Introduction
Desmoplastic small round cell tumor (DSRCT) was
originally described and reported in a study by Gerald and Rosai in
1989 (1). DSCRT is very uncommon and
only 15 cases in the English literature were reported until now
(2–10). The DSRCT primarily affects children
and young adults, particularly young men, with a reported male to
female ratio of 4:1 (11). The
initial presenting symptoms are associated with the tumor
involvement, such as pain and abdominal distention, however, the
majority of the patients present with widespread intra-abdominal
and pelvic involvement when first examined. The mass was
characterized as exhibiting divergent differentiation and was an
extremely aggressive tumor belonging to the family of ‘small round
blue cell tumors’ (12–15). Despite the aggressive nature of DSRCT,
it has low overall survival rates; the overall progression-free
5-year survival rate of patients is 18% (2,16). Since
there are no symptoms at the early stage it is very difficult to
make correct diagnosis at the early stage. The present study
describes a case of DSRCT in a young woman who initially presented
with ovarian masses accompanied with lymph node and lung
metastases.
Case report
Clinical manifestation
On November 11, 2013, a 30-year-old female (gravida
3, para 1) initially presented to West China Second University
Hospital (Chengdu, China) with abdominal fullness. Pelvic
examination revealed bilateral adnexal masses. The patient's past
medical history was unremarkable. Transabdominal and transvaginal
ultrasonography showed the adnexal masses were irregular, complex
and predominantly solid, but partially cystic. Chest, abdominal and
pelvic computed tomography (CT) scans demonstrated multiple,
bilateral intrapulmonary nodules, bilateral ovarian masses and
pleural involvement. The level of the tumor marker serum
carbohydrate antigen-125 (normal, <35 U/ml) was slightly
increased at 50.8 U/ml.
Treatment
The patient underwent an exploratory laparotomy.
Intraoperatively, large, irregular, bilateral ovarian masses
accompanying metastatic nodules that varied in size adhering to the
diaphragm, peritoneum, omentum, stomach surface and uterine surface
were observed. Analysis of the frozen sections revealed that the
tumor was composed of small and round tumor cells growing in
cluster and separated by desmoplastic stromal cells. The mitotic
count of the tumor cells was high (≤15 per 10 high power fields).
Thus, the tumor was diagnosed as a poorly-differentiated carcinoma.
A right salpingooophorectomy and partial omentectomy were
performed. The patient was still undergoing chemotherapy on
February 5, 2014 (intravenous administration of 2 mg vincristine,
50 mg/m2 doxorubicin and 500 mg/m2
cyclophosphamide on day 1, repeated every 3 weeks) at 3 months
after the initial presentation, and the follow-up showed a partial
response, with a decreased tumor nodule size and no evidence of
further metastasis. The patient refused further treatment and
follow-up.
Pathological characteristics
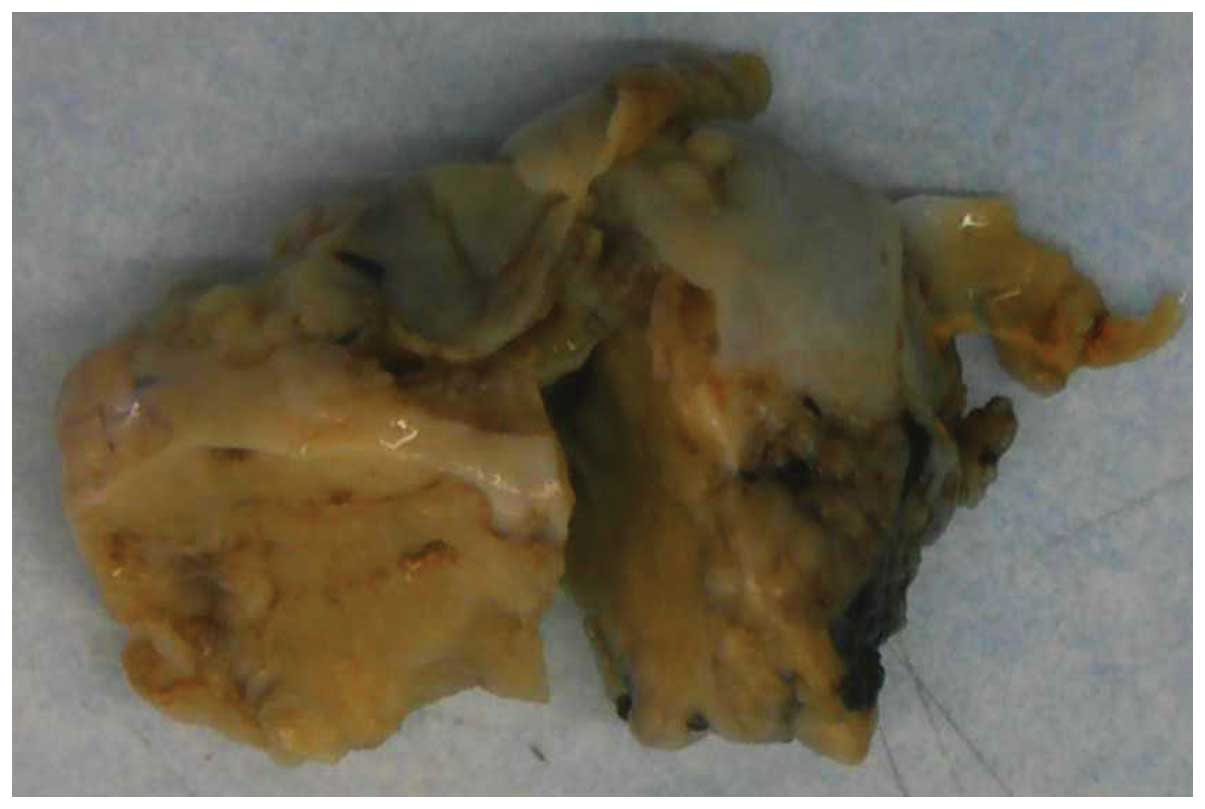
Macroscopically, the right ovarian mass exhibited a
smooth surface and measured 6.5×4.0×3.0 cm (Fig. 1). The cut surface was yellowish,
lobulated and predominantly solid, with partially mucoid cystic
areas and necrosis. The omental implant nodules were firm and
tan-white, with greatest diameters ranging from 0.5 to 2.5cm.
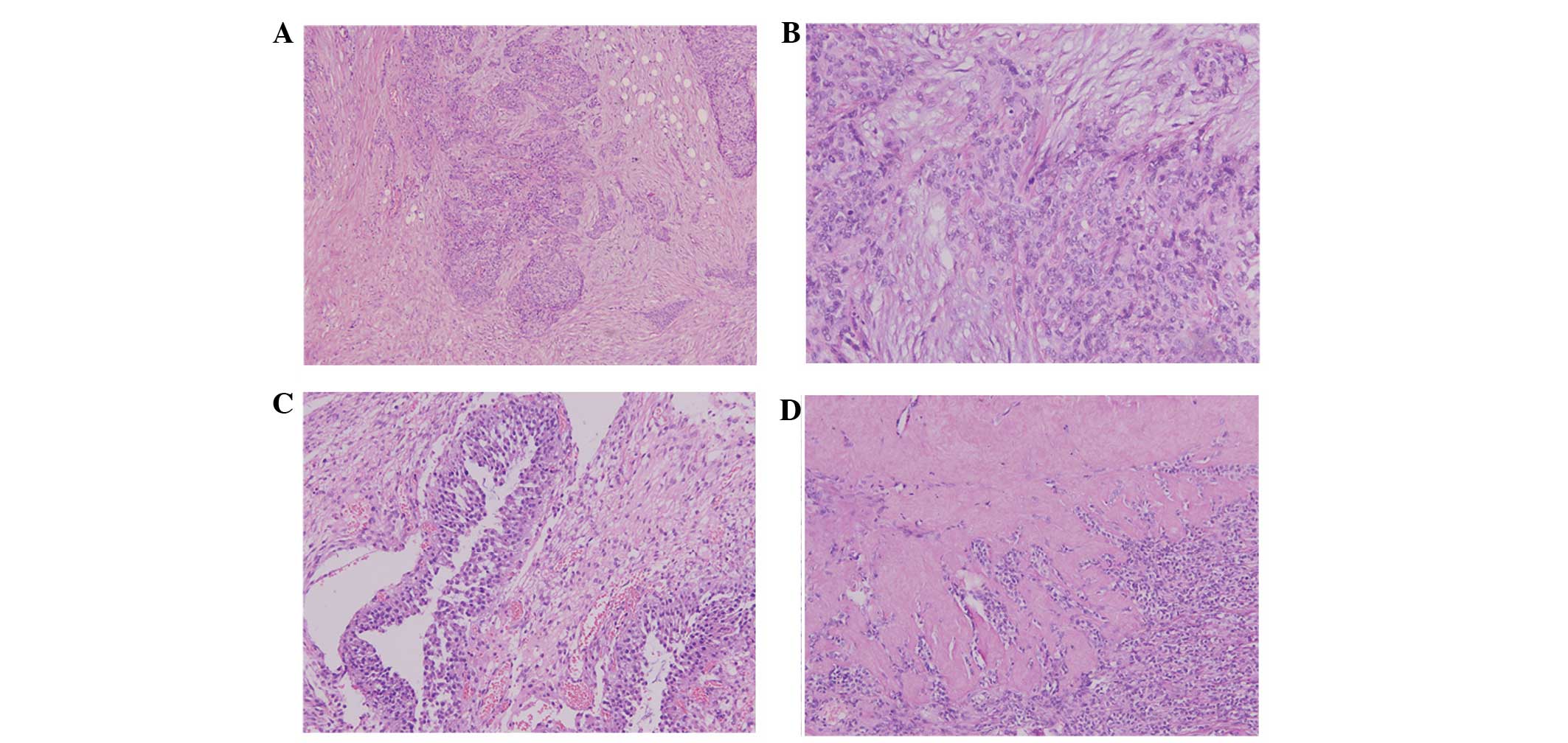
Microscopically, the tumor was characterized predominantly by nests
or/and clusters of small, round to oval-shaped cells separated by a
desmoplastic stroma (Fig. 2). The
tumor and stromal cells were distinctive: The aggressive tumor
cells exhibited hyperchromatic nuclei, inconspicuous nucleoli and
scant cytoplasm, while the desmoplastic stroma was composed of
elongated spindled cells with fibroblastic features. The mitotic
count was estimated up to 15 mitotic figures per 10 high-power
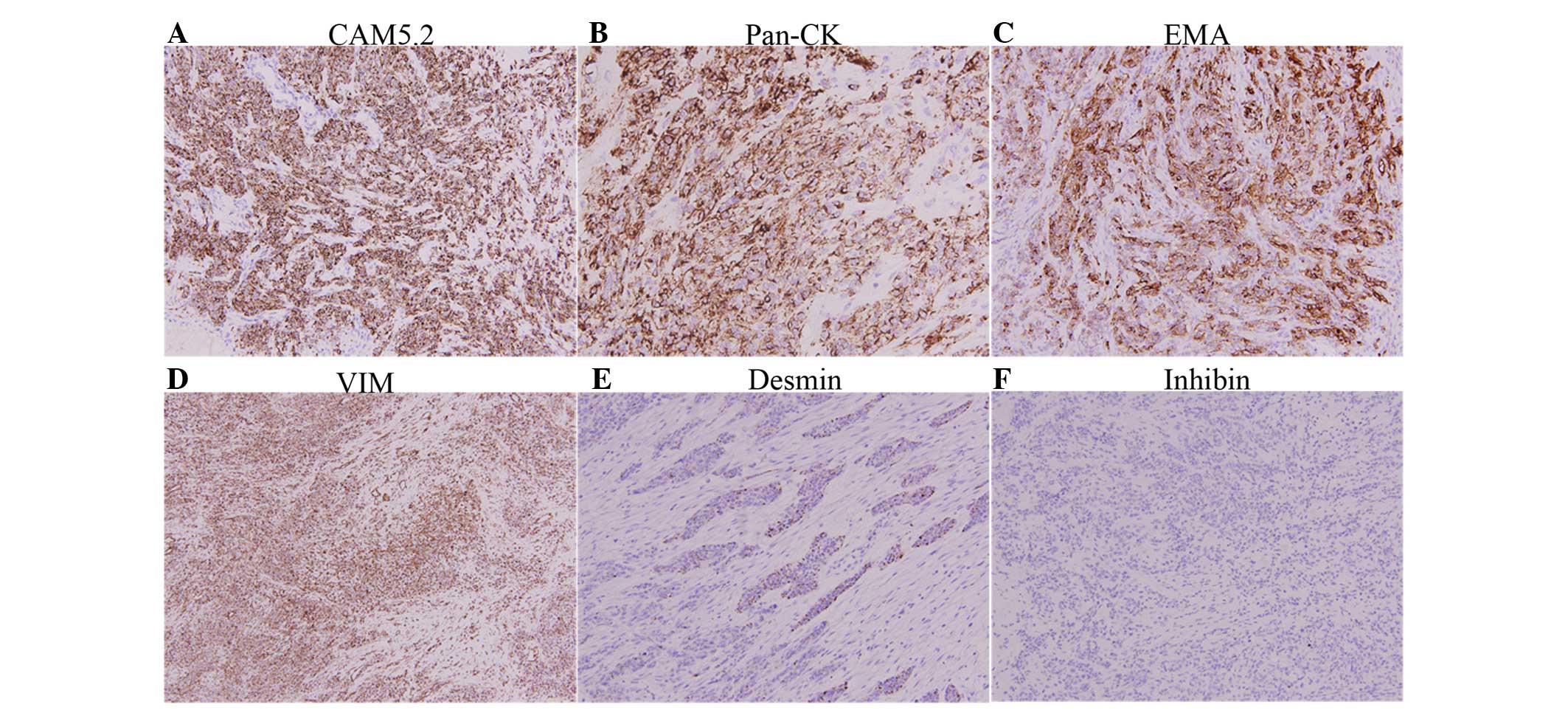
fields. Immunohistochemical staining was performed using the
antigen retrieval method. The small round cells were positive for
vimentin, broad-spectrum cytokeratins (AE1/AE3), CAM5.2, epithelial
membrane antigen (EMA) and desmin, with a dot-like staining pattern
(Fig. 3), but were negative for
inhibin, calretinin, cluster of differentiation (CD)99,
neuron-specific enolase (NSE), synaptophysin, Melan-A and HMB45,
among others. The overall immunohistochemical staining findings in
the small round cells are summarized in Table I. Based on the morphology and
immunohistochemistry findings, a final diagnosis of ovarian
involvement of a DSRCT was made.
 | Table I.Expression of immunolabelings in the
small round cells. |
Table I.
Expression of immunolabelings in the
small round cells.
| Immunolabeling | Expression |
|---|
| CAM5.2 | P |
| Pan-CK | P |
| EMA | P |
| VIM | P |
| Des | P (dot-like staining
pattern) |
| Ki-67 | P (~70%) |
| Inhibin | N |
| CD99 | N |
| NSE | N |
| Syn | N |
| HMB45 | N |
| S100 | N |
Discussion
DSRCT was first reported in 1989 as a rare tumor of
uncertain histogenesis arising in the pelvis or scrotum in young
men (1,12). Since then, further cases have been
described and reported. This rare, extremely aggressive tumor
usually affects individuals at adolescence and in early adulthood,
with a mean age of 25 years (1,17–19). The patient in the present study was
slightly older than this mean at 31 years old. Only 15 cases
previously reported in women in the English literature (3). A summary of the 15 cases is presented in
Table II (2–10).
Compared with the present case with the reported cases in Table II, the majority of the
characteristics of the present case were similar to the others,
including the locations, which were unilateral or bilateral ovarian
involved and presented as solid and/or cystic. All the patients
underwent surgical debulking and chemotherapy. The majority of
patients demonstrated partial remission, however, all the patients
succumbed to the disease 4–40 months following treatment due to
recurrence and metastases.
 | Table II.Summary of the 15 reported cases of
ovarian DSRCT. Modified from Ota et al (4) and Nakayama et al (3). |
Table II.
Summary of the 15 reported cases of
ovarian DSRCT. Modified from Ota et al (4) and Nakayama et al (3).
| Case, n | Reference | Age, years | Ovarian
involvement | CA-125, U/ml | Initial
treatment | Follow-up |
|---|
| 1 | Young et al
(5) | 15 | Unknown laterality: 9
and 8.5 cm (solid) | Not done | Surgical debulking
with multi-agent chemotherapy including carboplatin | Succumbed at 4
months |
| 2 | Young et al
(5) | 15 | Right: 15 cm
(solid/cystic) Left: 9 cm (solid) | Not done | Surgical debulking,
no chemotherapy | Secondary debulking
at 7 months |
| 3 | Young et al
(5) | 14 | Right: 5.3 cm
(solid) | Not done | Surgical
debulking | None |
| 4 | Zaloudek et al
(2) | 22 | Right: 8 cm (solid)
Left: 6.5 cm (solid) | 125 | Surgical debulking,
chemotherapy with BEP | Succumbed at 18
months |
| 5 | Solomovitz et
al (9) | 11 | Right: 12 cm
(solid/cystic) | 88.5 | Surgical debulking,
P6 and myeloablative chemotherapy | Succumbed at 11
months |
| 6 | Parker et al
(10) | 23 | Right: 6.8 cm
(solid) | 140 | Surgical debulking,
platinum and taxol chemotherapy | None |
| 7 | Elhajj et al
(6) | 27 | Right: 13 cm, Left:
20 cm | Not done | Surgical debulking,
delayed chemotherapy until symptomatic. C/E then VDC | Succumbed at 42
months |
| 8 | Ota et al
(4) | 26 | Bilateral
(solid) | 745.8 | Surgical debulking,
P6 chemotherapy | Succumbed at 23
months |
| 9 | Ota et al
(4) | 19 | Bilateral
(solid) | 2,823 | Surgical debulking,
BEP | Succumbed at 11
months |
| 10 | Fang et al
(7) | 13 | Left: 10 cm | Not done | Surgical debulking
BEP, radiotherapy | Succumbed at 21
months |
| 11 | Fang et al
(7) | 23 | Right: 11 cm Left: 9
cm | 51.7 | Surgical debulking,
myeloablative chemotherapy | Alive at 7
months |
| 12 | Engohan-Aloghe et
al (8) | 21 | Right: 18 cm (solid)
Left: unclear (solid) | Not done | Surgical debulking,
unknown chemotherapy | Alive at 7
months |
| 13 | Nakayama et al
(3) | 6 | Bilateral
(solid) | Not done | Surgical debulking,
P6 chemotherapy | Succumbed at 28
months |
| 14 | Nakayama et al
(3) | 28 | Right: 10 cm
(solid/cystic) | 42 | Neoadjuvant IE/VDC
chemotherapy, debulking surgery | Succumbed at 40
months |
| 15 | Nakayama et al
(3) | 17 | Right: 15 cm
(multicystic) | 35.9 | Surgical debulking,
IE/VDC chemotherapy with interval debulking | Alive at 11 months
followed by radiation therapy |
| 16 | Present case | 30 | Right: 6.5 cm
(soild/cystic) | 50.8 | Surgical debulking,
VAC chemotherapy | Alive at 15
months |
The majority of patients are characterized with
extensive peritoneal spread at the time of diagnosis, while the
initial presenting symptoms, such as pain, abdominal distension,
palpable mass and ascites, are associated with the tumor
involvement. In the majority of the reported cases (2,4–8), the clinical presentation of ovarian
DSRCT as a bilateral large ovarian mass accompanied with widespread
nodule implants throughout the peritoneum, with ascites, is
observed upon exploratory laparotomy. The most common sites of
metastasis are the liver, lymph nodes, lungs and bone marrow
(20). In the present case, the
initial symptom upon presentation was abdominal fullness, however,
bilateral intrapulmonary nodules and metastatic deposits were found
pre- and post-surgery.
It is difficult to correctly and rapidly form a
pathological diagnosis of DSRCT based on the initial histological
examination of the specimen due to the tumor cells exhibiting
divergent differentiation. As aforementioned, DSRCTs belong to the
family of ‘small round blue cell tumors’, which consist of several
tumor types, including extraskeletal Ewing's sarcoma/primitive
neuroectodermal tumors, rhabdomyosarcoma and lymphoma. All the
tumors have similar features. If the microscopic findings of a
young female show small, round and blue tumor cells, a common
ovarian tumor may first be highly suspected, such as a germ cell
tumor, a sex cord stromal tumor and Krukenberg's tumor. Therefore,
to make a correct diagnosis, a combination of immunohistochemical
staining and cytogenetic analysis can be useful and important. The
coexpression of cytokeratins, EMA, vimentin, desmin and NSE is a
unique immunophenotype of DSCRTs (3,4). By
contrast, negative immunohistochemical stains for Myogenin, MyoD1,
chromogranin, HMB45 and CD45 can assist in distinguishing DSRCT
from the aforementioned tumors. In the present case, a sex cord
tumor or a germ cell tumor were suspected at first, as the patient
was a young female. However, none of the immunophenotype markers
for these tumors were expressed. Next, a possible diagnosis of a
small round blue cell tumor was suggested and the corresponding
panel of markers was applied. The tumor cells exhibited
coexpression of the following markers: Epithelial staining for
cytokeratins and EMA, mesenchymal staining for vimentin and muscle
staining for desmin, which is characterized by a dot-like staining
pattern, but no expression of the rhabdomyosarcoma markers myogenin
and MyoD1. All the findings fulfilled the criteria for a DSRCT.
The optimal therapy for DSRCT patients has not yet
been determined yet due to the rarity of the disease, and the small
number of patients and multi-institutional clinical trials for the
tumor. It is important and useful for the attending physician to
seek the best therapy based on the individual responses and
clinical courses of the previously reported DSRCT patients who were
treated using various regimens. Although the majority of patients
undergo chemotherapy following surgery, the prognosis has been
shown to be independent of whether the surgical debulking process
preceded or followed chemotherapy. The overall progression-free
5-year survival rate of DSCRT patients is 18%. The aggressive
nature of the disease and the low overall survival rates achieved
should be carefully explained to affected patients and their
families, who should be included in the decision-making process
(2,16,18,21,22).
In conclusion, based on previous studies and the
present case, it is known that DSRCT is an uncommon and aggressive
tumor that affects adolescents, particularly young men. The
majority of patients are in the late stages of the disease upon
presentation. Currently, a combination of surgery and chemotherapy
are commonly used for treatment; however, it is difficult to
confirm whether surgery before or after chemotherapy is most
effective in such patients. Taken together, analysis of the
reported 15 cases and the present case, it appears that following
surgery with or without chemotherapy all the patients entered at
least partial remission; however, tumor recurrence and metastases
occurred in a number of cases. This may be due to the proliferative
nature and the divergent differentiation of the tumor cells, which
resulted in the cells losing sensitivity to the chemotherapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81101992).
References
|
1
|
Gerald WL and Rosai J: Case 2.
Histopathology. Pediatr Pathol. 9:177–183. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zaloudek C, Miller TR and Stern JL:
Desmoplastic small cell tumor of the ovary, A unique polyphenotypic
tumor with an unfavorable prognosis. Int J Gynecol Pathol.
14:260–265. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakayama J, Nassau S, Atkins K and
Modesitt SC: Desmoplastic small round cell tumor of the ovary, A
rare but devastating disease in young women. Gynecol Oncol Case
Rep. 7:16–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ota S, Ushijima K, Fujiyoshi N, Fujimoto
T, Hayashi R, Murakami F, Komai K, Fujiyoshi K, Hori D and Kamura
T: Desmoplastic small round cell tumor in the ovary: Report of two
cases and literature review. J Obstet Gynaecol Res. 36:430–434.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Young RH, Eichhorn JH, Dickersin GR and
Scully RE: Ovarian involvement by the intra-abdominal desmoplastic
small round cell tumor with divergent: differentiation. A report of
three cases. Hum Pathol. 23:454–464. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elhajj M, Mazurka J and Daya D:
Desmoplastic small round cell tumor presenting in the ovaries
Report of a case and review of the literature. Int J Gynecol
Cancer. 12:760–763. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang X, Rodabaugh K, Penetrante R, Wong M,
Wagner T, Sait S and Mhawech-Fauceglia P: Desmoplastic small round
cell tumor (DSRCT) with ovarian involvement in 2 young women. Appl
Immunohistochem Mol Morphol. 16:94–99. 2008.PubMed/NCBI
|
|
8
|
Engohan-Aloghe C Aubain, Sommerhausen Nde
S and Noël JC: Ovarian involvement by desmoplastic small round cell
tumor with leydig cell hyperplasia showing an unusual
immunophenotype (cytokeratin negative, calretinin and inhibin
positive) mimicking poorly differentiated sertoli leydig cell
tumor. Int J Gynecol Pathol. 28:579–583. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Slomovitz BM, Girotra M, Aledo A, Saqi A,
Soslow RA, Spigland NA and Caputo TA: Desmoplastic small round cell
tumor with primary ovarian involvement, case report and review.
Gynecol Oncol. 79:124–128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parker LP, Duong JL, Wharton JT, Malpica
A, Silva EG and Deavers MT: Desmoplastic small round cell tumor,
Report of a case presenting as a primary ovarian neoplasm. Eur J
Gynaecol Oncol. 23:199–202. 2002.PubMed/NCBI
|
|
11
|
Backer A, Mount SL, Zarka MA, Trask CE,
Allen EF, Gerald WL, Sanders DA and Weaver DL: Desmoplastic small
round cell tumour of unknown primary origin with lymph node and
lung metastases: histological, cytological, ultrastructural,
cytogenetic and molecular findings. Virchows Arch. 432:135–141.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerald WL, Miller HK, Battifora H,
Miettinen M, Silva EG and Rosai J: Intra-abdominal desmoplastic
small round-cell tumor. Histopathology. Am J Surg Pathol.
15:499–513. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Layfield LJ and Lenarsky C: Desmoplastic
small cell tumors of the pertoneum coexpressing mesenchymal and
epithelial markers. Am J Clin Pathol. 96:536–543. 1991.PubMed/NCBI
|
|
14
|
Norton J, Monaghan P and Carter RL:
Intra-abdominal desmoplastic small cell tumour with divergent
differentiation. Histopathology. 19:560–562. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ordóñez NG, el-Naggar AK, Ro JY, Silva EG
and Mackay B: Intra-abdominal desmoplastic small cell tumor, A
light microscopic, immunocytochemical, ultrastructural and flow
cytometric study. Hum Pathol. 24:850–865. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kushner BH, LaQuaglia MP, Wollner N,
Meyers PA, Lindsley KL, Ghavimi F, Merchant TE, Boulad F, Cheung
NK, Bonilla MA, et al: Desmoplastic small round-cell tumor:
Prolonged progression-free survival with aggressive multimodality
therapy. J Clin Oncol. 14:1526–1531. 1996.PubMed/NCBI
|
|
17
|
Robboy SJ, Anderson MC and Russel P:
Miscellaneous Primary Tumors, The Peritoneum, Pathology of the
Female Reproductive Tract. London: Churchill Livingstone. 809–810.
2002.
|
|
18
|
Churg A, Cagle PT and Roggli VL: Tumors of
the serosal membranes. Atlas of Tumor Pathology (4th). (Washington
DC). Armed Forces Institute of Pathology. 122–124. 2006.
|
|
19
|
Ordóñez NG: Desmoplastic small round cell
tumor. II. An ultrastructural and immunohistochemical study with
emphasis on new immunohistochemical markers. Am J Surg Pathol.
22:1314–1327. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Church DN, Bailey J, Hughes J and Williams
CJ: Desmoplastic small round cell tumour, obstetric and
gynecological presentations. Histopathology. Gynecol Oncol.
102:583–586. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slomovitz BM, Girotra M, Aledo A, Saqi A,
Soslow RA, Spigland NA and Caputo TA: Desmoplastic small round cell
tumor with primary ovarian involvement, Case report and review.
Gynecol Oncol. 79:124–128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwarz RE, Gerald WL, Kushner BH, Coit
DG, Brennan MF and La Quaglia MP: Desmoplastic small round cell
tumors. Prognostic indicators and results of surgical management.
Ann Surg Oncol. 5:416–422. 1998. View Article : Google Scholar : PubMed/NCBI
|