Introduction
Follicular lymphoma is an indolent B cell
lymphoproliferative disorder of transformed follicular center B
cells (1). Patients with follicular
lymphoma present with diffuse lymphadenopathy, bone marrow
involvement and splenomegaly; in rare cases, other extranodal sites
may also be involved (1). Follicular
lymphoma is one of the most common subtypes of non-Hodgkin lymphoma
(NHL) with an estimated incidence of 3.18 cases per 100,000
individuals in the USA (2).
Follicular lymphoma is the initial neoplasm for indolent lymphomas
and has a median overall survival of >10 years (1). Observation or radiation therapy is
adequate for asymptomatic and low tumor bulk disease cases
(3,4).
For patients needing therapy, the majority of patients are treated
with chemotherapy plus rituximab (5–7).
Myeloproliferative neoplasms are characterized by
terminal myeloid cell expansion in the peripheral blood, resulting
in various combinations of erythrocytosis, leukocytosis,
thrombocytosis, bone marrow hypercellularity/fibrosis and
splenomegaly (8). Polycythemia vera
is a Philadelphia-negative myeloproliferative disease associated
with JAK2 mutation characterized by erythrocytosis; patients are
treated by phlebotomy and/or cytotoxic drugs (9). Myeloproliferative neoplasms are
associated with lymphoproliferative disease following the
administration of cytotoxic drugs or exposure to radiation, but are
rarely observed prior to therapy (10,11).
In the present study, a case of a 61-year-old female
with a history of transient ischemic attack is reported. The
patient was simultaneously diagnosed with follicular lymphoma and
an unclassifiable myeloproliferative neoplasm. It is possible that
the coexistence of follicular lymphoma and an unclassifiable
myeloproliferative neoplasm prior to the administration of a
cytotoxic drug or exposure to radiation may involve 2 unrelated
clones of different lineages.
Case report
A 61-year-old Asian female visited the Ulsan
University Hospital (Dong-gu, Ulsan, Republic of Korea) in October
2013 with a painless but palpable left epitrochlear mass. The
patient had suffered a transient ischemic attack in August 2010,
and was prescribed aspirin (100 mg once per day) and cilostazol
(100 mg once per day) at the time. The 1.5-cm sized left
epitrochlear mass presented without systemic B symptoms, such as
fever, night sweat or unintentional weight loss. Laboratory
findings demonstrated leukocytosis [white blood cell (WBC) count,
16,040 cells/µl], erythrocytosis (hemoglobin, 17.5 g/dl;
hematocrit, 58.3%), thrombocytosis (platelet count, 782,000
cells/µl), reduced erythropoietin (6.26 mU/ml), elevated lactate
dehydrogenase (789 IU/l) and elevated serum β-2 microglobulin (2.8
mg/l). A computed tomography scan of the neck, chest and abdomen
identified an enlarged spleen, but no other lymphadenopathy. An
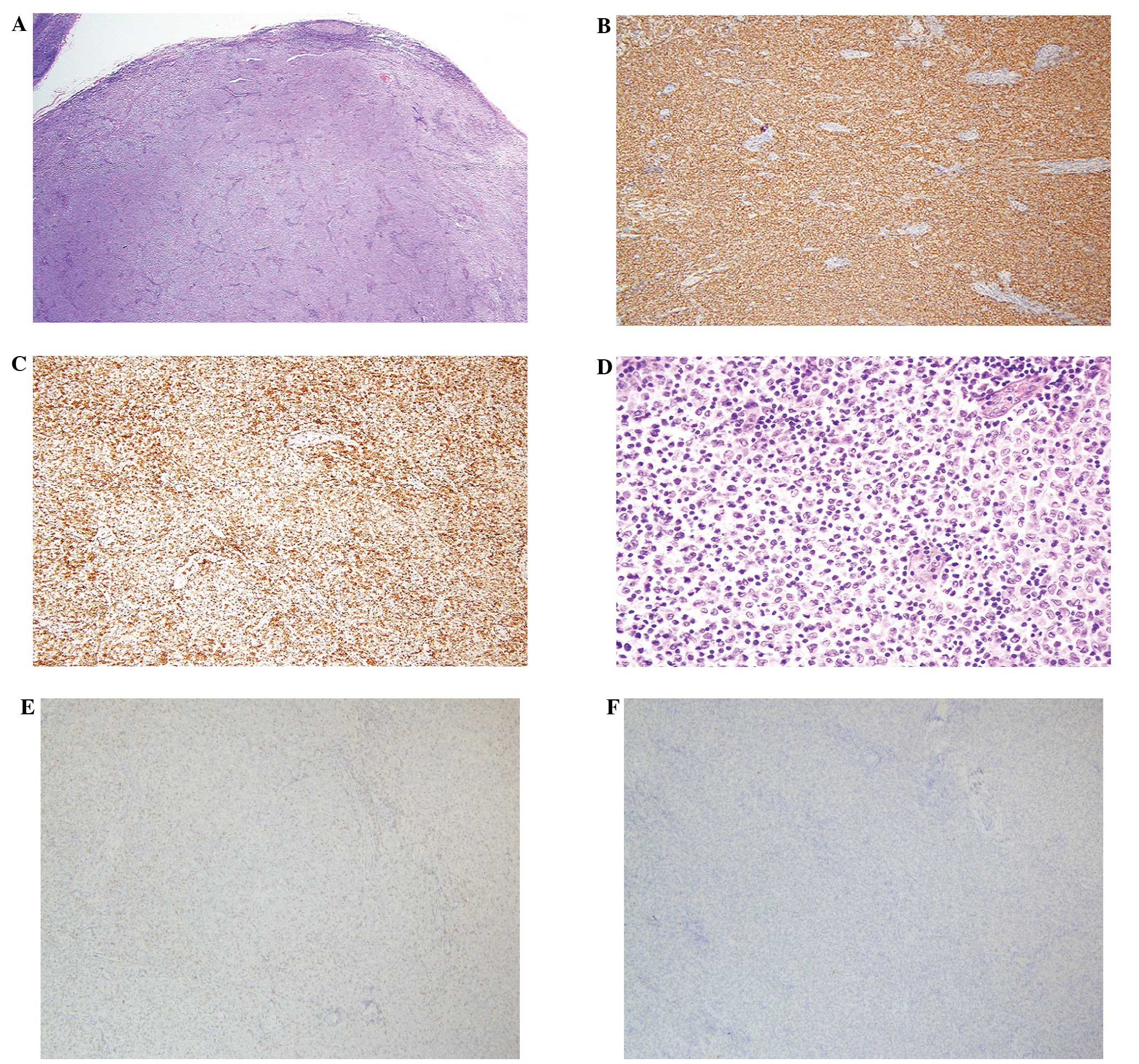
excisional lymph node biopsy demonstrated follicular lymphoma with
nodal growth pattern, which are closely packed and contained small
round cleaved cells and larger non-cleaved cells (grade 1)
(Fig. 1). The tumor cells were
positive for B-cell lymphoma 2 (Bcl-2), cluster of differentiation
(CD) 20, and negative for CD3 and cyclinD1. Positron emission
tomography identified a mild hypermetabolic lesion in the operation
bed of the left elbow, demonstrating post-surgery alteration.
Diffusely increased metabolism of the skeletal bone marrow was also
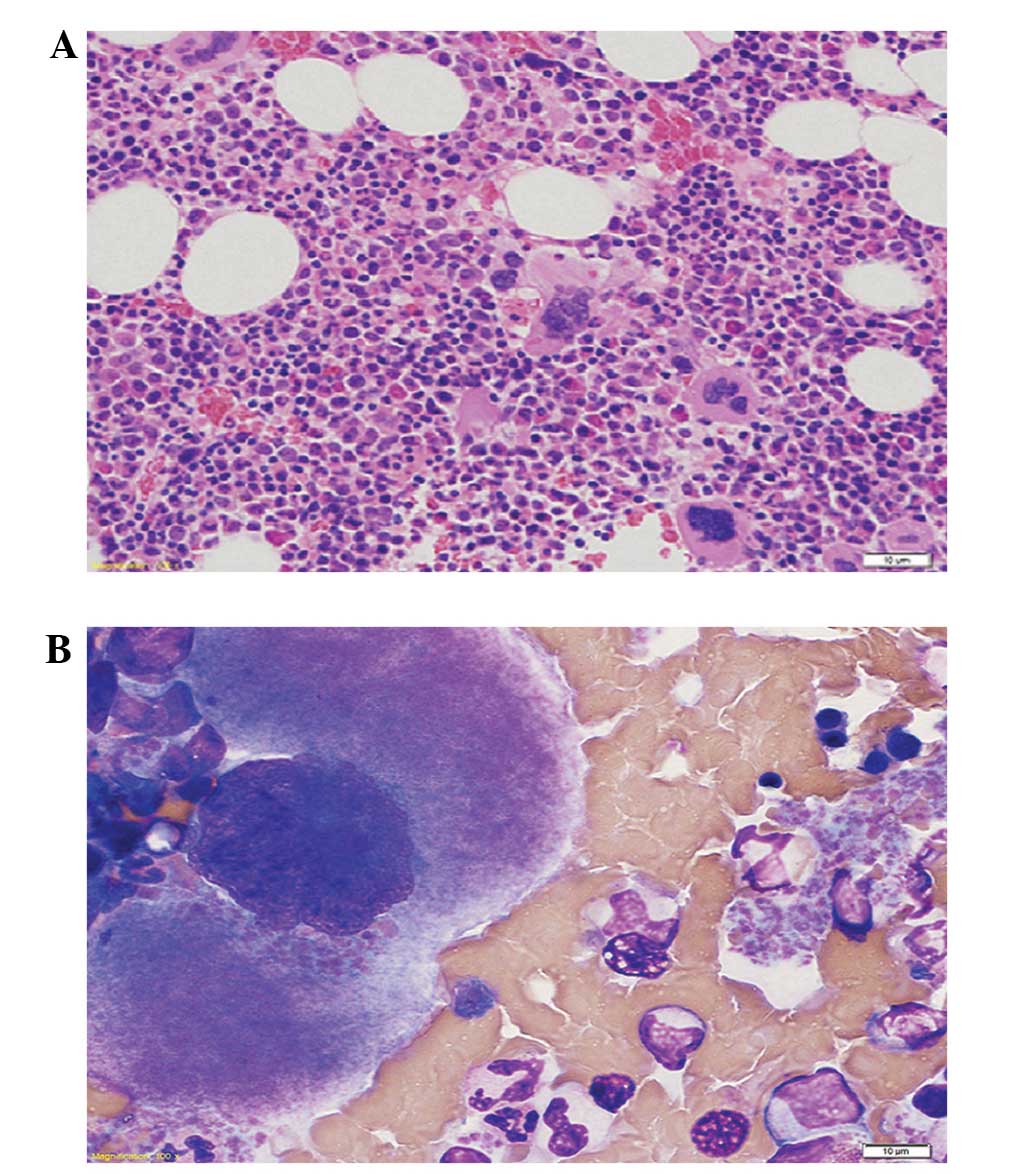
observed throughout the body. A biopsy of the bone marrow revealed
hypercellularity, with predominance of cells derived from the
erythroid series and large polylobated megakaryocytes with
increased mitotic figures. However, no evidence of lymphomatous
infiltration was observed (Fig. 2).
The V617F mutation in janus kinase 2 (JAK2) was also detected in
the cells of the bone marrow specimen. Based on these results, the
disease was considered to be at a clinical stage I, and the
follicular lymphoma international prognostic index score indicated
an intermediate risk (12). Following
local excision of the lymph node, radiation was delivered to the
involved field at a dose of 24 Gy in 12 fractions. A computed
tomography scan of the neck, chest and abdomen performed 3 months
later revealed no abnormalities. Following phlebotomy and plasma
apheresis, the patient was started on hydroxyurea (1 g twice per
day, orally) 2 weeks subsequent to the end of radiotherapy, and was
administered 500 mg twice per day of the drug as maintenance
therapy. On the sixth month of follow-up, the WBC count,
hemoglobin, hematocrit and platelet count were reduced to 7,430
cells/µl, 12.2 g/dl, 36.5% and 379,000 cells/µl, respectively, and
the patient was in a healthy condition at the time of
reporting.
Discussion
A small number of patients (<10%) diagnosed with
follicular lymphoma present stage I/II of the disease (13). In these cases, radiation therapy is
the treatment of choice, following which, the 10-year overall
survival rate is 60–80%, with a median survival time of ~19 years
(3). The therapies for polycythemia
vera are aimed at preventing the occurrence of thrombosis (19,20). The
initial therapy for patients at high risk of developing thrombosis
consists of phlebotomy plus a low dose of aspirin, supplemented
with a cytoreductive agent, such as hydroxyurea (21). In the present report, radiation was
delivered to the involved field, following local excision of the
lymph node in the left epitrochlear area. The patient was
administered hydroxyurea and aspirin subsequent to phlebotomy and
plasma apheresis.
Direct or indirect dysregulation of the tyrosine
kinase JAK2 signaling pathway, as a result of somatically acquired
mutations, is involved in the pathogenesis of myeloproliferative
neoplasms (22). JAK2 participates in
the signaling pathways initiated by the cytokine receptors that are
required for normal myelopoiesis, including those for
erythropoietin, thrombopoietin and granulocyte colony stimulating
factor (23). The JAK2 V617F variant
increases genomic instability by reducing apoptosis secondary to
DNA damage, and by altering the expression of a number of genes,
which in turn may increase the risk of genetic lesions that lead to
leukemic transformation (24).
The JAK2 V617F mutation is present in 95% of
patients with polycythemia vera and in ~50% of patients with
primary myelofibrosis or essential thrombocythemia (24,25). This
mutation has also been observed in patients affected by other
myeloid diseases, including 67% of patients with refractory anemias
and ring sideroblasts associated with thrombocytosis (26), 7.8% of patients with chronic
myelomonocytic leukemias and a low percentage of patients with
primary acute myeloblastic leukemias, myelodysplastic syndromes and
chronic myeloid leukemia (27).
However, to the best of our knowledge, the JAK2 V617F mutation has
not been detected in follicular lymphoma thus far. Furthermore, the
coexistence of follicular lymphoma and myeloproliferative neoplasm
is rare (14,28).
There are limited number of reports in the
literature that are relevant to the case described in the present
report (Table I). It is uncertain
whether there is a pathogenetic association between the
myeloproliferative and lymphoproliferative diseases: It is likely
that both are as a result of random mutations occurring in distinct
initiating cells. However, given the higher risk of
lymphoproliferative neoplasms development in myeloproliferative
neoplasms reported in larger studies, the genomic instability
characteristic to myeloproliferative neoplasms may contribute to
subsequent lymphoproliferative neoplasms occurrence (29,30). The
pathogenesis may be attributed to reduced immunocompetence and/or
anti oncogene suppression.
 | Table I.Review of the association between PV
and malignant lymphoma, prior to the administration of a cytotoxic
agent and/or exposure to radiation. |
Table I.
Review of the association between PV
and malignant lymphoma, prior to the administration of a cytotoxic
agent and/or exposure to radiation.
| Age
(years)/gender | Histological type of
lymphoma | Timing of
diagnoses | Treatment | Outcomes | Reference |
|---|
| 73/M | Plasmacytoid of the
colon | Simultaneous | Melphalan | Not available | (15) |
| 20/M | Immature T-cell | Simultaneous | CTX+ADM+VCR+VM26+PDN
plus CNS prophylaxis with MTX +DEX; PDN+VCR+DNM +asparaginase;
m-BACOD | No response and
progression of NHL, followed by close mortality | (16) |
| 78/M | Cutaneous T-cell | Sequential (PV
detected 1 year prior to NHL) | Phlebotomy;
photochemotherapy | Resolution of skin
lesion; stabilization of Hct; leukocytosis; thrombocytosis | (17) |
| 52/M | Hodgkin's | Sequential (PV
detected 9 months prior to NHL) | Phlebotomy; VLB;
CVPP | HL CR and reduction
of PV; recurrence of PV after 4 years of chemotherapy
withdrawal | (18) |
| 66/M | Follicle center cell
(grade 1) | Simultaneous | m-BACOD; MIT+PDM;
HU | NHL CR;
transformation of PV into secondary MMM | (14) |
| 61/F | Follicular (grade
1) | Simultaneous | Local excision;
radiation; phlebotomy; aspirin; HU | FL CR; stabilization
of Hct; leukocytosis; thrombocytosis | Present report |
In the present study, the JAK2 V617F mutation was
identified in the malignant cells of a patient with an
unclassifiable myeloproliferative neoplasm and coexisting
follicular lymphoma. This finding may indicate that the JAK2 V617F
mutation represents a secondary event to primary gene mutations in
the primitive stem cells, which leads to the development of a
follicular lymphoma and an unclassifiable myeloproliferative
neoplasm. Regardless, further studies using molecular and genetic
approaches may aid in the understanding of this rare occurrence of
neoplasms.
References
|
1
|
Freedman A: Follicular lymphoma: 2014
update on diagnosis and management. Am J Hematol. 89:429–436. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morton LM, Wang SS, Devesa SS, Hartge P,
Weisenburger DD and Linet MS: Lymphoma incidence patterns by WHO
subtype in the United States, 1992-2001. Blood. 107:265–276. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guadagnolo BA, Li S, Neuberg D, Ng A, Hua
L, Silver B, Stevenson MA and Mauch P: Long-term outcome and
mortality trends in early-stage, Grade 1-2 follicular lymphoma
treated with radiation therapy. Int J Radiat Oncol Biol Phys.
64:928–934. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Advani R, Rosenberg SA and Horning SJ:
Stage I and II follicular non-Hodgkin's lymphoma, long-term
follow-up of no initial therapy. J Clin Oncol. 22:1454–1459. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hiddemann W, Kneba M, Dreyling M, Schmitz
N, Lengfelder E, Schmits R, Reiser M, Metzner B, Harder H,
Hegewisch-Becker S, et al: Frontline therapy with rituximab added
to the combination of cyclophosphamide, doxorubicin, vincristine,
and prednisone (CHOP) significantly improves the outcome for
patients with advanced-stage follicular lymphoma compared with
therapy with CHOP alone: Results of a prospective randomized study
of the German Low-Grade Lymphoma Study Group. Blood. 106:3725–3732.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marcus R, Imrie K, Belch A, Cunningham D,
Flores E, Catalano J, Solal-Celigny P, Offner F, Walewski J, Raposo
J, et al: CVP chemotherapy plus rituximab compared with CVP as
first-line treatment for advanced follicular lymphoma. Blood.
105:1417–1423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marcus R, Imrie K, Solal-Celigny P,
Catalano JV, Dmoszynska A, Raposo JC, Offner FC, Gomez-Codina J,
Belch A, Cunningham D, et al: Phase III study of R-CVP compared
with cyclophosphamide, vincristine, and prednisone alone in
patients with previously untreated advanced follicular lymphoma. J
Clin Oncol. 26:4579–4586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dickstein JI and Vardiman JW:
Hematopathologic findings in the myeloproliferative disorders.
Semin Oncol. 22:355–373. 1995.PubMed/NCBI
|
|
9
|
Tefferi A and Barbui T: Polycythemia vera
and essential thrombocythemia: 2015 update on diagnosis
risk-stratification and management. Am J Hematol. 90:162–173. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Montefusco E, Fazi F, Cordone I, Ariola C,
Nanni M, Spadea A, Spiriti MA, Fenu S, Mandelli F, Petti MC, et al:
Molecular remission following high-dose hydroxyurea and fludarabine
plus cytarabine in a patient with simultaneous acute myeloid
leukemia and low-grade lymphoma. Leuk Lymph. 40:671–674. 2001.
View Article : Google Scholar
|
|
11
|
De Souza Ornellas MH, de Souza Fernandez
T, Diamond HR, Maioli MC, Pitanga Bacha PC and De Lucena SB:
Cytogenetic and immunophenotypic evidence of independent clonal
origins of concomitant chronic lymphocytic leukaemia and acute
myeloid leukaemia. Eur J Haematol. 66:281–283. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Solal-Céligny P, Roy P, Colombat P, White
J, Armitage JO, Arranz-Saez R, Au WY, Bellei M, Brice P, Caballero
D, et al: Follicular lymphoma international prognostic index.
Blood. 104:1258–1265. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Friedberg JW, Byrtek M, Link BK, Flowers
C, Taylor M, Hainsworth J, Cerhan JR, Zelenetz AD, Hirata J and
Miller TP: Effectiveness of first-line management strategies for
stage I follicular lymphoma, Analysis of the National LymphoCare
Study. J Clin Oncol. 30:3368–3375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rizzi R, Liso A, Pannunzio A, Carluccio P,
Specchia G and Liso V: Concomitant primary polycythemia vera and
follicle center cell non-Hodgkin lymphoma, A case report and review
of the literature. Leuk Lymph. 43:2217–2220. 2002. View Article : Google Scholar
|
|
15
|
Guzzini F, Cozzi C, Gasparini P, Giussani
R and Tomasi A: Spontaneous association of a chronic lympho- and
myeloproliferative disease in the same patient. Histopathology.
Recenti Prog Med. 79:365–369. 1988.PubMed/NCBI
|
|
16
|
Kurchan A, Somoza N, Pascuccelli H, Mide
S, Padros MR, Santiago J, Satz ML and Fainboim L: T lymphoma of
immature phenotype associated with polycythemia vera. Medicina (B
Aires). 51:151–154. 1991.PubMed/NCBI
|
|
17
|
Cottrill C, Geller A, diSpaltro FX,
Weissglass B, Klainer AS and Bisaccia E: Control of polycythaemia
vera with photochemotherapy in a patient with cutaneous T-cell
lymphoma. Br J Haematol. 86:225–226. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stolinsky DC: Twelve-year remission of
polycythemia vera followingH odgkin's disease and chemotherapy. CA
Cancer J Clin. 31:57–60. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chievitz E and Thiede T: Complications and
causes of death in polycythaemia vera. Acta Medica Scand.
172:513–523. 1962. View Article : Google Scholar
|
|
20
|
Patrono C, Rocca B and De Stefano V:
Platelet activation and inhibition in polycythemia vera and
essential thrombocythemia. Blood. 121:1701–1711. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hensley B, Geyer H and Mesa R:
Polycythemia vera, Current pharmacotherapy and future directions.
Expert Opin Pharmacother. 14:609–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oh ST and Gotlib J: JAK2 V617F and beyond:
Role of genetics and aberrant signaling in the pathogenesis of
myeloproliferative neoplasms. Expert Rev Hematol. 3:323–337. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muxi PJ and Oliver AC: Jak-2 positive
myeloproliferative neoplasms. Curr Treat Options Oncol. 15:147–156.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cross NC: Genetic and epigenetic
complexity in myeloproliferative neoplasms. Hematology Am Soc
Hematol Educ Program. 2011:208–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jones AV, Kreil S, Zoi K, Waghorn K,
Curtis C, Zhang L, Score J, Seear R, Chase AJ, Grand FH, et al:
Widespread occurrence of the JAK2 V617F mutation in chronic
myeloproliferative disorders. Blood. 106:2162–2168. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ceesay MM, Lea NC, Ingram W, Westwood NB,
Gäken J, Mohamedali A, Cervera J, Germing U, Gattermann N,
Giagounidis A, et al: The JAK2 V617F mutation is rare in RARS but
common in RARS-T. Leukemia. 20:2060–2061. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Levine RLI, Loriaux M, Huntly BJ, Loh ML,
Beran M, Stoffregen E, Berger R, Clark JJ, Willis SG, Nguyen KT, et
al: The JAK2V617F activating mutation occurs in chronic
myelomonocytic leukemia and acute myeloid leukemia, but not in
acute lymphoblastic leukemia or chronic lymphocytic leukemia.
Blood. 106:3377–3379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuroda HI, Abe T, Jomen W, Yoshida≈ M,
Matsuno T, Sato M, Yamada M, Sakurai T, Fujii S, Maeda M, et al:
[Follicular lymphoma complicated with myelofibrosis and
macroglobulinemia at initial presentation]. Rinsho ketsueki.
54:2068–2073. 2013.(In Japanese). PubMed/NCBI
|
|
29
|
Vannucchi AM, Masala G, Antonioli E,
Susini MC, Guglielmelli P, Pieri L, Maggi L, Caini S, Palli D,
Bogani C, et al: Increased risk of lymphoid neoplasms in patients
with Philadelphia chromosome-negative myeloproliferative neoplasms.
Cancer Epidemiol Biomarkers Prev. 18:2068–2073. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rumi E, Passamonti F, Elena C, Pietra D,
Arcaini L, Astori C, Zibellini S, Boveri E, Pascutto C and
Lazzarino M: Increased risk of lymphoid neoplasm in patients with
myeloproliferative neoplasm, A study of 1,915 patients.
Haematologica. 96:454–458. 2011.(In Italian). View Article : Google Scholar : PubMed/NCBI
|