Introduction
Gastric cancer is the fourth most common malignancy
and the second leading cause of cancer-related mortality worldwide
(1,2).
Patients with advanced gastric cancer frequently have metastases to
the lymph nodes and occasionally to distant organs. Lymph node
metastasis is controlled to a certain extent in these patients by
curative procedures, although gastric cancer patients with distant
organ metastasis exhibit a poor prognosis. Stage classification,
composed of the tumor depth, lymph node metastasis and distant
metastasis, generally predicts the post-operative prognosis of the
patient. However, the survival time differs among patients with the
same stage, as the biological characteristics of individual tumors
vary. Therefore, identifying novel biological molecules determining
the tumor aggressiveness of gastric cancer may be useful for
precisely predicting patient survival. Moreover, clarifying further
biological markers for gastric cancer would improve individual
pathogenetic treatment matching for patients with gastric
cancer.
A hypoxic environment is frequently present in solid
tumors and has been recognized to be associated with high-grade
cancers and anticancer drug resistance (3). Hypoxia inducible factor-1 (HIF-1) is a
transcription factor that plays a central role in the hypoxic
environment by controlling the expression of target genes that
regulate energy metabolism, cell proliferation, cell death, cell
migration and angiogenesis (4–10). HIF-1
is a heterodimer composed of a constitutively expressed HIF-1β
subunit and an O2 level-regulated HIF-1α subunit
(3–7).
At present, HIF-1α is widely known to be a master regulator that
accelerates tumor invasion and metastasis in solid tumors,
including gastric cancers (3–5). In our recent study, a gastric cancer
cell line, 58As9-KD, was established in which HIF-1α expression is
completely knocked down by small interfering (si)RNA transfection,
and this transfectant was used to describe the critical role of
HIF-1α expression in the development of the peritoneal metastasis
of gastric cancer (11). HIF-1α
overexpression has been immunohistochemically detected in a variety
of cancer types, including prostate, breast, lung, brain, gastric,
and head and neck cancers (3,4,5,10), and is associated with tumor
aggressiveness, vascularity, treatment failure and mortality,
resulting in a poor patient prognosis (3,4,5,8,9,10,12).
Angiopoietin-like protein 4 (ANGPTL4) is a member of
the angiopoietin family and is also known as peroxisome
proliferator-activated receptor γ-induced angiopoietin-related
protein, fasting-induced adipose factor or hepatic
fibrinogen/angiopoietin-related protein. This protein is a
circulating glycoprotein that is highly expressed within adipose
tissue, the liver and the placenta (13–16). The
native full-length ANGPTL4 (flANGPTL4) is a fusion protein
consisting of an N-terminal coiled-coil domain (nANGPTL4) and a
large ANG/fibrinogen-like COOH-terminal domain (cANGPTL4) (17–19); these
three domains have been shown to exhibit distinct biological
functions (18). Furthermore, ANGPTL4
has been reported to exhibit diverse effects, including lipid
metabolism, glucose metabolism, vascular permeability,
angiogenesis, wound healing and tumorigenesis, in normal and
malignant cells (17,20–23). Among
these biological effects, recent studies have focused on the
critical roles of ANGPTL4 in tumor progression in various cancers,
including hepatocellular carcinoma (HCC) (24), colorectal cancer (25–27),
breast cancer (28,29), prostate cancer (30), renal cell carcinoma (31,32) and
Kaposi's sarcoma (33,34). In addition, ANGPTL4 is known to be a
hypoxia-induced gene. It was previously reported that HIF-1α
directly upregulates ANGPTL4 in HCC cells, and a high level of
ANGPTL4 secretion in HCC patients is correlated with intrahepatic
metastasis (24). However, there have
been few studies on the association between gastric cancer and
ANGPTL4 expression (35).
Furthermore, the association between ANGPTL4 and the HIF-1α
expression has not yet been studied in gastric cancer.
The present study evaluated the hypoxia-induced
expression of ANGPTL4 in various gastric cancer cell lines. Using
58As9-KD cells, the study assessed whether ANGPTL4 expression is
dependent of HIF-1α under hypoxic conditions (1% O2).
Immunohistochemical examinations were also performed using
surgically excised specimens obtained from 170 patients with
gastric cancer in order to determine the the association between
ANGPTL4 and HIF-1α expression and the clinicopathological
factors.
Materials and methods
Cell lines and treatment
A total of 10 gastric cancer cell lines (MKN1, MKN7,
MKN28, MKN45, MKN74, HSC45, HSC57, 44As3, 58As9 and KATO-III) were
used for the following studies. HSC45, HSC57, 44As3 and 58As9 were
provided by Dr K. Yanagihara (National Cancer Institute, Tokyo,
Japan), while the remaining six cell lines were purchased from Cell
Bank, Riken BioResource Center (Ibaraki, Japan). The cells were
cultured in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA)
and maintained under either normoxic (20% O2 and 5%
CO2 in air) or hypoxic (1% O2, 5%
CO2 and 94% N2) conditions.
Patients
A total of 170 patients with advanced gastric cancer
who consecutively underwent curative surgery at the Department of
Surgery, Saga University Hospital (Saga, Japan) between June 2000
and December 2008 were enrolled in the present study. None of the
patients presented with hepatic, peritoneal or distant metastasis
or tumor cells in the peritoneal fluid. Stage classification was
performed according to the guidelines of the Japanese Gastric
Cancer Association (36). The
clinicopathological characteristics of the patients were recorded
(Table I).
 | Table I.Characteristics of the patients and
tumors. |
Table I.
Characteristics of the patients and
tumors.
|
Characteristics | Value |
|---|
| Patients, n
(%) | 170 (100.0) |
| Age, years |
|
|
Median | 71 |
|
Range | 26–88 |
| Gender, n (%) |
|
|
Male | 113 (66.5) |
|
Female | 57
(33.5) |
| Surgery, n (%) |
|
| Distal
gastrectomy | 71
(41.8) |
| Total
gastrectomy | 98
(57.6) |
|
Proximal gastrectomy | 1
(0.59) |
| Histology, n
(%) |
|
|
Differentiated | 68
(40.0) |
|
Undifferentiated | 102 (60.0) |
| Tumor depth, n
(%) |
|
| 2 | 46
(27.0) |
| 3 | 69
(40.6) |
| 4a | 51
(30.0) |
| 4b | 4
(2.4) |
| Lymph node
metastasis, n (%) |
|
| 0 | 63
(37.1) |
| 1 | 36
(21.2) |
| 2 | 26
(15.3) |
| 3a | 16 (9.4) |
| 3b | 29
(17.0) |
| Lymphatic invasion,
n (%) |
|
| − | 36
(21.2) |
| + | 134 (78.8) |
| Vascular invasion,
n (%) |
|
| − | 90
(52.9) |
| + | 80
(47.1) |
| Stage, n (%) |
|
| IB | 28
(16.5) |
|
IIA | 36
(21.2) |
|
IIB | 26
(15.3) |
|
IIIA | 29
(17.0) |
|
IIIB | 26
(15.3) |
|
IIIC | 25
(14.7) |
| Adjuvant |
|
| − | 102 (60.0) |
| + | 68
(40.0) |
Informed consent to use the tissue specimens was
obtained from each patient, and the study protocol was approved by
the Ethics Committee of Saga University Faculty of Medicine (no.
26–45).
Establishment of the HIF-1α-knockdown
cell line, 58As9-KD, using siRNA
The pBAsi-hU6 Pur DNA plasmid vector (Takara
Biotechnology, Shiga, Japan) was used to construct a HIF-1α siRNA
plasmid by inserting a siRNA-coding sequence under the U6 promoter.
The sequences of siRNA targeting HIF-1α and control scrambled siRNA
were designed as follows: HIF-1α (5′-CCACATTCACGTATATGAT-3′) and
scrambled (5′-TCTTAATCGCGTATAAGGC-3′). The 58As9 cells were
transfected using a MicroPorator-mini (MP-100; Digital Bio
Technology, Seoul, Korea) according to the manufacturer's
instructions. In order to generate HIF-1α-knockdown cells
(58As9-KD) and control cells (58As9-SC) with stable transfection of
the aforementioned sequences, the cells were selected with
puromycin (Sigma-Aldrich) at a concentration of 1.0–2.5 µg/ml and
maintained in complete medium supplemented with puromycin, as
previously described (11).
Western blot analysis
Whole cell lysates from cultured cells were prepared
using lysis buffer, as described previously (11). Aliquots containing 30 µg of protein
were subjected to 4–12% Bis-Tris gel electrophoresis (NuPAGE;
Invitrogen) and transferred onto Amersham Hybond-ECL membranes (GE
Healthcare, Buckinghamshire, UK) in transfer buffer. Subsequent to
being blocked with 5% skimmed milk for 30 min, the membranes were
incubated with primary antibodies overnight at 4°C. The primary
antibodies were monoclonal rabbit anti-human HIF-1α (cat. no.
EP1215Y; 1:1,000 dilution; Epitomics, Burlingame, CA, USA) and
anti-β-actin (cat. no. A5441; 1:10,000 dilution; Sigma-Aldrich).
Following incubation with the corresponding goat anti-rabbit IgG
secondary antibodies (cat. no. sc-2004; 1:10,000 dilution; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), the signals were
developed using the Amersham ECL Plus Western Blotting Detection
System (GE Healthcare).
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from each cell line using an
extraction kit (ISOGEN; Nippon Gene, Osaka, Japan). For each cell
line, 1 µg RNA was converted into complementary (c)DNA using a
reverse transcription reaction kit (ReverTra Ace; Toyobo Co., Ltd.,
Osaka, Japan). The cDNA was used as a template for PCR, and RT-qPCR
was performed using the Light Cycler instrument system (Roche
Diagnostics GmbH, Mannheim, Germany), as previously described
(11). The primers were designed
according to the cDNA sequences (GenBank, Bethesda, MD) as follows:
ANGPTL4 sense, 5′-TCCGTACCCTTCTCCACTTG-3′ and antisense,
5′-AGTACTGGCCGTTGAGGTTG-3′ (124 bp); carbonic anhydrase 9 (CA9)
sense, 5′-CCGAGCGACGCAGCCTTTGA-3′ and antisense,
5′-GGCTCCAGTCTCGGCTACCT-3′ (252 bp); and β-actin sense,
5′-TCGTGCGTGACATTAAGGAG-3′ and antisense,
5′-GTCAGGCAGCTCGTAGCTCT-3′ (109 bp). After performing a
denaturation step at 95°C for 3 min, 50 cycles PCR amplification
was conducted (15 sec of denaturation at 95°C, 5 sec of annealing
at 60°C and 10 sec of extension at 72°C). The quantitative values
were normalized to the β-actin expression. All experiments were
performed in triplicate, and the mean values calculated.
Immunohistochemistry
The immunohistochemical analyses of ANGPTL4 and
HIF-1α were performed as previously described (4,35). In
brief, formalin-fixed, paraffin-embedded samples were sectioned to
4-µm wide. For antigen retrieval, the tissue sections were heated
in 1 mM EDTA (pH 8.0) in a microwave for 5 min. The slides were
then incubated in the humidified chamber at room temperature for 2
h with a primary polyclonal goat anti-human ANGPTL4 antibody (cat.
no. AF3485; 1:500 dilution; R&D Systems, Inc., Minneapolis, MA,
USA) or primary monoclonal mouse anti-human HIF-1α antibody (cat.
no. NB100-105; 1:200 dilution; Novus Biologicals, Littleton, CO,
USA). Subsequent to being washed in phosphate-buffered saline,
biotinylated anti-goat immunoglobulin G (IgG) for ANGPTL4 and
anti-mouse IgG for HIF-1α, conjugated to a peroxidase-labeled
dextran polymer (Dako EnVision, Carpinteria, CA, USA), were used as
secondary antibodies. The 3,3′-diaminobenzidine substrate kit
(Nichirei Co., Tokyo, Japan) was used for color development.
Finally, nuclear counterstaining was performed with Mayer's
hematoxylin solution. The cells in the fundic gland in the normal
gastric tissue served as the internal positive control for ANGPTL4
immunostaining (35). The ANGPTL4
expression was divided into three categories according to the
percentage of positively-stained tumor cells as follows: 0–10%,
negative; 11–30%, weakly positive; and >31%, strongly positive.
The HIF-1α expression was divided into positive and negative
categories, as previously described (4). The HIF-1α expression was assessed in the
center, as well as at the invasive front of the tumor in each
section. Positive HIF-1α expression was determined if nuclear
staining was observed in the cancer center and at the invasive
front.
Statistical analysis
The statistical analyses were performed using the
computer software program IBM SPSS Statistics 19 for Windows (IBM
SPSS, Armonk, NY, USA). Differences in the mean values were
evaluated using Student's t-test. Analyses comparing the ANGPTL4
expression levels were performed with the χ2 test for
independence. Univariate and multivariate analyses for
disease-specific survival were performed using Cox's proportional
hazards model. Survival curves were generated using the
Kaplan-Meier method, and statistical differences were compared
using the log-rank test. P<0.05 was considered to indicate a
statistically significant difference.
Results
ANGPTL4 expression in the gastric
cancer cell lines
The ANGPTL4 mRNA expression levels in the 10 gastric
cancer cell lines under normoxic and hypoxic conditions are shown
in Fig. 1A. Under normoxia, ANGPTL4
was expressed in 7 cell lines (MKN1, MKN7, 44As3, 58As9, HSC45,
HSC57 and KATOIII), but not 3 other cell lines (MKN28, MKN45 and
MKN74). Notably, the expression levels were significantly elevated
under hypoxia in the 7 positive cell lines. Meanwhile, hypoxia did
not induce any expression in the 3 negative cell lines.
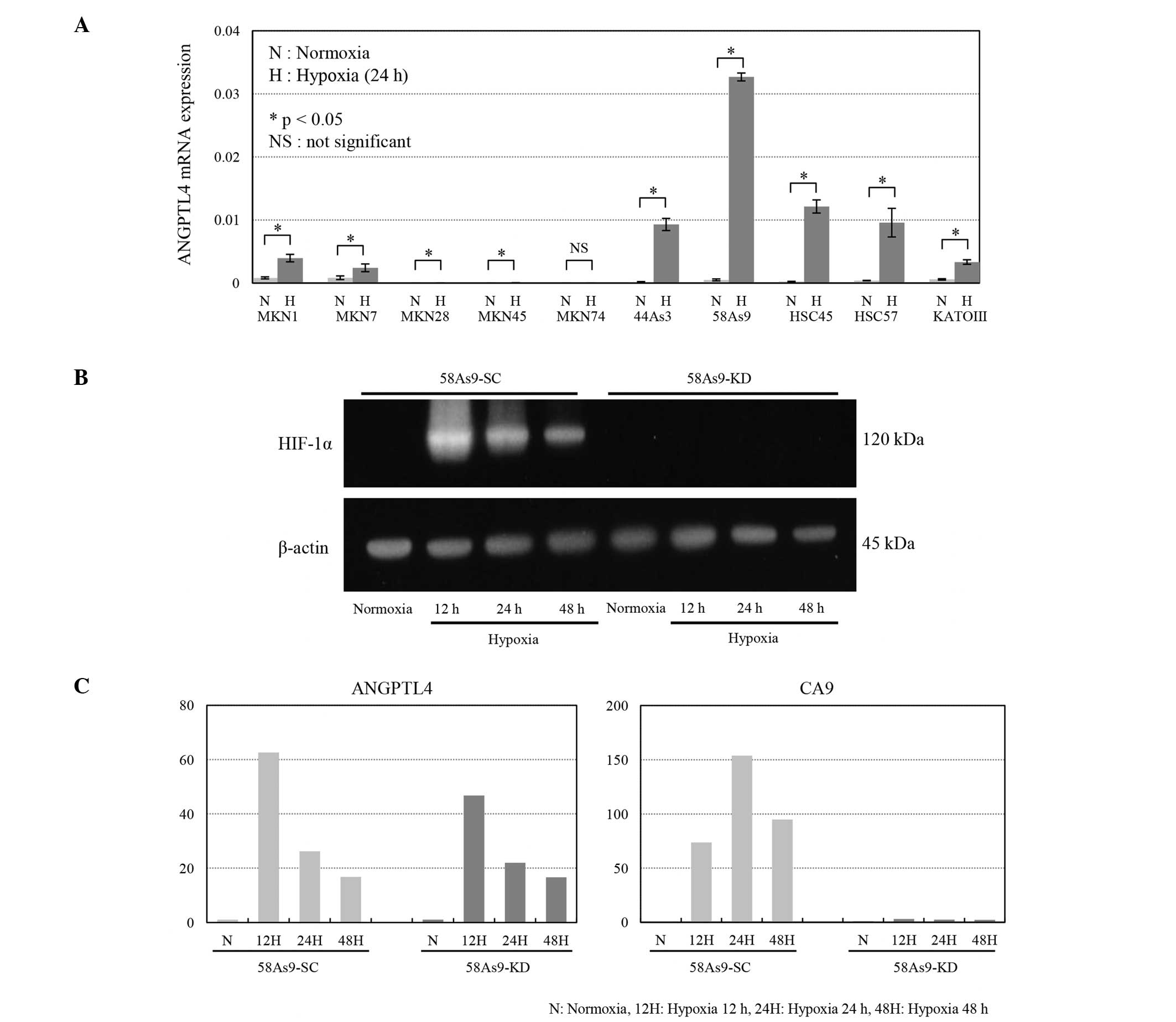 | Figure 1.ANGPTL4 expression in the gastric
cancer cell lines and HIF-1α-knockdown cells. (A) RT-qPCR for
ANGPTL4 was performed in 10 gastric cancer cell lines exposed to
normoxia (N; 20% O2) or hypoxia (H; 1% O2)
for 24 h. ANGPTL4 mRNA expression was significantly induced under
hypoxia in the MKN1, MKN7, 44As3, 58As9, HSC45, HSC57 and KATOIII
cells, but not in the MKN28, MKN45 or MKN74 cells. *P<0.05; NS,
not significant. (B) Western blot analysis of HIF-1α expression in
the HIF-1α-knockdown 58As9-KD cells and control 58As9-SC cells
under normoxia and hypoxia. The HIF-1α expression was strongly
induced in the 58As9-SC cells under hypoxia for 12, 24 and 48 h. By
contrast, HIF-1α expression was completely suppressed in the
58As9-KD cells under hypoxia for 12, 24 and 48 h. (C) RT-qPCR
analysis of the CA9 and ANGPTL4 expression in the 59As9-SC and
58As9-KD cells under normoxia (N) and hypoxia (H) for 12, 24 and 48
h. The hypoxia-induced expression of CA9 mRNA was drastically
suppressed in the 58As9-KD cells compared with that noted in the
58As9-SC cells. By contrast, mRNA expression was preserved under
hypoxia (at 12, 24 and 48 h) in the 58As9-KD cells compared with
that observed in the 58AS9-SC cells. HIF-1α, hypoxia inducible
factor 1α; ANGPTL4, angiopoietin-like protein 4; CA9, carbonic
anhydrase 9; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction. |
In order to investigate the effects of
HIF-1α-knockdown on gene expression, HIF-1α-knockdown 58As9-KD and
control 58As9-SC cells were used. Fig.
1B shows the complete knockdown of HIF-1α expression in the
58As9-KD cells under hypoxia for 12, 24 and 48 h, compared with the
strong induction of HIF-1α expression noted in the 58As9-SC cells.
Between these two transfectants, the mRNA expression levels of the
known HIF-1α target gene, CA9, and ANGPTL4 were compared under
conditions of normoxia and hypoxia (Fig.
1C). Consequently, the mRNA expression of CA9 in the 58As9-SC
cells was significantly induced under hypoxia, where the fold
induction was 74 times for 12 h, 154 times for 24 h and 95 times
for 48 h. The hypoxic induction of CA9 mRNA was markedly decreased
in the 58As9-KD cells, in which the fold induction was 1.3 times
for 12 h, 2.4 times for 24 h and 2.3 times for 48 h. On the other
hand, the ANGPTL4 mRNA expression in the 58As9-SC cells was induced
under hypoxia, with a fold induction of 63 times for 12 h, 26 times
for 24 h and 17 times for 48 h. The hypoxic induction of ANGPTL4
mRNA in the 58As9-KD cells was decreased by only a small amount
compared with that observed in the 58As9-SC cells, as the estimated
fold induction remained at 47 times for 12 h, 22 times for 24 h and
17 times for 48 h.
Immunohistochemistry for ANGPTL4 and
HIF-1α
The ANGPTL4 and HIF-1α expression levels were
evaluated using immunohistochemistry in 170 advanced gastric cancer
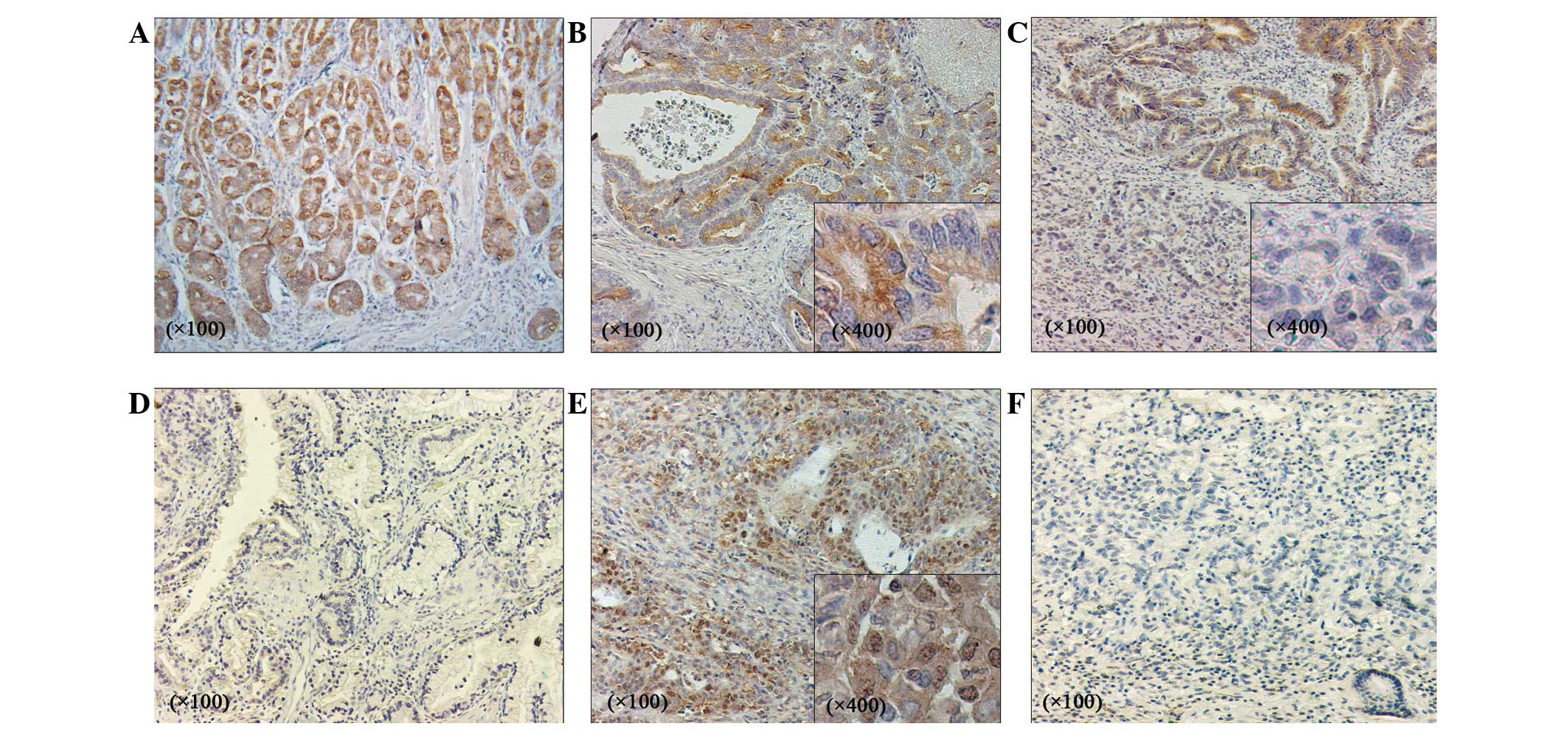
tissues (Fig. 2). In the normal
stomach tissues, ANGPTL4 expression was observed in the cytoplasm
of the fundic gland cells (Fig. 2A).
Strongly positive ANGPTL4 expression was observed in 21 out of 170
(12.4%) gastric adenocarcinoma tissues, showing strong staining in
the cytoplasm of the cancer cells (Fig.
2B). By contrast, weakly positive ANGPTL4 staining was observed
in 60 out of 170 (35.3%) samples (Fig.
2C), whereas negative staining was noted in 89 out of 170
(52.3%) samples (Fig. 2D). In
addition, the HIF-1α expression was immunohistochemically evaluated
in the 170 cancer specimens. Consequently, positive HIF-1α
expression with cytoplasmic and nuclear staining of the cancer
cells was noted in 95 out of 170 (55.9%) specimens (Fig. 2E), while negative staining was found
in 75 out of 170 (44.1%) specimens (Fig.
2F).
Patient characteristics and
clinicopathological features
The clinicopathological characteristics of the
gastric cancer patients who underwent gastrectomy are summarized in
Table I. The 170 patients consisted
of 113 males and 57 females, ranging in age from 26 to 88 years
(median, 71 years). The performed surgeries included distal
gastrectomy in 71 patients (41.8%), total gastrectomy in 98
patients (57.6%) and proximal gastrectomy in 1 patient (0.59%). The
histological diagnosis of the resected cancer tissues was
classified as a differentiated type of carcinoma in 68 cases
(40.0%) and an undifferentiated type in 102 cases (60.0%). The
tumor depth (T) was histologically determined as T2 in 46 cases
(27.1%), T3 in 69 cases (40.6%), T4a in 51 cases (30.0%) and T4b in
4 cases (2.4%), and the degree of lymph node metastasis was defined
as N0 in 63 cases (37.1%), N1 in 36 cases (21.2%), N2 in 26 cases
(15.3%), N3a in 16 cases (9.4%) and N3b in 29 cases (17.1%).
Positive vascular invasion into the lymph and blood vessels was
detected in 36 (21.2%) and 90 (52.9%) tissues, respectively. The
tumor stage was determined to be IB in 28 cases (16.5%), IIA in 36
cases (21.2%), IIB in 26 cases (16.5%), IIIA in 29 cases (15.3%),
IIIB in 26 cases (15.3%) and IIIC in 25 cases (14.7%).
Comparison between the
clinicopathological factors and ANGPTL4 and HIF-1α expression in
the 170 gastric cancer patients
Table II shows the
correlations between several clinicopathological factors and the
ANGPTL4 and HIF-1α expression levels. Strongly positive ANGPTL4
expression was found to be significantly correlated with the tumor
depth (T) (Table II). In addition,
cancer invasion was significantly deeper in the cases with weakly
positive or negative ANGPTL4 expression (n=149) compared with the
strongly positive cases (n=21) (P=0.032). Meanwhile, the tumor
stage tended to be lower in the strongly positive ANGPTL4cases
compared with the other cases; however, the difference was not
statistically significant (P=0.067). A comparative analysis of the
patients with strongly or weakly positive ANGPTL4 expression and
those with negative expression did not reveal any significant
differences among the clinicopathological factors (data not shown).
On the other hand, the patients with positive HIF-1α expression
presented with a significantly higher degree of vascular invasion
compared with those with negative expression (P=0.013) (Table II). There were no significant
correlations between strongly positive ANGPTL4 expression and
HIF-1α expression (Table II).
 | Table II.Correlations between the ANGPTL4 and
HIF-1α expression levels and clinicopathological factors. |
Table II.
Correlations between the ANGPTL4 and
HIF-1α expression levels and clinicopathological factors.
|
| ANGPTL4 | HIF-1α |
|---|
|
|
|
|
|---|
| Factor | Strongly positive
(n=21) | Weakly positive
plus negative (n=149) |
P-valuea | Positive
(n=95) | Negative
(n=75) |
P-valuea |
|---|
| Age, years
(mean±SD) | 69.2±9.91 | 68.4±11.4 | 0.759 | 68.5±12.0 | 68.6±10.2 | 0.975 |
| Gender, n |
|
|
|
|
|
|
|
Male | 13 | 100 | 0.629 | 64 | 49 | 0.870 |
|
Female | 8 | 49 |
| 31 | 26 |
|
| Histology, n |
|
|
|
|
|
|
|
Differentiated | 11 | 57 | 0.240 | 36 | 32 | 0.533 |
|
Undifferentiated | 10 | 92 |
| 59 | 43 |
|
| Tumor depth, n |
|
|
|
|
|
|
| 2 | 10 | 35 | 0.032 | 20 | 25 | 0.082 |
|
3/4 | 11 | 114 |
| 75 | 50 |
|
| Lymph node
metastasis, n |
|
|
|
|
|
|
| − | 9 | 54 | 0.631 | 37 | 26 | 0.632 |
| + | 12 | 95 |
| 58 | 49 |
|
| Lymphatic invasion,
n |
|
|
|
|
|
|
| − | 3 | 32 | 0.573 | 21 | 14 | 0.703 |
| + | 18 | 117 |
| 74 | 61 |
|
| Vascular invasion,
n |
|
|
|
|
|
|
| − | 14 | 78 | 0.249 | 43 | 49 | 0.013 |
| + | 7 | 71 |
| 52 | 26 |
|
| Stage, n |
|
|
|
|
|
|
|
I/II | 15 | 74 | 0.067 | 47 | 42 | 0.441 |
|
III | 6 | 75 |
| 48 | 33 |
|
| Adjuvant, n |
|
|
|
|
|
|
| − | 14 | 88 | 0.636 | 56 | 46 | 0.875 |
| + | 7 | 61 |
| 39 | 29 |
|
| ANGPTL4, n |
|
|
|
|
|
|
|
Strongly positive | − | − | − | 8 | 13 | 0.101 |
| Weakly
positive |
|
|
| 87 | 62 |
|
| plus
negative |
|
|
|
|
|
|
ANGPTL4 and HIF-1α expression levels
and patient outcomes
The associations between the patient outcomes and
the levels of ANGPTL4 and HIF-1α expression were statistically
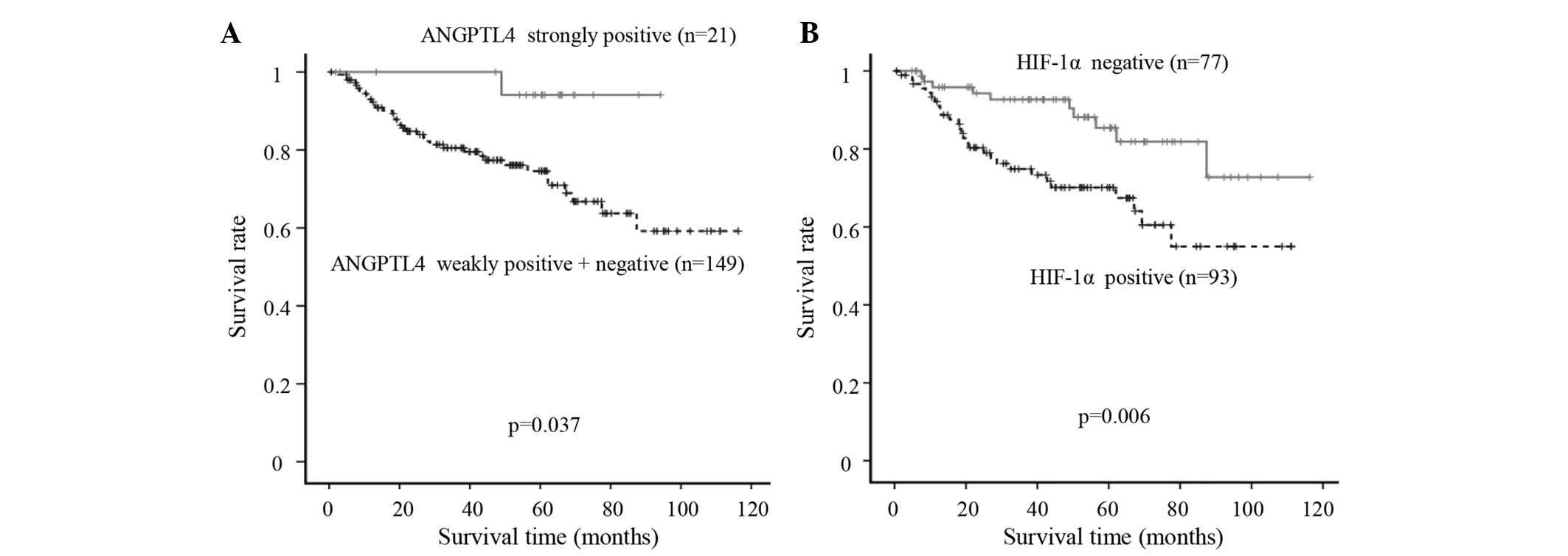
analyzed in 170 patients with advanced gastric cancer (Fig. 3). The disease-specific survival of the
patients with strongly positive ANGPTL4 expression (n=21) was
significantly more favorable than that of the other patients
(n=149) (P=0.037). By contrast, the disease-specific survival rate
of the HIF-1α-positive patients (n=93) was significantly worse than
that of the HIF-1α-negative patients (n=77) (P=0.006).
Univariate and multivariate analyses
of disease-specific survival
A univariate analysis of the 170 patients revealed
that the tumor depth, lymph node metastasis, lymphatic invasion,
vascular invasion, tumor stage, adjuvant chemotherapy and HIF-1α
expression were significantly associated with the disease-specific
survival (Table III). A
multivariate analysis using these factors was carried out according
to Cox's proportional hazards model. Consequently, the multivariate
analysis confirmed that lymph node invasion and HIF-1α expression
were independent predictive factors for disease-specific survival
(P=0.006 and P=0.024, respectively) (Table III).
 | Table III.Univariate and multivariate analyses
of the disease-specific survival of the 170 gastric cancer
patients. |
Table III.
Univariate and multivariate analyses
of the disease-specific survival of the 170 gastric cancer
patients.
|
| Disease-specific
survival |
|---|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
<65 | 1 |
|
|
|
|
≥65 | 1.245
(0.633–2.451) | 0.524 |
|
|
| Gender |
|
|
|
|
|
Male | 1 |
|
|
|
|
Female | 0.972
(0.500–1.891) | 0.934 |
|
|
| Histology |
|
|
|
|
|
Differentiated | 1 |
|
|
|
|
Undifferentiated | 0.876
(0.461–1.645) | 0.669 |
|
|
| Tumor depth |
|
|
|
|
| 2 | 1 |
| 1 |
|
|
3/4 | 4.854
(1.493–15.635) | 0.009 | 1.767
(0.498–6.250) | 0.379 |
| Lymph node
metastasis |
|
|
|
|
| − | 1 |
| 1 |
|
| + | 14.493
(3.497–58.824) | <0.001 | 10.204
(1.927–52.632) | 0.006 |
| Lymphatic
invasion |
|
|
|
|
| − | 1 |
| 1 |
|
| + | 4.016
(1.236–12.987) | 0.021 | 0.842
(0.212–3.344) | 0.807 |
| Vascular
invasion |
|
|
|
|
| − | 1 |
| 1 |
|
| + | 2.392
(1.245–4.587) | 0.009 | 1.727
(0.847–3.521) | 0.132 |
| Stage |
|
|
|
|
|
I/II | 1 |
| 1 |
|
|
III | 5.780
(2.646–12.658) | <0.001 | 1.883
(0.720–4.926) | 0.197 |
| Adjuvant |
|
|
|
|
| − | 1 |
| 1 |
|
| + | 2.169
(1.157–4.065) |
0.016 | 0.771
(0.390–1.524) | 0.455 |
| ANGPTL4 |
|
|
|
|
| Weakly
positive plus negative | 1 |
|
|
|
|
Strongly positive | 0.142
(0.019–1.034) | 0.054 |
|
|
| HIF-1α |
|
|
|
|
|
Negative | 1 |
| 1 |
|
|
Positive | 2.375
(1.186–4.762) | 0.015 | 2.336
(1.119–4.878) | 0.024 |
Discussion
The present study first investigated the expression
levels of ANGPTL4 mRNA in 10 gastric cancer cell lines under
normoxic and hypoxic conditions. Notably, ANGPTL4 expression was
significantly induced under hypoxia in 7 of the cell lines (MKN1,
MKN7, 44As3, 58As9, HSC45, HSC57 and KATOIII), whereas no such
expression was found in the remaining 3 cell lines (MKN8, MKN45 and
MKN74), under normoxia and hypoxia. In order to investigate whether
hypoxia-induced ANGPTL4 expression is HIF-1α dependent in gastric
cancer cells, ANGPTL4 expression was compared between the
HIF-1α-knockdown 58As9-KD cells and the control 58As9-SC cells
established from ANGLTL4-expressing 58As9 parent cells (11). The results of the analysis showed that
the hypoxic induction of ANGPTL4 was weakened with a small amount
of 58As9-KD cells compared with that observed in the 58As9-SC
cells. By contrast, the hypoxia-induced expression of CA9, which is
known to be directly regulated by HIF-1α, was markedly diminished
in the 58As9-KD cells compared with that observed in the 58As9-SC
cells. These results indicate that hypoxia-induced ANGPTL4
expression may be preserved without HIF-1α in hypoxic 58As9 gastric
cancer cells. It is possible that HIF-1α, as well as other HIF-a
family members, such as HIF-2α and HIF-3α, or other factors
regulate the hypoxia-induced ANGPTL4 expression in 58As9 cells
(7). Although HIF-1α dependency was
not determined in the 6 other cell lines expressing ANGPTL4, the
present results suggest that ANGPTL4 is induced under hypoxia
predominantly via an HIF-1α-independent pathway in gastric cancer
cells. In the immunohistochemical analysis, tumors with strongly
positive ANGPTL4 expression exhibited significantly less tumor
invasion. By contrast, those with positive HIF-1α expression
demonstrated significantly greater venous invasion. These results
reflect the inverse effect of ANGPTL4 and HIF-1α expression on
cancer invasiveness. Furthermore, the survival time of the patients
with strongly positive ANGPTL4 expression was significantly longer
than that associated with the other expression patterns.
Conversely, the survival time of the HIF-1α-positive patients was
significantly shorter than that of the HIF-1α-negative patients.
Moreover, the multivariate analysis revealed HIF-1α to be an
independent prognostic factor. Taken together, these results
suggest that the hypoxic induction of ANGPTL4 is independently
regulated by HIF-1α and that ANGPTL4 expression may inhibit cancer
invasion into the gastric wall, thus resulting in a longer survival
time among patients with strongly positive ANGPTL4 expression.
To date, several studies have addressed the emerging
roles of ANGPTL4 under conditions of tumor hypoxia. For example,
Kim et al reported that ANGPTL4 induction by prostaglandin
E2 under hypoxia promotes colorectal cancer growth (27), and Zhang et al demonstrated
that the inhibition of HIF-1α expression in breast cancer cells by
RNA interference disturbs primary tumor growth and metastasis in
severe combined immunodeficiency mice by blocking ANGPTL4
expression (28). Meanwhile, Li et
al reported that HIF-1α-activated ANGPTL4 expression
contributes to tumor metastasis via vascular cell adhesion
molecule-1/integrin β1 signaling in the setting of HCC (24). These studies demonstrate that
HIF-1α-induced ANGPTL4 expression increases cancer cell
aggressiveness under hypoxic conditions. By contrast, various
studies have also shown that increased ANGPTL4 expression inhibits
melanoma, lung and colorectal tumor growth, as well as metastasis
and angiogenesis (37,38). High ANGPTL4 expression in mouse tumors
also impairs tumor cell migration and invasiveness, thereby
inhibiting metastasis (37). These
studies demonstrate the inhibitory roles of ANGPTL4 in cancer
progression and support the findings of the present study. Although
the reasons for the aforementioned discrepancies are unclear, it
can be speculated that the flANGPTL4, nANGPTL4 and cANGPTL4
domains, which have distinct biological functions, are
differentially expressed in various cancers.
In conclusion, the present study demonstrated for
the first time that ANGPTL4 expression is predominantly regulated
via an HIF-1α-independent pathway under hypoxia in gastric cancer
cells. High ANGPTL4 expression may inhibit tumor invasion and
potentially serves as a favorable marker for predicting a long
survival time in advanced gastric cancer patients. Gastric cancer
tissues, which are exposed to a hypoxic environment, and HIF-1α
expression may increase malignant behavior by upregulating target
genes. By contrast, a hypoxic environment may induce ANGPTL4
expression via an HIF-1α-independent pathway and thus suppress
tumor invasion. Recombinant ANGPTL4 may therefore be useful as a
novel pharmacological agent for inhibiting the invasion of gastric
cancer cells.
References
|
1
|
Guggenheim DE and Shah MA: Gastric cancer
epidemiology and risk factors. J Surg Oncol. 107:230–236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality and prevalence across five
continents, Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical biologic and molecular aspects. J
Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitajima Y and Miyazaki K: The Critical
Impact of HIF-1α on Gastric Cancer Biology. Cancers (Basel).
5:15–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Semenza GL: Hypoxia-inducible factors: M
ediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Semenza GL: Hypoxia-inducible factor 1:R
egulator of mitochondrial metabolism and mediator of ischemic
preconditioning. Biochim Biophys Acta. 1813:1263–2168. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keith B, Johnson RS and Simon MC: HIF1α
and HIF2α: Sibling rivalry in hypoxic tumour growth and
progression. Nat Rev Cancer. 12:9–22. 2011.PubMed/NCBI
|
|
8
|
Rey S and Semenza GL: Hypoxia-inducible
factor-1-dependent mechanisms of vascularization and vascular
remodelling. Cardiovasc Res. 86:236–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koh MY, Lemos R Jr, Liu X and Powis G: The
hypoxia-associated factor switches cells from HIF-1α- to
HIF-2α-dependent signaling promoting stem cell characteristics,
aggressive tumor growth and invasion. Cancer Res. 71:4015–4027.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakamura J, Kitajima Y, Kai K, Hashiguchi
K, Hiraki M, Noshiro H and Miyazaki K: HIF-1alpha is an unfavorable
determinant of relapse in gastric cancer patients who underwent
curative surgery followed by adjuvant 5-FU chemotherapy. Int J
Cancer. 127:1158–1171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyake S, Kitajima Y, Nakamura J, Kai K,
Yanagihara K, Tanaka T, Hiraki M, Miyazaki K and Noshiro H: HIF-1α
is a crucial factor in the development of peritoneal dissemination
via natural metastatic routes in scirrhous gastric cancer. Int J
Oncol. 43:1431–1440. 2013.PubMed/NCBI
|
|
12
|
Yoshimura H, Dhar DK, Kohno H, Kubota H,
Fujii T, Ueda S, Kinugasa S, Tachibana M and Nagasue N: Prognostic
impact of hypoxia-inducible factors 1alpha and 2alpha in colorectal
cancer patients, Correlation with tumor angiogenesis and
cyclooxygenase-2 expression. Clin Cancer Res. 10:8554–8560. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm
CS, Lee ZH and Koh GY: Hepatic expression, synthesis and secretion
of a novel fibrinogen/angiopoietin-related protein that prevents
endothelial-cell apoptosis. Biochem J. 346:603–610. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mandard S, Zandbergen F, Tan NS, Escher P,
Patsouris D, Koenig W, Kleemann R, Bakker A, Veenman F, Wahli W, et
al: The direct peroxisome proliferator-activated receptor target
fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in
blood plasma as a truncated protein that is increased by
fenofibrate treatment. J Biol Chem. 279:34411–34420. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoon JC, Chickering TW, Rosen ED, Dussault
B, Qin Y, Soukas A, Friedman JM, Holmes WE and Spiegelman BM:
Peroxisome proliferator-activated receptor gamma target gene
encoding a novel angiopoietin-related protein associated with
adipose differentiation. Mol Cell Biol. 20:5343–5349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida K, Shimizugawa T, Ono M and
Furukawa H: Angiopoietin-like protein 4 is a potent
hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein
lipase. J Lipid Res. 43:1770–1772. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan MJ, Teo Z, Sng MK, Zhu P and Tan NS:
Emerging roles of angiopoietin-like 4 in human cancer. Mol Cancer
Res. 10:677–688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lei X, Shi F, Basu D, Huq A, Routhier S,
Day R and Jin W: Proteolytic processing of angiopoietin-like
protein 4 by proprotein convertases modulates its inhibitory
effects on lipoprotein lipase activity. J Biol Chem.
286:15747–15756. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ge H, Yang G, Huang L, Motola DL,
Pourbahrami T and Li C: Oligomerization and regulated proteolytic
processing of angiopoietin-like protein 4. J Biol Chem.
279:2038–2045. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oike Y, Akao M, Kubota Y and Suda T:
Angiopoietin-like proteins, Potential new targets for metabolic
syndrome therapy. Trends Mol Med. 11:473–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Katoh Y and Katoh M: Comparative
integromics on Angiopoietin family members. Int J Mol Med.
17:1145–1149. 2006.PubMed/NCBI
|
|
22
|
Hato T, Tabata M and Oike Y: The role of
angiopoietin-like proteins in angiogenesis and metabolism. Trends
Cardiovasc Med. 18:6–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu P, Goh YY, Chin HF, Kersten S and Tan
NS: Angiopoietin-like 4: A decade of research. Biosci Rep.
32:211–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Ge C, Zhao F, Yan M, Hu C, Jia D,
Tian H, Zhu M, Chen T, Jiang G, et al: Hypoxia-inducible factor 1
alpha-activated angiopoietin-like protein 4 contributes to tumor
metastasis via vascular cell adhesion molecule-1/integrin β1
signaling in human hepatocellular carcinoma. Hepatology.
54:910–919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakayama T, Hirakawa H, Shibata K, Nazneen
A, Abe K, Nagayasu T and Taguchi T: Expression of angiopoietin-like
4 (ANGPTL4) in human colorectal cancer, ANGPTL4 promotes venous
invasion and distant metastasis. Oncol Rep. 25:929–935. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akishima-Fukasawa Y, Ishikawa Y, Akasaka
Y, Uzuki M, Inomata N, Yokoo T, Ishii R, Shimokawa R, Mukai K,
Kiguchi H, et al: Histopathological predictors of regional lymph
node metastasis at the invasive front in early colorectal cancer.
Histopathology. 59:470–481. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SH, Park YY, Kim SW, Lee JS, Wang D
and DuBois RN: ANGPTL4 induction by prostaglandin E2 under hypoxic
conditions promotes colorectal cancer progression. Cancer Res.
71:7010–7020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Wong CC, Wei H, Gilkes DM,
Korangath P, Chaturvedi P, Schito L, Chen J, Krishnamachary B,
Winnard PT Jr, et al: HIF-1-dependent expression of
angiopoietin-like 4 and L1CAM mediates vascular metastasis of
hypoxic breast cancer cells to the lungs. Oncogene. 31:1757–1770.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Padua D, Zhang XH, Wang Q, Nadal C, Gerald
WL, Gomis RR and Massagué J: TGFbeta primes breast tumors for lung
metastasis seeding through angiopoietin-like 4. Cell. 133:66–77.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ifon ET, Pang AL, Johnson W, Cashman K,
Zimmerman S, Muralidhar S, Chan WY, Casey J and Rosenthal LJ: U94
alters FN1 and ANGPTL4 gene expression and inhibits tumorigenesis
of prostate cancer cell line PC3. Cancer Cell Int. 5:192005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Le Jan S, Amy C, Cazes A, Lamandé N,
Favier J, Philippe J, Sibony M, Gasc JM, Corvol P and Germain S:
Angiopoietin-like 4 is a proangiogenic factor produced during
ischemia and in conventional renal cell carcinoma. Am J Pathol.
162:1521–1528. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Verine J, Lehmann-Che J, Soliman H,
Feugeas JP, Vidal JS, Mongiat-Artus P, Belhadj S, Philippe J,
Lesage M, Wittmer E, et al: Determination of angptl4 mRNA as a
diagnostic marker of primary and metastatic clear cell renal-cell
carcinoma. PLoS One. 5:e104212010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu J, Jham BC, Ma T, Friedman ER, Ferreira
L, Wright JM, Accurso B, Allen CM, Basile JR and Montaner S:
Angiopoietin-like 4: A novel molecular hallmark in oral Kaposi's
sarcoma. Oral Oncol. 47:371–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma T, Jham BC, Hu J, Friedman ER, Basile
JR, Molinolo A, Sodhi A and Montaner S: Viral G protein-coupled
receptor up-regulates Angiopoietin-like 4 promoting angiogenesis
and vascular permeability in Kaposi's sarcoma. Proc Natl Acad Sci
USA. 107:14363–14368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakayama T, Hirakawa H, Shibata K, Abe K,
Nagayasu T and Taguchi T: Expression of angiopoietin-like 4 in
human gastric cancer, ANGPTL4 promotes venous invasion. Oncol Rep.
24:599–606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Japanese Gastric: CancerA ssociation.
Histopathology. Gastric Cancer. 14:101–112. 2011.PubMed/NCBI
|
|
37
|
Galaup A, Cazes A, Le Jan S, Philippe J,
Connault E, Le Coz E, Mekid H, Mir LM, Opolon P, Corvol P, et al:
Angiopoietin-like 4 prevents metastasis through inhibition of
vascular permeability and tumor cell motility and invasiveness.
Proc Natl Acad Sci USA. 103:18721–18726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ito Y, Oike Y, Yasunaga K, Hamada K,
Miyata K, Matsumoto S, Sugano S, Tanihara H, Masuho Y and Suda T:
Inhibition of angiogenesis and vascular leakiness by
angiopoietin-related protein 4. Cancer Res. 63:6651–6657.
2003.PubMed/NCBI
|