Introduction
Plants have long been used in the treatment of a
variety of diseases, including cancer. Several anticancer drugs,
such as vincristine, etoposide, vinblastine, irinotecan, paclitaxel
(taxol) and topotecan are botanical secondary metabolites or
semi-synthetic derivatives (1).
Apigenin (2), deguelin (3), kaempferol (4), luteolin (5–7), quercetin
(8–11), rutin (12–16),
tricin (17), xanthomicrol (18), α-copaene (19), α-humulene (20,21) and
β-himachalene (22) are some
botanical ingedients that have been investigated as possible future
remedies for use in chemotherapy. Xanthomicrol and calycopterin
have also been shown to exert potent inhibitory effects on
microvessel outgrowth and that these anti-angiogenic effects
enhance the antitumor activity (18).
Cyperaceae is a large family of plants known as
sedges, with 5,500 species described. They are known as traditional
medicines; however they have to be more extensively investigated
(23). From Cyperaceae, Cyperus
kyllingia has been shown to exert cytotoxic effects on NCI-H187
cells (small cell lung cancer cells) (24) and the main chemical constituents of
its effective essential oil (EO) are α-humulene, an agent with
reported anticancer activity (21)
and caryophyllene, that facilitates the penetration of α-humulene
through the cell membrane and potentiates its anticancer activity
(25). Furthermore, Cyperus
rotundus has been shown to exert cytotoxic effects on SH-SY5Y
human neuroblastoma (26) and K562
erythroleukemia cells (27). However,
the total oligomeric flavonoids (TOFs) and ethyl acetate (EtOAc)
extract of Cyperus rotundus have exhibited weak anticancer
effects on L1210 cells (IC50=240 and 200 µg/ml,
respectively) (28).
Cyperus longus L. (Cyperaceae) is an Egyptian
traditional plant that is used as a diuretic and tonic herbal
medicine. It is widely distributed in the Middle East (29). Several flavonoids (30), terpenoids (31,32) and
stilbenes (33) have been isolated
from this plant. However, to date, and to the best of our
knowledge, there is no available scientific report of the
anticancer acivity of Cyperus longus. al-Samarqandi (13–14th
century, Iran) and al-Kindi (10th century, Iraq) had been
prescribing this plant as a traditional remedy in cases that were
suspected to be cancer (34). In
2012, Ait-Ouazzou et al (31)
evaluated the chemical composition of Cyperus longus EO.
α-humulene (16.7%), γ-himachalene (10.1) and β-himachalene (46.6%)
were found to be the main constituents and they all exhibited
anticancer activity (31). Flavonoids
as botanical ingredients exhibit a wide range of biological
effects, such as anticancer activities (5,12,35). Luteolin and tricin are two flavonoids
that are isolated from Cyperus longus (30), and they have been reported to possess
cytotoxic activity against cancer cell lines (6,7,17). In 2010, Morikawa et al
determined the antioxidant activity of resveratrol (a polyphenol or
stilbene) and its oligomer, pallidol, in the methanol extract of
Cyperus longus (29). These
two stilbenes have been shown to exert anticancer effects on cancer
cell lines (36). The resveratrol
analogue, DMU-212, was shown to inhibit HepG2 and MCF7 cell
proliferation by inducing apoptosis and G2/M arrest through the
upregulation of p53 and Bax/Bcl-xL (37). Pallidol has also been shown to exert
significant cytotoxic effects against A549 cells (38).
Based on these data, it seems Cyperus longus
possesses significant anticancer activity. An important step in
determining the chemical constituents of plants is preliminary
phytochemical screening. Thus, in this study, we examined the plant
extract, fraction by fraction, for its anticancer activity against
two cancer cell lines (MCF7 and PC3) and one transformed
non-malignant (normal) cell line (L929). After comparing the
fractions, we evaluated the major constituents that may be
responsible for the cytotoxic effects. Finally, we introduced these
suspected ingredients as possible candidates for further
investigation in cancer chemotherapy.
Materials and methods
Reagents
Propidium iodide (PI), dimethylsulphoxide (DMSO),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution, Triton X-100, paclitaxel and other chemicals of
analytical grade were purchased from Sigma (St. Louis, MO, USA),
Roswell Park Memorial Institute (RPMI)-1640 medium, fetal bovine
serum (FBS), antibiotic solution (penicillin 1,000 IU and
streptomycin 10 mg/ml) were obtained from Gibco (Grand Island, NY,
USA).
Collection of plant material
The total plant was collected from Bojnurd and the
surrounding areas in North Khorasan, Iran. The plant was identified
by researchers from the Research Center of Natural Product Health,
North Khorasan University of Medical Sciences, Bojnurd, Iran. The
herbarium code was MP96.
Preparation of extracts
The plant was shade-dried and then ground to a
powder using a mortar and pestle. The powder was stored in
air-tight sealed bottles. The shade-dried (100 g) powder of the
plant was suspended in absolute methanol (350 ml) at room
temperature for 7 days. The whole extract was filtered through a
paper filter and the solvent was evaporated under a vacuum at 45°C,
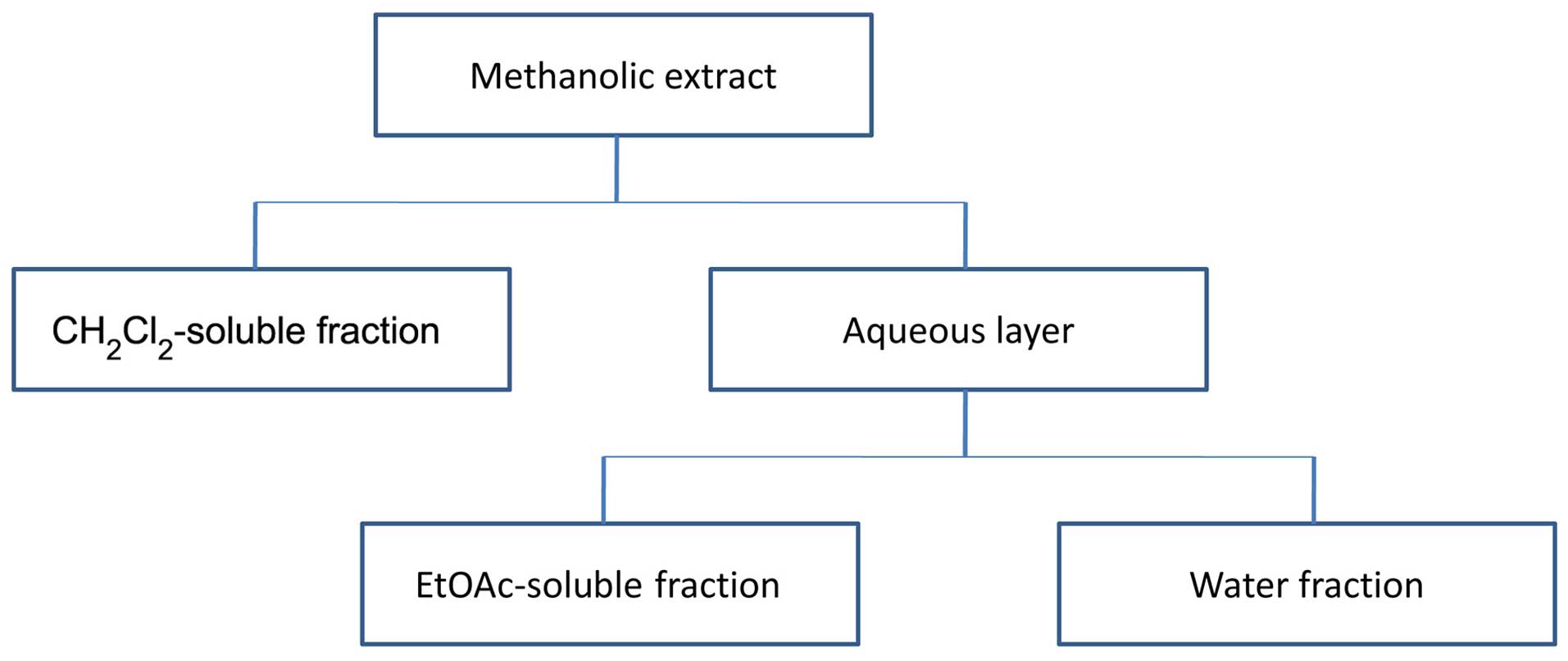
to yield 12.6 g crude (yield 12.6%) extract. Fractionation was
carried out as described in the study by Parsaee et al
(39). Briefly, the solution was
successively partitioned with dichloromethane
(CH2Cl2), EtOAc and finally, water. The
CH2Cl2, EtOAc and water fractions were
evaporated under a vacuum to yield residues of 3.31, 4.22 and 4.35
g, respectively. The extracts were stored at 4°C until analysis. A
partitioning scheme of the methanol extract is presented in
Fig. 1.
Extraction of EO
The EO was extracted according to a previously
described method (40) with minor
modifications. Briefly, the dried whole plant (480 g) was ground
and hydro-distilled for 5 h using a Clevenger apparatus (ISO
glass®; Sina Shisheh Co., Tehran, Iran). The upper oily layer of
the extract was separated and dried with anhydrous sodium sulfate.
The sample obtained was stored in tightly closed dark vials at
−20°C until analysis. The EO was a light yellow transparent liquid
with a 0.45% (v/w) yield.
Cell lines and culture
The human prostate cancer cell line (PC3), human
breast cancer cell line (MCF-7) and mouse fibroblast cell line
(L929; as a non-malignant cell line) were obtained from Pasteur
Institute (Tehran, Iran). The cells were maintained at 37°C in a
humidified atmosphere 95% containing 5% CO2. All cell
lines were cultured in RPMI-1640 with 10% v/v FBS, 100 U/ml
penicillin and 100 mg/ml streptomycin, as previously described
(41).
MTT assay
The evaluation of cytotoxicity was peformed using
MTT assay, as previously described (42,43). The
cells were plated in a 96-well culture plate with various
concentrations (12.5–200 µg/ml) of the methanol extract and
fractions. The cultured plates were incubated for 24, 48 and 72 h
at 37°C and 5% CO2. Following incubation, 20 µl MTT
solution in phosphate-buffered saline (PBS) were added to each well
at a final concentration of 0.5 mg/ml followed by further
incubation for 3 h at 37°C. The medium was then removed, and 100 ml
DMSO were added to each well for solubilizing the formazan. The
absorbance was measured at 490 nm (630 nm as a reference) using an
ELISA reader (Start Fax 2100; Awareness Technology Inc., Fisher
Bioblock Scientific, Tournai, Belgium). Three independent
experiments were carried out and 8 replicates were taken for each
experiment. The concentration of the methanol extract and fractions
which resulted in a 50% reduction of cell viability, the half
maximal inhibitory concentration (IC50 value), was
calculated using the following formula: % inhibition = (control abs
- sample abs)/(control abs) × 100. Paclitaxel was used as a
positive control at the concentration of 0.2–50 µg/ml.
DNA fragmentation assay
For in vitro DNA fragmentation assay, all
cell lines (1×105 cells/ml) were incubated in a 12-well
plate with 75 µg/ml of methanol extract and the fractions, for 48 h
at 37°C and 5% CO2. The optimal concentration (75 µg/ml)
and time point (48 h) for this method were selected based on the
data resulting from MTT assay. Paclitaxel was used as a positive
control at the concentration of 0.35 µM. Floating and adherent
cells were harvested and incubated for 5 h at 4°C in the dark with
750 ml of a hypotonic buffer (50 µg/ml PI in 0.1% sodium citrate
plus 0.1% Triton X-100 PBS) prior to flow cytometric analysis using
a FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
The sub-G1 peak was analyzed by FACScan using CellQuest software
(BD Biosciences), as previously described (42).
Gas chromatography-mass spectrometry
(GC-MS) analysis
For the EO of the plant, GC-MS analysis was carried
out using a Shimadzu-QP2010SE chromatograph mass spectrometer (qp
2010 ultra; serial no. 01139; Shimadzu, Kyoto, Japan) operating at
70 eV ionization energy, equipped with a HP-5 capillary column
(serial no. 1107908; Restek Corp., Bellefonte, PA, USA;
phenylmethyl siloxane, 30 m × 0.25 mm; with 0.25 µm film thickness)
with helium as the carrier gas, flow rate 1 ml/min and a split
ratio of 1:20. The acquisition mass range was 35–300 and the scan
time was 0.5 sec/scan. The retention indices were determined using
the retention times of n-alkanes as a standard that had been
injected after the sample under the same chromatographic
conditions. The compounds were identified by comparison of the
retention indices (RRI, HP-5) as well as by comparison of their
mass spectra with the Wiley and Mass Finder 3 libraries or with the
published mass spectra (44).
Statistical analysis
Data are expressed as the means ± standard deviation
of at least 3 independent determinations in 8 replicates for each
experimental point. Statistical tests were performed using one-way
ANOVA followed by the Tukey-Kramer post hoc test for multiple
comparisons. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of the extract, different
fractions and EO of Cyperus longus on cell viability
The effects of Cyperus longus were examined in a
time-response experiment after 24, 48 and 72 h, at concentrations
of 12.5–200 µg/ml. The viability of the MCF7 and PC3 cells was
significantly inhibited by the methanol extract, the
CH2Cl2 and EtOAc fractions and the EO of the
plant in a time-dependent manner at 24, 48 and 72 h, as shown in
Table I. There was no significant
activity in the L929 normal cells (IC50 >100 µg/ml)
(data not shown). The critical time point for the cytotoxic
activity was 48 h following exposure; this indicates that there was
a delay in reaching the maximum effect in both cell lines for all
the samples. In the MCF7 cell line, the most effective fraction was
the CH2Cl2 fraction (IC50 after 48
h, 25.34±2.01) followed by the EtOAc fraction (IC50
after 48 h, 35.2±2.69); these fractions were both more effective
than the primary methanol extract (IC50 after 48 h,
64.64±1.64). In the PC3 cells, the IC50 values were
37.97±3.87, 51.57±3.87 and 70.33±2.36, for the
CH2Cl2 fraction, EtOAc fraction and methanol
extract, respectively. The water (aqueous) fraction did not exhibit
an acceptable cytotoxic activity in any of the two cancer cell
lines (IC50 values >100 µg/ml).
 | Table I.Half maximal inhibitory
concentrations (IC50) of the extract, fractions and
essential oil of Cyperus longus in the MCF7 and PC3 cell
lines following 24, 48 and 72 h of exposure. |
Table I.
Half maximal inhibitory
concentrations (IC50) of the extract, fractions and
essential oil of Cyperus longus in the MCF7 and PC3 cell
lines following 24, 48 and 72 h of exposure.
|
| IC50 in
MCF7 cells | IC50 in
PC3 cells |
|---|
|
|
|
|
|---|
| Treatment
agent | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
|---|
| Aqueous
fraction | >100 | >100 | >100 | >100 | >100 | >100 |
| Methanolic
extract | >100 | 64.64±1.64 | 93.47±5.11 | >100 | 70.33±2.36 | 95.82±1.96 |
|
CH2Cl2 fraction |
22.17±2.33a |
25.34±2.01b |
43.15±3.02a | 58.01±6.39 | 37.97±3.87 | 72.39±6.84 |
| Ethyl acetate
fraction |
42.38±2.54b |
35.2±2.69a |
55.38±4.25a | 72.37±3.69 | 51.57±3.89 | 73.22±3.64 |
| Essential oil |
21.17±2.01b |
12.55±3.65c,d | 31.35±3.69 | 43.65±4.12 |
22.25±4.25e | 39.91±3.21 |
| Paclitaxel
(positive control) |
6.34±0.81a |
3.45±0.39a |
6.75±0.57a |
0.10±0.02 |
0.09±0.03 |
0.08±0.03 |
Based on these data and on the polarity of the most
effective fraction (CH2Cl2), we decided to
determine the cytotoxic activity of the plant EO. Our results
revealed that the EO was more effective than even the
CH2Cl2 fraction in both cell lines
(IC50 after 48 h, 22.25±4.25 in the PC3 cells and
12.55±3.65 in the MCF7 cell). As shown in Table I, the EO, and the EtOAc and
CH2Cl2 fractions were significantly more
effective at exerting cytotoxic effects on the MCF7 cells compared
to the PC3 cells (p<0.05, p<0.001 and p<0.01,
respectively). There was a significant difference in the
IC50 values between the plant EO and paclitaxel in the
MCF7 cells (12.55±3.65 and 3.45±0.39, respectively; p<0.05);
however, in the PC3 cells, this difference was even more
significant (22.25±4.25 and 0.09±0.03, respectively; p<0.001).
This indicated that the plant EO exerted more potent inhibitory
effects on the MCF7 cells compared to the PC3 cells. However, in
the case of paclitaxel, this difference was partly due to the very
high cytotoxicity of paclitaxel to PC3 cells
(IC50=0.09±0.03 compared to 3.45±0.39 for the MCF7
cells).
Effects of the extract, different
fractions and EO of Cyperus longus on cell apoptosis
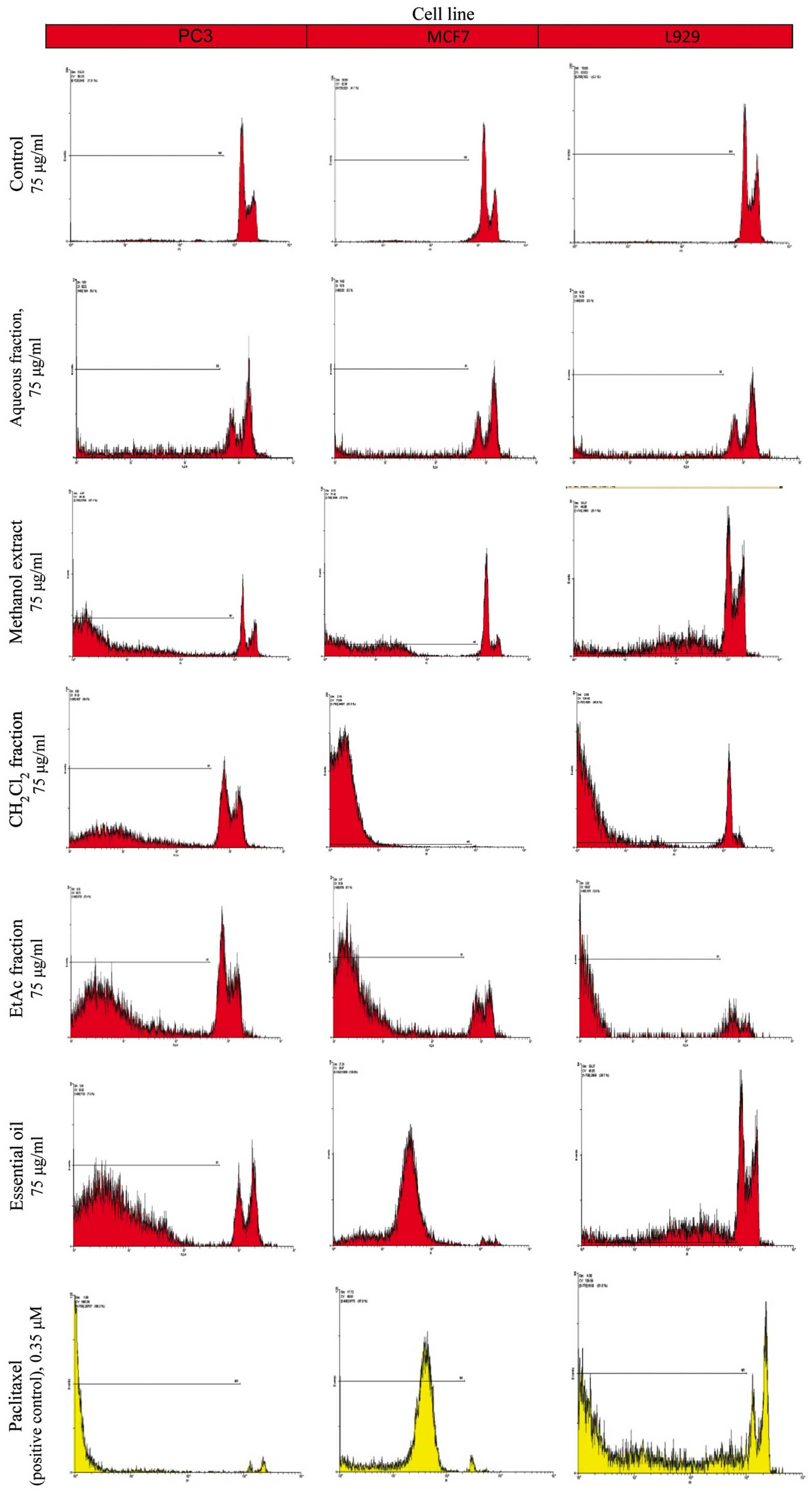
The proportion of apoptotic cells was measured after
PI staining of the DNA fragments using flow cytometry. The sub-G1
peak (one of the reliable biochemical markers of apoptosis) was
observed at 48 h following treatment of the cells with the extract,
the different fractions and the EO of Cyperus longus (Fig. 2). Our results indicated that the EO
and the CH2Cl2 fraction at the same
concentration (75 µg/ml) were the most effective inducers of
apoptosis among the plant extracts examined in both the PC3 and
MCF7 cancer cell lines (Table II).
These data support the results obtained from MTT assay.
 | Table II.Effects of the extract, fractions and
essential oil of Cyperus longus on the sub-G1 cell
population (apoptosis, %) in the MCF7 and PC3 cell lines following
48 h of exposure. |
Table II.
Effects of the extract, fractions and
essential oil of Cyperus longus on the sub-G1 cell
population (apoptosis, %) in the MCF7 and PC3 cell lines following
48 h of exposure.
|
| Percentage of
apoptotic cells |
|---|
|
|
|
|---|
| Treatment
agent | L929 cells | MCF7 cells | PC3 cells |
|---|
| Control |
2.72±3.19 |
3.2±1.59 |
3.65±1.61 |
| Aqueous fraction,
75 µg/ml |
6.25±3.21 |
7.59±1.54 |
9.87±1.31 |
| Methanolic extract,
75 µg/ml | 20.85±3.07 |
51.72±7.2a | 25.07±4.21 |
|
CH2Cl2 fraction, 75
µg/ml | 22.69±4.35 |
78.35±5.65b | 60.39±5.32 |
| Ethyl acetate
fraction, 75 µg/ml | 27.35±6.65 |
68.89±3.34b | 50.89±6.87 |
| Essential oil, 75
µg/ml | 29.22±3.68 |
78.23±2.86c | 65.35±2.35 |
| Paclitaxel
(positive control), 0.35 µM | 90.15±2.33 |
89.1±5.79 |
98.66±3.43d |
Chemical composition of EO
Hydro-distillation of the dried powder of the plant
yielded a pale yellow-colored oil with a pleasant aroma, yield
0.45% (v/w). A total of 32 components comprising 83.24% of the EO
was identified (Table III).
β-himachalene (10.81%), α-caryophyllene oxide (7.6%), irisone
(4.78%), β-caryophyllene oxide (4.36%), humulene oxide (12%),
viridiflorol (4.73%), aristolone (6.39%) and longiverbenone (6.04%)
were identified as the major constituents of the EO. Among the
identified components, 7 components comprising 9.13% of the EO were
oxygenated monoterpenes, 11 components comprising 20.89% were
sesquiterpene hydrocarbons, 11 components comprising 46.83% were
oxygenated sesquiterpenes, 1 component comprising 1.71% was
diterpene hydrocarbon and 2 components comprising 4.68% were
oxygenated diterpenes.
 | Table III.Chemical composition of the essential
oil of Cyperus longus from North Khorasan, Iran. |
Table III.
Chemical composition of the essential
oil of Cyperus longus from North Khorasan, Iran.
| No. | Compound name | Area % | RI | Type of
compound |
|---|
| 1 | Linalool |
0.15 | 1,000.423 | Monoterpene
oxygenated |
| 2 |
cis-Dihydrocarvone |
0.22 | 1,100.42 | Monoterpene
oxygenated |
| 3 |
trans-Dihydrocarvone |
0.12 | 1,100.50 | Monoterpene
oxygenated |
| 4 | α-Cubebene |
0.23 | 1,200.90 | Sesquiterpene |
| 5 | α-Copaene |
2.35 | 1,300.21 | Sesquiterpene |
| 6 | β-Elemene |
0.05 | 1,300.25 | Sesquiterpene |
| 7 | Germacrene-D |
0.61 | 1,300.33 | Sesquiterpene |
| 8 |
Megastigmatrienone |
0.06 | 1,300.37 | Monoterpene
oxygenated |
| 9 | α-Gurjunene |
0.56 | 1,300.49 | Sesquiterpene |
| 10 | α-Guaiene |
0.71 | 1,300.79 | Sesquiterpene |
| 11 | α-Humulene |
1 | 1,300.99 | Sesquiterpene |
| 12 | α-Amorphene |
1.16 | 1,400.18 | Sesquiterpene |
| 13 | Spathulenol |
0.97 | 1,400.41 | Sesquiterpene
oxygenated |
| 14 | δ-Guaiene |
1.27 | 1,400.48 | Sesquiterpene |
| 15 | Torreyol |
1.61 | 1,400.59 | Sesquiterpene
oxygenated |
| 16 |
9-methyl-10-methylenetricyclo[4,2,1,1]decane-9-ol |
1.65 | 1,400.87 | Monoterpene
oxygenated |
| 17 | β-Himachalene | 10.81 | 1,500.02 | Sesquiterpene |
| 18 |
γ-Gurjunenepoxide |
1.4 | 1,500.11 | Sesquiterpene
oxygenated |
| 19 | α-Caryophyllene
oxide |
7.6 | 1,500.22 | Sesquiterpene
oxygenated |
| 20 | Irisone |
4.78 | 1,500.30 | Monoterpene
oxygenated |
| 21 | β-Caryophyllene
oxide |
4.36 | 1,500.35 | Sesquiterpene
oxygenated |
| 22 | Humulene oxide |
12 | 1,500.65 | Sesquiterpene
oxygenated |
| 23 |
Retinol-acetate |
1.58 | 1,500.73 | Diterpene
oxygenated |
| 24 |
Limonene-1,2-epoxide |
2.15 | 1,500.80 | Monoterpene
oxygenated |
| 25 | Viridiflorol |
4.73 | 1,600.02 | Sesquiterpene
oxygenated |
| 26 | α-Himachalene |
2.14 | 1,600.18 | Sesquiterpene |
| 27 | Longiverbenone |
6.04 | 1,600.29 | Sesquiterpene
oxygenated |
| 28 | Aristolone |
6.39 | 1,600.57 | Sesquiterpene
oxygenated |
| 29 | Cembrene |
1.71 | 1,600.67 | Diterpene |
| 30 | Viridiflorol |
0.79 | 1,600.75 | Sesquiterpene
oxygenated |
| 31 |
Aromadendrenepoxide |
1.1 | 1,600.92 | Sesquiterpene
oxygenated |
| 32 |
17-Acetoxy-19-kauranal |
3.1 | 1,800.64 | Diterpene
oxygenated |
|
| Total
compounds | 83.24 |
|
|
|
| Monoterpene
oxygenated |
9.13 |
|
|
|
| Sesquiterpene | 20.89 |
|
|
|
| Sesquiterpene
oxygenated | 46.83 |
|
|
|
| Diterpene |
1.71 |
|
|
|
| Diterpene
oxygenated |
4.68 |
|
|
Discussion
The data of the present study demonstrated that
partially non-polar components from Cyperus longus exert
more potent cytotoxic effects on the MCF7 than on the PC3 cells.
The most effective fraction was the CH2Cl2
fraction followed by the EtOAc fraction and the methanol extract.
The water (aqueous) fraction did not exhibit any significant
anticancer activity in any cell lines (IC50>100
µg/ml). All the effective fractions had more potent inhibitory
effects on the MCF7 cells compared to the PC3 cells. The critical
time point for cytotoxic activity was 48 h following exposure; this
indicated that there was a delay in reaching the maximum effect in
both cell lines. The GC-MS data revealed that 32 components
comprising 83.24% of the EO were identified (Table III). β-himachalene (10.81%),
α-caryophyllene oxide (7.6%), irisone (4.78%), β-caryophyllene
oxide (4.36%), humulene oxide (12%), viridiflorol (4.73%),
aristolone (6.39%) and longiverbenone (6.04%) were identified as
the major constituents of the EO. As we already mentioned,
β-himachalene (22), caryophyllene
oxide (25) and α-humulene (20,21) have
been shown to exert cytotoxic effects against cancer cell lines.
The evaluation of the chemical composition of Cyperus longus
EO from Morocco by the same method (GC-MS analysis) revealed a
different spectrum of ingredients: α-humulene (16.7%),
γ-himachalene (10.1%) and β-himachalene (46.6%) (31); however, as we have already mentioned,
these agents also possess a satisfactory cytotoxic activity. Based
on the percentage ratio of viridiflorol (4.73%), aristolone
(6.39%), longiverbenone (6.04%) and irisone (4.78%) in our
Cyperus longus EO, we searched the databases for the
potential cytotoxicity of these two agents in PC3, MCF7 or other
cell lines. It has been reported that longiverbenone isolated from
the rhizome of Cyperus scariosus (45) exerts cytotoxic effects in newborn
brine shrimp (Artemia salina) bioassay with a lethal
concentration (LC50) of 14.38 µg/ml. These data support
our results on the cytotoxic effects of Cyperus longus
EO.
It has been reported in previous studies that
aristolone does not exert cytotoxic effects against cancer cell
lines, including human hepatocellular carcinoma (HepG2 and Hep3B),
human breast carcinoma (MCF7 and MDA-MB-231) and human lung
carcinoma (A-549) cells (46,47). In a recent study, using MTT and
lactate dehydrogenase (LDH) cytotoxic assays in human epithelial
gastric cells (AGS cell line), viridiflorol fucoside, as a
sesquiterpene glycoside from Calendula officinalis L., was
shown to exert potent cytotoxic effects (48). Concerning irisone (β-ionone), this
agent was previously shown to exert toxic effects on the
photosynthetic system of Microcystis aeruginosa NIES-843
(Cyanobacteria) with a half maximal effective concentration
(EC50) of 21.23±1.87 µg/m (49); however, to the very best of our
knowledge, there is no availabe study to date on the cytotoxicity
of this agent in cancer cell lines. Thus, further investigations
are warranted to determine its exact cytotoxic effects on cancer
cell lines.
In conclusion, in the present study, our findings
demonstrated that the EO isolated from Cyperus longus exerts
satisfactory cytotoxic effects on the PC3 and MCF7 cancer cell
lines. Based on the chemical composition of the EO and since
iridiflorol and longiverbenone belong to the constituents that make
up at least 5% of the effective essential oil, it would be of
interest to investigate the effects of viridiflorol and
longiverbenone for their possible use as anticancer agents in the
future.
Acknowledgements
This study was supported financially by a research
grant from the Vice Chancellor for Research of North Khorasan
University of Medical Sciences, Bojnurd, Iran.
References
|
1
|
Katzung BG, Masters SB and Trevor AJ:
Basic and Clinical Pharmacology (12th). New York, NY: McGraw-Hill
Companies. 2012.
|
|
2
|
Shao H, Jing K, Mahmoud E, Huang H, Fang X
and Yu C: Apigenin sensitizes colon cancer cells to antitumor
activity of ABT-263. Mol Cancer Ther. 12:2640–2650. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu H, Li X, Ding W, Zeng X, Kong H, Wang H
and Xie W: Deguelin induces the apoptosis of lung cancer cells
through regulating a ROS driven Akt pathway. Cancer Cell Int.
15:252015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Youssef Moustafa AM, Khodair AI and Saleh
MA: Isolation, structural elucidation of flavonoid constituents
from Leptadenia pyrotechnica and evaluation of their
toxicity and antitumor activity. Pharm Biol. 47:539–552. 2009.
View Article : Google Scholar
|
|
5
|
Seelinger G, Merfort I, Wölfle U and
Schempp CM: Anti-carcinogenic effects of the flavonoid luteolin.
Molecules. 13:2628–2651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Samy RP, Gopalakrishnakone P and
Ignacimuthu S: Anti-tumor promoting potential of luteolin against
7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Chem
Biol Interact. 164:1–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu J, Li G, He K, et al: Luteolin exerts a
marked antitumor effect in cMet-overexpressing patient-derived
tumor xenograft models of gastric cancer. J Transl Med. 13:422015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Zhang M, Yu L, Zhao Y, He N and
Yang X: Antitumor activities of quercetin and
quercetin-5′,8-disulfonate in human colon and breast cancer cell
lines. Food Chem Toxicol. 50:1589–1599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang P, Zhang K, Zhang Q, Mei J, Chen CJ,
Feng ZZ and Yu DH: Effects of quercetin on the apoptosis of the
human gastric carcinoma cells. Toxicol In Vitro. 26:221–228. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borska S, Chmielewska M, Wysocka T,
Drag-Zalesinska M, Zabel M and Dziegiel P: In vitro effect of
quercetin on human gastric carcinoma: targeting cancer cells death
and MDR. Food Chem Toxicol. 50:3375–3383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chan ST, Yang NC, Huang CS, Liao JW and
Yeh SL: Quercetin enhances the antitumor activity of trichostatin A
through upregulation of p53 protein expression in vitro and in
vivo. PLoS One. 8:e542552013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dixit S: Anticancer Effect of Rutin
Isolated from the Methanolic Extract of Triticum aestivum
Straw in Mice. Med Sci. 2:153–160. 2014.
|
|
13
|
Chen H, Miao Q, Geng M, Liu J, Hu Y, Tian
L, Pan J and Yang Y: Anti-tumor effect of rutin on human
neuroblastoma cell lines through inducing G2/M cell cycle arrest
and promoting apoptosis. Scientific World Journal.
29:269152013.
|
|
14
|
Al-Dhabi NA, Arasu MV, Park CH and Park
SU: An up-to-date review of rutin and its biological and
pharmacological activities. EXCLI J. 14:59–63. 2015.PubMed/NCBI
|
|
15
|
Slavov A, Trifonov A, Peychev L, Dimitrova
S, Peycheva S, Gotcheva V and Angelov A: Biologically active
cCompounds with antitumor activity in propolis extracts from
different geographic regions. Biotechnol Biotechnol Equip.
27:4010–4013. 2013. View Article : Google Scholar
|
|
16
|
Sahpazidou D, Geromichalos GD, Stagos D,
Apostolou A, Haroutounian SA, Tsatsakis AM, Tzanakakis GN, Hayes AW
and Kouretas D: Anticarcinogenic activity of polyphenolic extracts
from grape stems against breast, colon, renal and thyroid cancer
cells. Toxicol Lett. 230:218–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Henderson AJ, Ollila CA, Kumar A, Borresen
EC, Raina K, Agarwal R and Ryan EP: Chemopreventive properties of
dietary rice bran: current status and future prospects. Adv Nutr.
3:643–653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abbaszadeh H, Ebrahimi SA and Akhavan MM:
Antiangiogenic activity of xanthomicrol and calycopterin, two
polymethoxylated hydroxyflavones in both in vitro and ex vivo
models. Phytother Res. 28:1661–1670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Turkez H, Togar B, Tatar A, Geyıkoglu F
and Hacımuftuoglu A: Cytotoxic and cytogenetic effects of α-copaene
on rat neuron and N2a neuroblastoma cell lines. Biologia.
69:936–942. 2014. View Article : Google Scholar
|
|
20
|
Costa EV, Menezes LR, Rocha SL, Baliza IR,
Dias RB, Rocha CA, Soares MB and Bezerra DP: Antitumor properties
of the leaf essential oil of Zornia brasiliensis. Planta
Med. 81:563–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
del Hadri A, del Río MAG, Sanz J,
González-Coloma A, Idaomar M, Ozonas BR, González JB and Reus MIS:
Cytotoxic activity of α-humulene and transcaryophyllene from
Salvia officinalis in animal and human tumor cells. An R
Acad Nac Farm. 76:343–356. 2010.
|
|
22
|
Saab AM, Guerrini A, Sacchetti G, Maietti
S, Zeino M, Arend J, Gambari R, Bernardi F and Efferth T:
Phytochemical analysis and cytotoxicity towards multidrug-resistant
leukemia cells of essential oils derived from Lebanese medicinal
plants. Planta Med. 78:1927–1931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Babu HR and Savithramma N: Screening of
secondary metabolites of underutilized species of Cyperaceae. Int J
Pharm Sci Rev Res. 24:182–187. 2014.
|
|
24
|
Khamsan S, Liawruangrath B, Liawruangrath
S, Teerawutkulrag A, Pyne SG and Garson MJ: Antimalarial,
anticancer, antimicrobial activities and chemical constituents of
essential oil from the aerial parts of Cyperus kyllingia
Endl. Rec Nat Prod. 5:324–327. 2011.
|
|
25
|
Legault J and Pichette A: Potentiating
effect of β-caryophyllene on anticancer activity of α-humulene,
isocaryophyllene and paclitaxel. J Pharm Pharmacol. 59:1643–1647.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar KH, Razack S, Nallamuthu I and
Khanum F: Phytochemical analysis and biological properties of
Cyperus rotundus L. Ind Crops Prod. 52:815–826. 2014.
View Article : Google Scholar
|
|
27
|
Kilani-Jaziri S, Neffati A, Limem I, et
al: Relationship correlation of antioxidant and antiproliferative
capacity of Cyperus rotundus products towards K562
erythroleukemia cells. Chem Biol Interact. 181:85–94. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kilani S, Sghaier Ben M, Limem I, Bouhlel
I, Boubaker J, Bhouri W, Skandrani I, Neffatti A, Ammar Ben R,
Dijoux-Franca MG, et al: In vitro evaluation of antibacterial,
antioxidant, cytotoxic and apoptotic activities of the tubers
infusion and extracts of Cyperus rotundus. Bioresour
Technol. 99:9004–9008. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morikawa T, Xu F, Matsuda H and Yoshikawa
M: Structures of novel norstilbene dimer, longusone A, and three
new stilbene dimers, longusols A, B, and C, with antiallergic and
radical scavenging activities from Egyptian natural medicine
Cyperus longus. Chem Pharm Bull (Tokyo). 58:1379–1385. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harborne J: Distribution and taxonomic
significance of flavonoids in the leaves of the cyperaceae.
Phytochemistry. 10:1569–1574. 1971. View Article : Google Scholar
|
|
31
|
Ait-Ouazzou A, Lorán S, Arakrak A,
Laglaoui A, Rota C, Herrera A, Pagán R and Conchello P: Evaluation
of the chemical composition and antimicrobial activity of Mentha
pulegium, Juniperus phoenicea, and Cyperus longus
essential oils from Morocco. Food Res Int. 45:313–319. 2012.
View Article : Google Scholar
|
|
32
|
Xu F, Morikawa T, Matsuda H, Ninomiya K
and Yoshikawa M: Structures of new sesquiterpenes and
hepatoprotective constituents from the Egyptian herbal medicine
Cyperus longus. J Nat Prod. 67:569–576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharma R and Gupta R: Cyperus
rotundus extract inhibits acetylcholinesterase activity from
animal and plants as well as inhibits germination and seedling
growth in wheat and tomato. Life Sci. 80:2389–2392. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ben-Arye E, Schiff E, Hassan E, Mutafoglu
K, Lev-Ari S, Steiner M, Lavie O, Polliack A, Silbermann M and Lev
E: Integrative oncology in the Middle East: from traditional herbal
knowledge to contemporary cancer care. Ann Oncol. 23:211–221. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shao H, Jing K, Mahmoud E, Huang H, Fang X
and Yu C: Apigenin sensitizes colon cancer cells to antitumor
activity of ABT-263. Mol Cancer Ther. 12:2640–2650. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Apostolou A, Stagos D, Galitsiou E, Spyrou
A, Haroutounian S, Portesis N, Trizoglou I, Hayes Wallace A,
Tsatsakis AM and Kouretas D: Assessment of polyphenolic content,
antioxidant activity, protection against ROS-induced DNA damage and
anticancer activity of Vitis vinifera stem extracts. Food
Chem Toxicol. 61:60–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Androutsopoulos VP, Ruparelia KC,
Papakyriakou A, Filippakis H, Tsatsakis AM and Spandidos DA:
Anticancer effects of the metabolic products of the resveratrol
analogue, DMU-212: Structural requirements for potency. Eur J Med
Chem. 46:2586–2595. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xue YQ, Di JM, Luo Y, Cheng KJ, Wei X and
Shi Z: Resveratrol oligomers for the prevention and treatment of
cancers. Oxid Med Cell Longev. 2014:7658322014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parsaee H, Asili J, Mousavi SH, Soofi H,
Emami SA and Tayarani-Najaran Z: Apoptosis Induction of Salvia
chorassanica Root Extract on Human Cervical Cancer Cell Line.
Iran J Pharm Res. 12:75–83. 2013.PubMed/NCBI
|
|
40
|
Diao WR, Hu QP, Zhang H and Xu JG:
Chemical composition, antibacterial activity and mechanism of
action of essential oil from seeds of fennel (Foeniculum
vulgare Mill.). Food Contr. 35:109–116. 2014. View Article : Google Scholar
|
|
41
|
Malaekeh-Nikouei B, Mousavi SH, Shahsavand
S, Mehri S, Nassirli H and Moallem SA: Assessment of cytotoxic
properties of safranal and nanoliposomal safranal in various cancer
cell lines. Phytother Res. 27:1868–1873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mousavi SH, Moallem SA, Mehri S,
Shahsavand S, Nassirli H and Malaekeh-Nikouei B: Improvement of
cytotoxic and apoptogenic properties of crocin in cancer cell lines
by its nanoliposomal form. Pharm Biol. 49:1039–1045. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Olaru OT, Venables L, VAN DE, Venter M,
Nitulescu GM, Margina D, Spandidos DA and Tsatsakis AM: Anticancer
potential of selected Fallopia Adans species. Oncol Lett.
10:1323–1332. 2015.PubMed/NCBI
|
|
44
|
Boussaada O, Ammar S, Saidana D, Chriaa J,
Chraif I, Daami M, Helal AN and Mighri Z: Chemical composition and
antimicrobial activity of volatile components from capitula and
aerial parts of Rhaponticum acaule DC growing wild in
Tunisia. Microbiol Res. 163:87–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rahman MS and Anwar MN: Antibacterial and
Cytotoxic Activity of Longiverbenone Isolated from the Rhizome of
Cyperus scariosu. Bangladesh J Microbiol. 25:82–84.
2008.
|
|
46
|
Su JH, Dai CF, Huang HH, Wu YC, Sung PJ,
Hsu CH and Sheu JH: Terpenoid-related metabolites from a formosan
soft coral Nephthea chabrolii. Chem Pharm Bull (Tokyo).
55:594–597. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kamada T and Vairappan CS: New bioactive
secondary metabolites from Bornean red alga, Laurencia
similis (Ceramiales). Nat Prod Commun. 8:287–288.
2013.PubMed/NCBI
|
|
48
|
D'Ambrosio M, Ciocarlan A, Colombo E,
Guerriero A, Pizza C, Sangiovanni E and Dell'Agli M: Structure and
cytotoxic activity of sesquiterpene glycoside esters from
Calendula officinalis L.: Studies on the conformation of
viridiflorol. Phytochemistry. 117:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shao J, Xu Y, Wang Z, Jiang Y, Yu G, Peng
X and Li R: Elucidating the toxicity targets of β-ionone on
photosynthetic system of Microcystis aeruginosa NIES-843
(Cyanobacteria). Aquat Toxicol. 104:48–55. 2011. View Article : Google Scholar : PubMed/NCBI
|