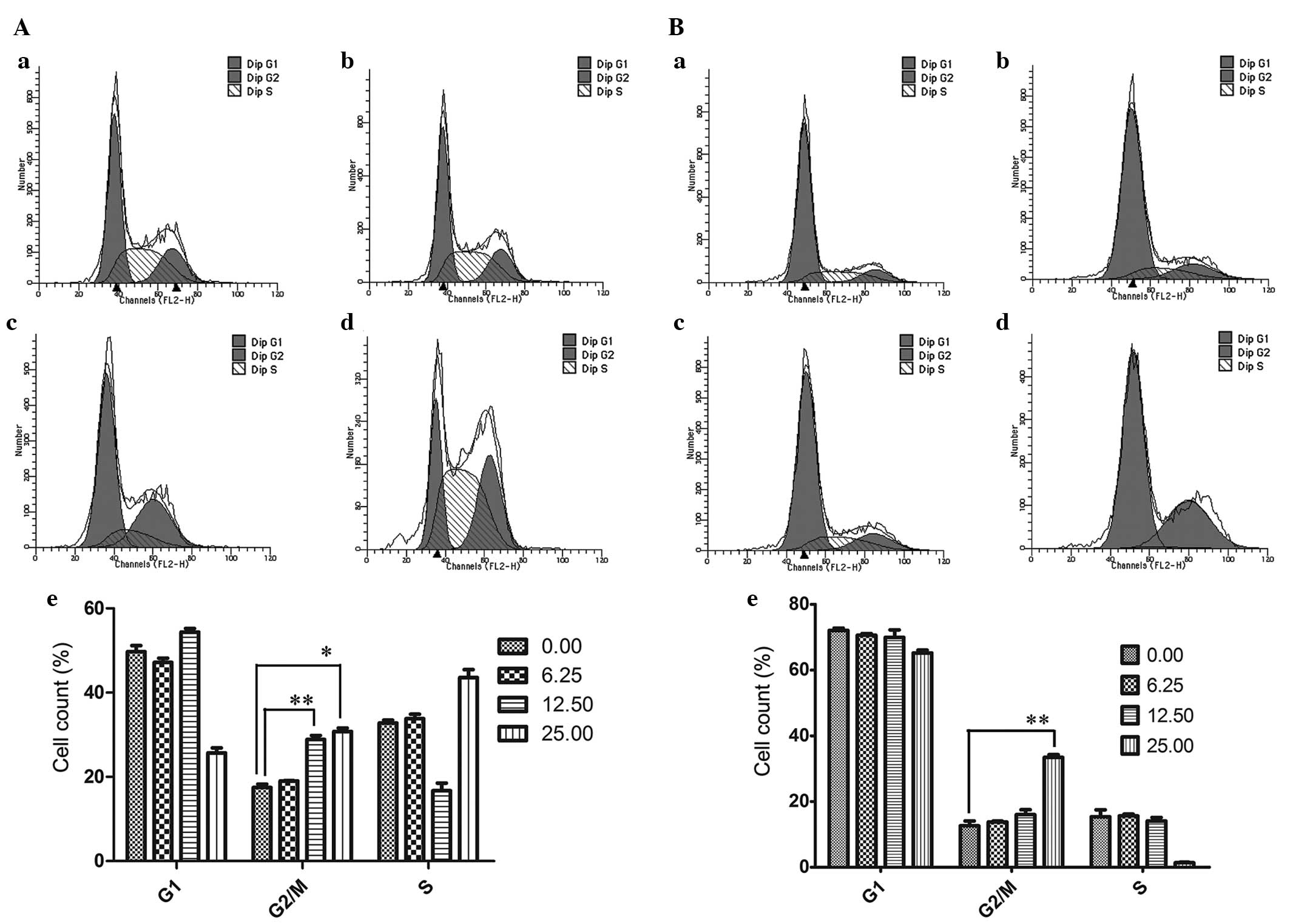
GLA induced G2/M arrest in liver
cancer cells
The effect of GLA on cell cycle progression in Focus
and SMMC-7721 cells was determined following 24 h of GLA treatment
(0.00, 6.25, 12.50 and 25.00 µmol/l) using FCM. As indicated in
Fig. 5A, there was a significant
increase in the proportion of Focus cells in the G2/M phase when
the concentration of GLA was >12.50 µmol/l. The alteration of
cell cycle distribution in SMMC-7721 cells was similar to that in
Focus cells, but the increase of the proportion of SMMC-7721 cells
in the G2/M phase was not significant until the cells were treated
with 25.00 µmol/l GLA (Fig. 5B).
Discussion
GLA is isolated from the leaves of R.
umbrosa, which has been used in Asia for centuries as a
medicinal herb for diminishing inflammation, invigorating the
circulation of blood and relieving swelling and pain (20,21).
Consequently, verifying the effect that GLA has against liver
cancer cells would be valuable. GLA is a natural form of
ent-kaurene diterpenoids. Ent-kaurene diterpenoids
isolated from the same Isodon plants were selectively toxic
against tumor cell lines (22). The
present study confirmed that GLA is toxic to SMMC-7721 cells
(Fig. 3), which supported the results
of a previous study (23). In
addition, the present study demonstrated that GLA is also toxic to
various cancer cells; however, the sensitivity to GLA varied
between the cancer types. This may be due to GLA being involved in
various gene expression pathways, which vary between cancer cells.
For the same reason, certain liver cancer cells are more sensitive
to GLA, including the Focus and SK-HEP1 cells.
Kaurene diterpenes exhibit toxicity against tumor
cells through various methods. The ent-kaurene diterpenoid
ent-16β-17α-dihydroxykaurane triggered apoptosis of MCF-7
cells by a decrease in telomerase mRNA (24). Oridonin, ponicidin, xindongnin A and
xindoninin have been demonstrated to be potent inhibitors of NF-κB
transcription activity (25). To
investigate the possible mechanism for the anti-proliferative
ability of GLA, the present study examined the DNA content of liver
cancer cells by FCM. Apoptotic cells were identified by the
appearance of a sub-G1 peak. Focus cells treated with GLA
demonstrated a significant increase in the sub-G1 fraction
(Fig. 4A), which indicated that GLA
induced apoptosis in liver cancer cells.
One of the major apoptosis pathways involves the
release of cytochrome c from mitochondria into the cytosol
of cells. Cytosolic cytochrome c induces caspase 9-dependent
activation of caspase 3 and cleavage of the DNA repair protein PARP
(26). As shown in Fig. 4B, the expression of caspase 3 and
full-length PARP was decreased in GLA-treated cells, while the
expression of cleaved PARP increased in a dose-dependent manner.
Together, the slight alteration of caspase 3 expression and
significant alteration of the sub-G1 fraction suggest that GLA may
affect the mitochondrial pathway, including the caspase cascade, to
induce apoptosis in Focus cells.
Cell cycle arrest is also an effective method of
inhibiting the proliferation of cells. The deregulation of the cell
cycle is one of the most frequent alterations observed during tumor
development (27), and a cell cycle
blockade is regarded as an effective strategy for eliminating
cancer cells (28). The G1/S and G2/M
checkpoints are two major regulated cell cycle checkpoints. The
present study analyzed the cell cycle progression in Focus and
SMMC-7721 cells treated with GLA for 24 h. The results indicate a
significant G2/M stage arrest in Focus and SMMC-7721 cells in a GLA
dose-dependent manner (Fig. 5). This
indicates that GLA induces G2/M arrest and therefore apoptosis of
Focus and SMMC-7721 cells.
In summary, the present study, to the best of our
knowledge, is the first to demonstrate that GLA has an inhibitory
effect on the growth of Focus and SMMC-7721 cells. This may be due
to G2/M cell cycle arrest and apoptosis being induced in cancer
cells, although the target molecule of the GLA remains unknown.
Acknowledgements
This present study was supported by the National Key
Sci-Tech Special Project of China (grant no. 2013ZX10002010).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhong JH, Li H, Xiao N, Ye XP, Ke Y, Wang
YY, Ma L, Chen J, You XM, Zhang ZY, et al: Hepatic resection is
safe and effective for patients with hepatocellular carcinoma and
portal hypertension. PloS One. 9:e1087552014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahbari NN, Ulrich AB, Bruckner T, Münter
M, Nickles A, Contin P, Löffler T, Reissfelder C, Koch M, Büchler
MW and Weitz J: Surgery for locally recurrent rectal cancer in the
era of total mesorectal excision, Is there still a chance for cure?
Ann Surg. 253:522–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takayama T: Surgical treatment for
hepatocellular carcinoma. Jpn J Clin Oncol. 41:447–454. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagai T, Arao T, Furuta K, et al:
Sorafenib inhibits the hepatocyte growth factor-mediated epithelial
mesenchymal transition in hepatocellular carcinoma. Mol Cancer
Ther. 10:169–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Usami Y, Takaoka I, Ichikawa H, Horibe Y,
Tomiyama S, Ohtsuka M, Imanishi Y and Arimoto M: First total
synthesis of antitumor natural product (+)- and (−)-pericosine A,
Determination of absolute stereo structure. J Org Chem.
72:6127–6134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Satooka H, Isobe T, Nitoda T and Kubo I:
Melanogenesis inhibitors from Rabdosia japonica.
Phytomedicine. 19:1016–1023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun HD, Huang SX and Han QB: Diterpenoids
from Isodon species and their biological activities. Nat Prod Rep.
23:673–698. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim BW, Koppula S, Hong SS, Jeon SB, Kwon
JH, Hwang BY, Park EJ and Choi DK: Regulation of microglia activity
by glaucocalyxin-A: Attenuation of lipopolysaccharide-stimulated
neuroinflammation through NF-κB and p38 MAPK signaling pathways.
PloS One. 8:e557922013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao X, Cao W, Jiang X, Zhang W, Zhang Y,
Liu B, Cheng J, Huang H, Huo J and Zhang X: Glaucocalyxin A, a
negative Akt regulator, specifically induces apoptosis in human
brain glioblastoma U87MG cells. Acta Biochim Biophys Sin
(Shanghai). 45:946–952. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang B and Long K: Effects of
glaucocalyxin A on aggregation and cAMP levels of rabbit platelets
in vitro. Zhongguo Yao Li Xue Bao. 14:347–350.
1993.PubMed/NCBI
|
|
14
|
Liu MJ, Sun Q, Yu LJ, Bao GL and Zhao M:
Protective effect of glaucocalyxin A inhibits H2O2-induced H9c2
cardiomyocytes injury. Latin American J Pharmacy. 33:1216–1220.
2014.
|
|
15
|
Gao LW, Zhang J, Yang WH, Wang B and Wang
JW: Glaucocalyxin A induces apoptosis in human leukemia HL-60 cells
through mitochondria-mediated death pathway. Toxicol In Vitro.
25:51–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang WH, Zhang Z, Sima YH, Zhang J and
Wang JW: Glaucocalyxin A and B-induced cell death is related to GSH
perturbation in human leukemia HL-60 cells. Anticancer Agents Med
Chem. 13:1280–1290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Q, Wang X, Zhang Y, Li CJ, Hu LH and
Shen X: Leukamenin F suppresses liver fibrogenesis by inhibiting
both hepatic stellate cell proliferation and extracellular matrix
production. Acta Pharmacol Sin. 31:839–848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ying N, Xu CL, Zhang J, Fu Q and Ma SP:
The effects of glaucocalyxin A in hepatic cancer HepG2 cell line.
Strait Pharm J. 24:235–238. 2012.
|
|
19
|
Wigmore PM, Mustafa S, El-Beltagy M, Lyons
L, Umka J and Bennett G: Effects of 5-FU. Adv Exp Med Biol.
678:157–164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuji K, Node M, Ito N, Fujita E, Takeda S
and Unemi N: Terpenoids L. antitumor-activity of diterpenoids from
Rabdosia shikokiana var. occidentalis. Chem Pharm Bull.
33:1038–1042. 1985. View Article : Google Scholar
|
|
21
|
Takeda Y, Fujita T and Ueno A: Structures
Of Leukamenins. Chem Lett. 10:1229–1232. 1981. View Article : Google Scholar
|
|
22
|
Ding L, Hou Q, Zhou Q, Zhang Q, Hou T and
Liu G: Structure-activity relationships of eight ent-kaurene
diterpenoids from three Isodon plants. Res Chem Intermed.
36:443–452. 2010. View Article : Google Scholar
|
|
23
|
Yang J, Liu Y, Xue C, Yu W, Zhang J and
Qiao C: Synthesis and biological evaluation of glaucocalyxin A
derivatives as potential anticancer agents. Eur J Med Chem.
86:235–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morales A, Pérez P, Mendoza R, Compagnone
R, Suarez AI, Arvelo F, Ramírez JL and Galindo-Castro I: Cytotoxic
and proapoptotic activity of ent-16beta-17alpha-dihydroxykaurane on
human mammary carcinoma cell line MCF-7. Cancer Lett. 218:109–116.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geng S, Sun B, Lu R and Wang J: Coleusin
factor, a novel anticancer diterpenoid, inhibits osteosarcoma
growth by inducing bone morphogenetic protein-2-dependent
differentiation. Mol Cancer Ther. 13:1431–1441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
27
|
Park MT and Lee SJ: Cell cycle and cancer.
J Biochem Mol Biol. 36:60–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Buolamwini JK: Cell cycle molecular
targets in novel anticancer drug discovery. Curr Pharm Des.
6:379–392. 2000. View Article : Google Scholar : PubMed/NCBI
|