Introduction
Breast cancer is the most prevalent cancer in women
around the world today (1). In 2008,
it caused the most cancer-associated mortalities among women
(13.7%) (2). From 2005–2009, the
age-adjusted incidence rate for breast cancer was 124.3 cases per
100,000 women per year (3). For 2012,
it was estimated that 226,870 women would be diagnosed and 39,510
women would succumb to breast cancer (4). From 2002–2008, the 5-year relative
survival rate of breast cancer patients with distant cancer
metastasis was very low (23.8%) (3).
Chemotherapy is widely used in the neoadjuvant and
adjuvant treatment for breast cancer, and also for advanced breast
cancer. Docetaxel is a standard chemotherapy and is one of the most
active drugs used in breast cancer treatment. However, it is
difficult to further improve the efficacy of the drug.
microRNAs (miRNAs/miRs) are endogenously processed
non-coding RNAs that are able to regulate the expression of genes
by blockage of the translation of mRNA or by decreasing its
stability. miRNA can be incorporated into RNA-induced silencing
complex and guides the complex to target mRNAs, leading to
post-transcription repression (5). A
number of studies have found that miRNAs exert diverse functions in
a broad range of biological events, which affect the sensitivity of
different ex vivo cancer cell lines and nude mice models to
chemotherapeutic drugs by regulating different target genes that
play important roles in proliferation, cell cycle regulation,
apoptosis, differentiation and angiogenesis in breast cancers
(6–13). In vivo and in vitro
trials have shown that miR-21, miR-10b and miR-27 can stimulate the
growth of breast cancer, while miR-125a, miR-125b and miR-205 can
inhibit the proliferation of breast cancer by decreasing the
expression of HER-2 or HER-3. miR-206 may be associated with
estrogen receptor-α (6–13), but the exact mechanisms remain
unclear. All the aforementioned results suggest that miRNAs may act
as novel potential diagnostic and treatment targets. mir-205, which
directly targets the HER-3 receptor, has been found to be
downregulated in breast cancer tumors (7). Recent studies have also reported that
the reduced expression of miR-205 may cause docetaxel resistance in
prostate cancer (14). Therefore, the
present study analyzed whether docetaxel sensitivity could be
increased in breast cancer therapy by reintroducing miR-205.
Materials and methods
Lentiviral constructs and
transduction
To generate the miR-205 expression vector, a
fragment carrying pre-miR-205 was amplified as referenced (15). Briefly, a ~600 bp fragment carrying
pre-miR-205 was amplified from MCF-10A genomic DNA by the Phusion®
High-Fidelity DNA Polymerase enzyme (New England Biolabs, Ipswich,
MA, USA) using the following PCR primers: miR-205-5.1,
5′-GAATTCCTTATCTGGGTGGCTGTTTTG-3′ and miR-205-3.1,
5′-GGTACCGCGGTGCTTTTTCCAATCTGC-3′. The amplified fragment was first
cloned into a pBS-hU6 vector with fusion green fluorescent protein
(GFP) expression. To construct the miR-205 lentiviral expression
vector, the pre-miR-205 fragment was subcloned into an FG12 vector,
and then co-transfected into 293T cells with pMDLg/RRE, pRSV/rev
and pHCMV-G. All the plasmids were kindly provided by Mr. Yu (The
Shanghai Cancer Institute, Shanghai, China). Cell supernatants were
collected at 48 h post-transfection and passed through a 0.22-mm
filter. The titer of purified virus was 3.0×108
IU/ml.
Cell culture and transfection
The human breast cancer MDA-MB-231 and MCF-7 cell
lines and the normal human embryonic kidney 293T cell line were all
obtained from the American Type Culture Collection (Manassas, VA,
USA). All the cells were maintained in Dulbecco's modified Eagle's
medium (DMEM)/F12 medium (Invitrogen Life Technologies, Carlsbad,
CA, USA) supplemented with 10% fetal bovine serum and 1% penicillin
and streptomycin (Invitrogen Life Technologies) and under a
standard gas atmosphere of humidified air/5% CO2.
Transient transfection was performed with lipofectamine 2000
(Invitrogen Life Technologies).
Cell proliferation assay
A CellTiter-Glo® Luminescent Cell Viability Assay
kit (Promega Corporation, Madison, WI, USA) was used for cell
growth measurements. A total of 1×104 cells were seeded
in a 96-well plate, in 100 µl medium for each well. Docetaxel
(Sigma-Aldrich, St. Louis, MO, USA) was added at 0, 0.5, 1.0, 2.0,
4.0 and 8.0 µM after a 24-h regular incubation. The cells were
cultured for 36 h, and then 100 µl/well CellTiter-Glo reagent was
added to measure cell growth according to the manufacturer's
instructions.
Colony formation assay
The cells (1×105/well) were treated with
docetaxel (Sigma-Aldrich) at a concentration of 0, 0.5, 1.0, 2.0,
4.0 and 8.0 mM for 48 h. Next, the cells were re-seeded at 100
cells/well in a 24-well plate and regularly cultured in DMEM/F12
medium supplemented with 10% fetal bovine serum, and 1% penicillin
and streptomycin (Invitrogen Life Technologies) 14 days. The
colonies were stained with crystal violet (Sangon Biotech Co.,
Ltd., Shanghai, China) and counted under a microscope (SZ61-ILST;
Olympus, Tokyo, Japan). Cells were tested in four groups: The LacZ
control, miR205 alone, docetaxel (0.5 µM) and LacZ, and docetaxel
(0.5 µM) and miR205.
In vivo xenograft study and
immunohistochemistry
The in vivo animal procedure was approved by
the Animal Ethics Committee at the Chinese People's Liberation Army
General Hospital (Beijing, China). The MDA-MB-231 cells and the
cells stably expressing miRNA-205 were grown to log phase. A total
of 1×107 microplasma free cells in 0.2 ml
phosphate-buffered saline were subcutaneously injected into the
flank region of female athymic nude mice (four groups, 4 mice per
group). Mice were housed and submitted to an inverse 12-h day-night
cycle, with lights on at 8:30 PM, and maintained in a temperature
(22±1°C) and humidity (55±5%) controlled room. Animals were housed
in four different cages (Beijing ZS Dichuang Co., Ltd., Beijing,
China), with 4 mice in each. The cages were filled with sterilized
wood shavings, bedding and a cardboard tube for environmental
enrichment. All mice were allowed free access to water and a
maintenance diet (SLACOM for mouse and rat; SLAC Laboratory Animal
Co., Ltd., Shanghai, China) Docetaxel (5 µM) was injected directly
into the xenografts from day 9 once every three days. Tumor growth
was measured using calipers and body weight was monitored
simultaneously. Next, formalin-fixed tissue sections were prepared
for GFP detection.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies). RNA quality was confirmed by
Nanodrop 2000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
The following PCR primers were used: Forward,
5′-TCCTCAGACAATCCATGTGC-3′ and reverse, 5′-TGCCTCCTGAACTTCACTCC-3′.
The miR-205 expression was detected by Platinum PCR Super Mix
(Invitrogen Life Technologies) and amplified by Bio-Rad PCR T100
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Results were analyzed by performing Student t-tests
using Microsoft Excel 2010 (Microsoft, Redmond, WA, USA) and
P<0.05 indicated a statistically significant difference.
Results
Overexpression of miRNA-205 increases
docetaxel sensitivity in breast cancer cell lines
To investigate the function of miRNA-205 in breast
cancer cell lines, docetaxel sensitivity was detected in two breast
cancer cell lines with or without miRNA-205 overexpression.
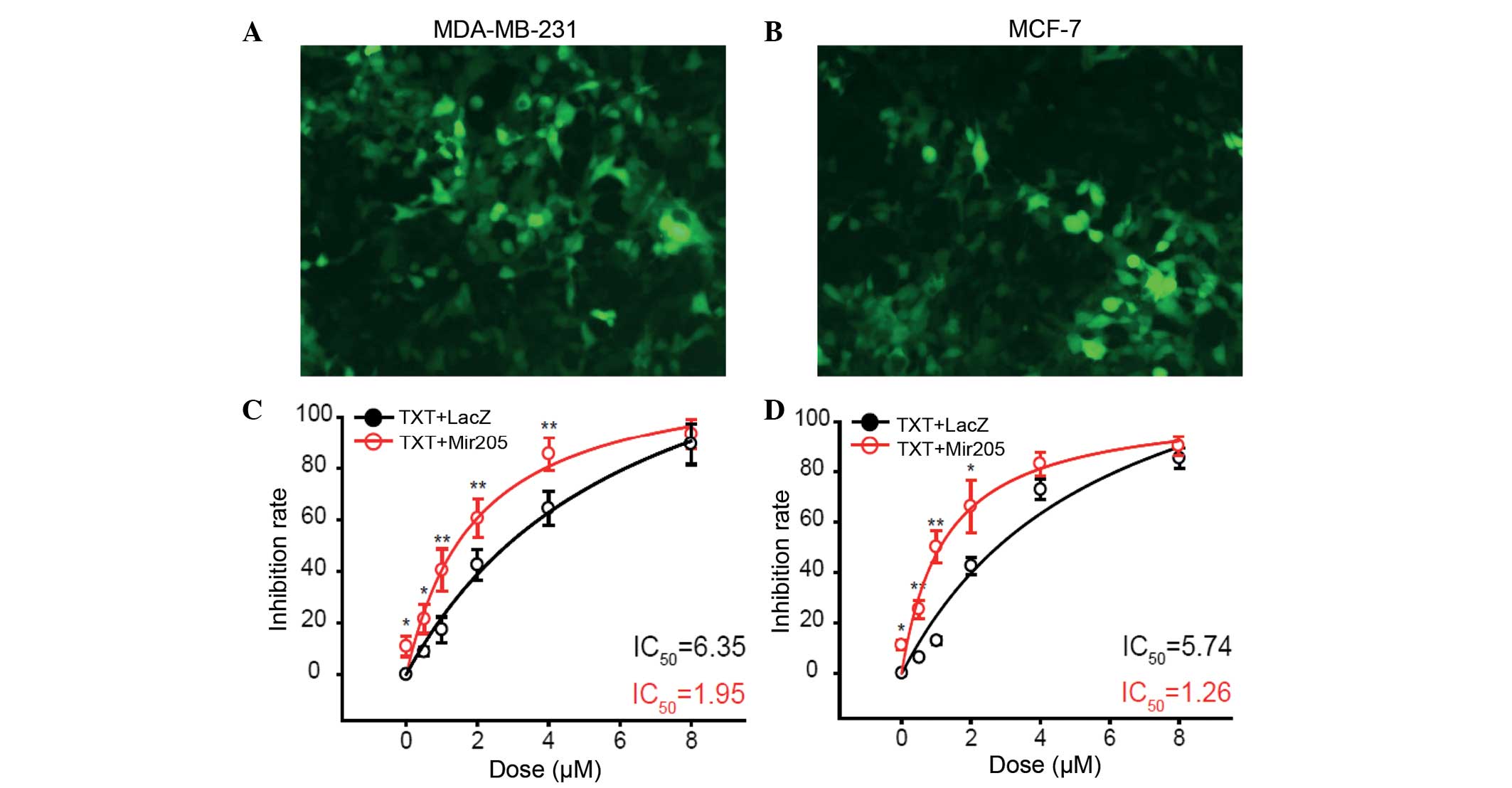
miRNA-205 was expressed fused with GFP in the MDA-MB-231 (Fig. 1A) and MCF-7 (Fig. 1B) cell lines. The overexpression of
miR-205 inhibited the cell growth of the breast cancer MDA-MB-231
(Fig. 1C) and MCF-7 (Fig. 1D) cell line. miR-205 was shown to
increase cell sensitivity of MDA-MB-231 cells to 0, 0.5, 1, 2 and 4
µM docetaxel (P=0.0453, P=0.0386, P=0.00526, P=0.00613 and
P=0.00571, respectively). In MCF-7 cells, cell sensitivity to
docetaxel was also increased following treatment with 0, 0.5, 1 and
2 µM docetaxel (P=0.0367, P=0.00836, P=0.00578 and P=0.0127,
respectively).
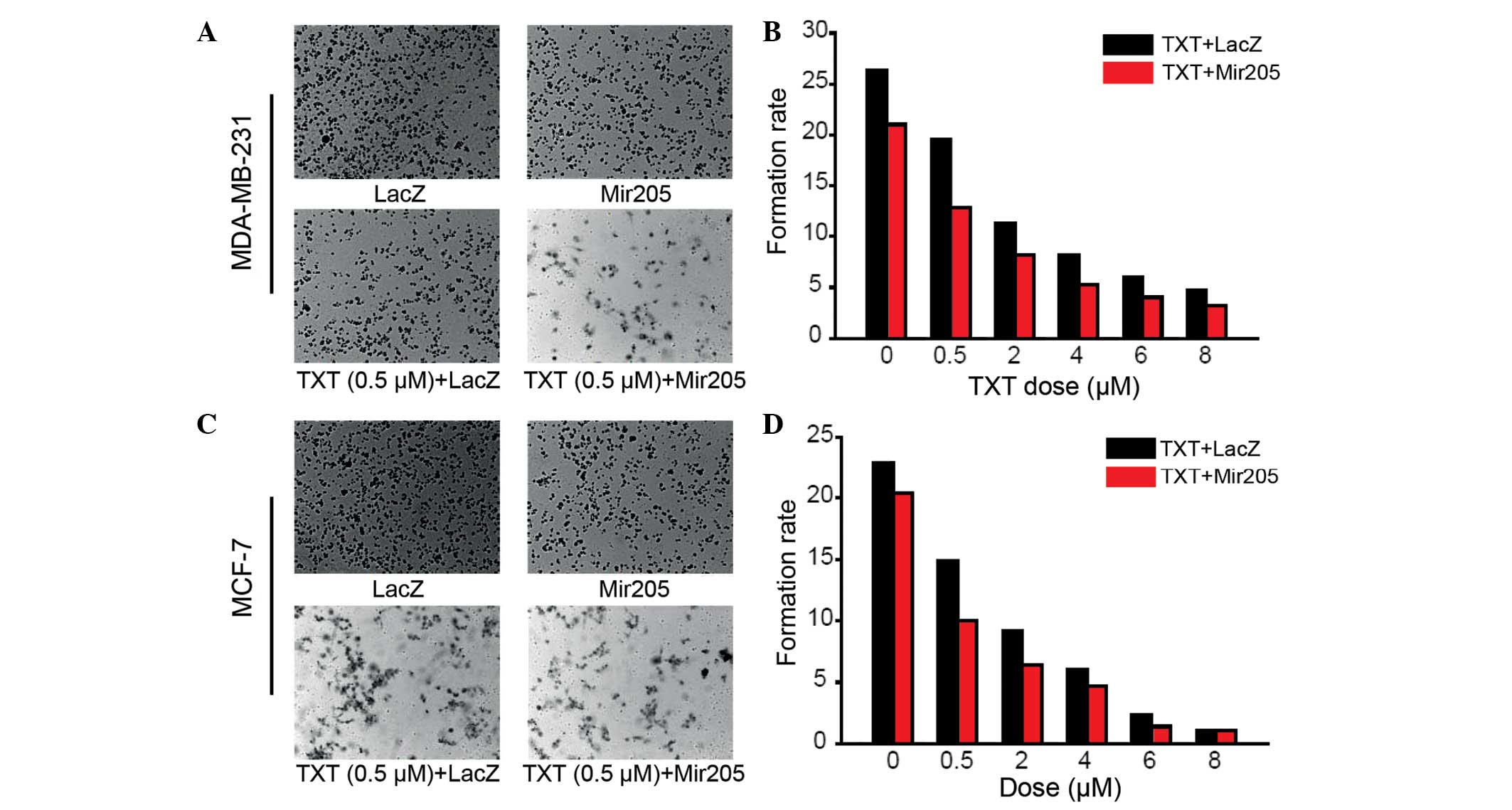
A colony formation assay was performed to assess
whether miR-205 could inhibit the clonogenic survival of the
MDA-MB-231 cancer cells. Compared with the LacZ control, docetaxel
alone and miR-205 alone groups, the cells treated with miR-205
combined with docetaxel showed a significantly decreased colony
formation ability (Fig. 2A and
B).
Similar results were acquired with another breast
cancer cell line, MCF-7 (Fig. 2C and
D). These results suggested that miR-205 suppresses breast
cancer cell proliferation and has a synergistic effect with
docetaxel treatment.
miRNA-205 has a synergistic inhibition
effect with docetaxel treatment in vivo
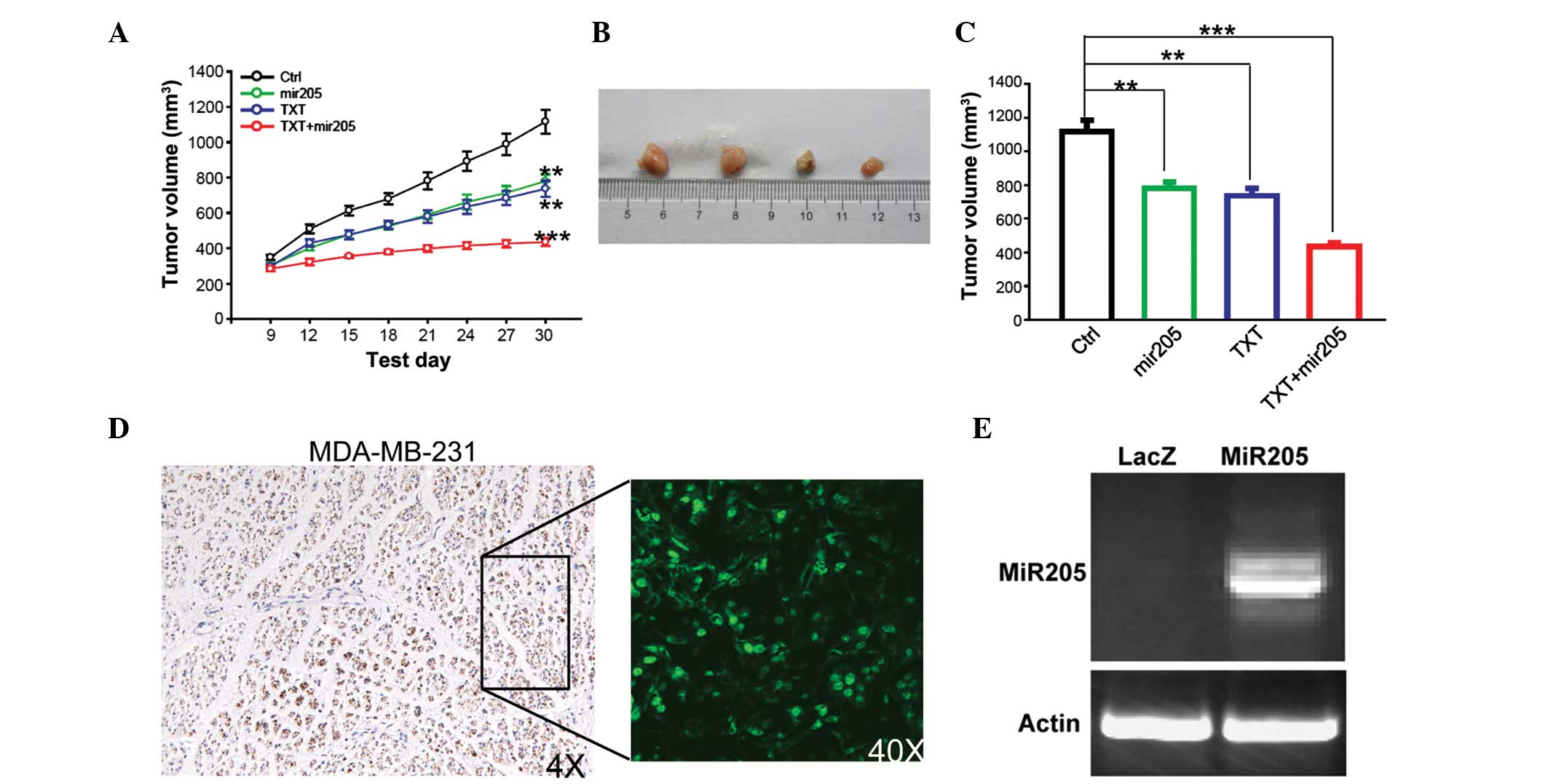
Next, the study investigated whether miR-205 can
inhibit cell growth and whether it has a synergistic effect with
docetaxel in vivo. Since it is difficult to form xenograft
tumors in nude mice with MCF-7 cells, the study was focused on
MDA-MB-231 cells. It was found that miR-205 overexpression or
docetaxel treatment inhibited tumor cell growth in vivo, and
that the sensitivity to docetaxel was significantly increased when
combined with miR-205 reintroduction (Fig. 3A–C). When compared with the control
group, miR-205 and docetaxel treatment alone inhibited tumor growth
(miR-205, P=0.00845; TXT, P=0.00648). Docetaxel exhibited a greater
inhibitory effect on tumor growth when combined with miR-205
(P=0.000641). miR-205 expression was confirmed by GFP detection and
RT-PCR (Fig. 3D and E).
Discussion
With the occurrence of taxanes, the chemotherapeutic
efficacy in breast cancer has been significantly improved. However,
drug resistance and further improvements in efficacy remain great
challenges in breast cancer medical oncology. Biomarker diagnosis
and biotarget therapy have provided a novel direction of study
since the clinical use of Herceptin, a HER-2 monoclonal antibody,
in 1997. Herceptin was confirmed to provide significantly improved
clinical benefits and formed the foundation of modern biotarget
therapy in breast cancer. Clinical trials have confirmed that
Herceptin can reduce the risk of recurrence in HER-2-positive
breast cancer post-operative patients by ~50%, and improve the
progression-free and overall survival of HER-2-positive advanced
breast cancer patients (16,17). Lapatinib, a multi-inhibitor, which can
inhibit the tyrosin kinases of EGFR1 and EGFR2 (HER-2) was
permitted to be used in Herceptin failure HER-2-positive advanced
breast cancer patients by the FDA in 2001 (18–20).
Inspired by the aforementioned results, research into multi-target
therapy attracts much attention. Although a number of targets are
currently being processed, progress and studies have thus far been
primarily focused on the EGFR family.
Research efforts in human breast cancer have been
focused on studying the role of altered expression. miRNA
expression signatures appear to represent promise with regard to
tumor characterization, and could be potential diagnostic and
treatment tools. Additionally, approaches that interfere with miRNA
function are also being considered. It is known that certain miRNAs
confer drug resistance or sensitivity to cancer cells. However,
this drug resistance is a hindrance to the effective curative
treatment of solid tumors, and occurs frequently through a number
of actions, including suppressed apoptosis, improved proliferation
and crosstalk between different signal transduction pathways.
Several miRNAs have been reported to be involved in these processes
or signal pathways. In the present study, miR-205, which decreases
the expression of HER-3, is suggested to function as a tumor
suppressor in breast cancer development. miR-205 can increase the
sensitivity of breast cancer to chemotherapeutic or biochemical
drugs (5). Recently, Iorio et
al identified that miR-205 can inhibit the proliferation of
breast cancer cells, possibly by inhibiting the formation of
heterodimer with HER-2, and increase the sensitivity of breast
cancer to gefitinib and lapatinib (7). Kastl et al confirmed that miR-34a
can improve the sensitivity of breast cancer to docetaxel by
inhibiting the expression of the BCL-2 target gene, which is an
anti-apoptotic family member (21).
The present study observed that miR-205 could
improve the inhibition ability of docetaxel to the breast cancer
MDA-MB-231 cell line and the MDA-MB-231 nude mouse model. The
synergistic action may be due to the role of docetaxel in
inhibiting the deregulation of spindle fibres (by stabilizing the
microtubules in spindle fibres, arresting the cell at M phase and
by inducing cell apoptosis), and the role of miR-205 in
downregulating the post-transcriptional expression of HER-3. As
observed in previous studies (7,15),
transferring miR-205 alone also can inhibit the proliferation of
the breast cancer MDA-MB-231 cell line, which be may be ascribed to
blocking of the PI3K/AKT pathway. At the same time, the present
study data also showed that the synergistic action is most
significant when the concentration of docetaxel is between 1 and 4
µmol/l, while the concentration of miR-205 is constant. When the
concentration of docetaxel is above a certain high level, the
effect of miR-205 is no more significant; the reason behind this
may lie in the fact that a high concentration of docetaxel can kill
the entire breast cancer cell without the action of miR-205.
In summary, the present results strongly suggested
that miR-205 may have a synergistic action with docetaxel by the
downregulation of the post-transcriptional expression of HER-3.
Elevated miR-205 expression shows promise as a novel strategy for
the treatment of HER-2-positive breast cancer.
References
|
1
|
Azambuja E, Durbecq V, Rosa DD, Colozza M,
Larsimont D, Piccart-Gebhart M and Cardoso F: HER-2
overexpression/amplification and its interaction with taxane-based
therapy in breast cancer. Ann Oncol. 19:223–232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health: Organization: W orld Cancer
Report 2008. http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/wcr_2008.pdfAccessed.
February 26–2011
|
|
3
|
Howlader N, Noone AM, Krapcho M, Neyman N,
Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, et al:
SEER Cancer Statistics Review, 1975-2009. (Vintage 2009
Populations). http://seer.cancer.gov/csr/1975_2009_pops09/Accessed.
May 20–2013
|
|
4
|
Cancer Facts & Figures 2012. Atlanta:
American Cancer Society. 42012.
|
|
5
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khoshnaw SM, Green AR, Powe DG and Ellis
IO: MicroRNA involvement in the pathogenesis and management of
breast cancer. J Clin Pathol. 62:422–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iorio MV, Casalini P, Piovan C, Di Leva G,
Merlo A, Triulzi T, Ménard S, Croce CM and Tagliabue E:
MicroRNA-205 regulates HER3 in human breast cancer. Cancer Res.
69:2195–2200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng F, Henson R, Lang M, Wehbe H,
Maheshwari S, Mendell JT, Jiang J, Schmittgen TD and Patel T:
Involvement of human micro-RNA in growth and response to
chemotherapy in human cholangiocarcinoma cell lines.
Gastroenterology. 130:2113–2129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu S, Si ML, Wu H and Mo YY: MicroRNA-21
targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol
Chem. 282:14328–14336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adams BD, Furneaux H and White BA: The
micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen
receptor-alpha (ERalpha) and represses ERalpha messenger RNA and
protein expression in breast cancer cell lines. Mol Endocrinol.
21:1132–1147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kondo N, Toyama T, Sugiura H, Fujii Y and
Yamashita H: MiR-206 expression is down-regulated in estrogen
receptor alpha-positive human breast cancer. Cancer Res.
68:5004–5008. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tavazoie SF, Alarcón C, Oskarsson T, Padua
D, Wang Q, Bos PD, Gerald WL and Massagué J: Endogenous human
microRNAs that suppress breast cancer metastasis. Nature.
451:147–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang N, Li Q, Feng NH, Cheng G, Guan ZL,
Wang Y, Qin C, Yin CJ and Hua LX: MiR-205 is frequently
downregulated in prostate cancer and acts as a tumor suppressor by
inhibiting tumor growth. Asian J Androl. 15:735–741. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu H, Zhu S and Mo YY: Suppression of cell
growth and invasion by miR-205 in breast cancer. Cell Res.
19:439–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, et al: Trastuzumab plus adjuvant chemotherapy for operable
HER2-positive breast cancer. N Engl J Med. 353:1673–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Slamom DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER-2
for metastatic breast cancer that overexpresses HER-2. J Engl J
Med. 344:783–792. 2001. View Article : Google Scholar
|
|
18
|
Burris HA: Dual kinase inhibition in the
treatment of breast cancer: initial experience with the EGFR/ErbB-2
inhibitor lapatinib. Oncologist. 9(Suppl 3): 10–15. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higa GM and Abraham J: Lapatinib in the
treatment of breast cancer. Expert Rev Anticancer Ther.
7:1183–1192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
[no authors: listed]:B reast cancer drug
approved for new indication. Womens Health (Lond Engl).
6:1732011.
|
|
21
|
Kastl L, Brown I and Schofield AC:
MiRNA-34a is associated with docetaxel resistance in human breast
cancer cells. Breast Cancer Res Treat. 131:445–454. 2012.
View Article : Google Scholar : PubMed/NCBI
|