Introduction
Reversible posterior leukoencephalopathy syndrome
(RPLS) was first described by Hinchey et al (1) as an acute illness that causes symptoms
such as hypertension, headaches, seizures, altered mental status
and visual disturbances, and which is usually reversible following
the removal of the causative agents and the control of the
patient's blood pressure. RPLS is characterized by white matter
edema, particularly involving the bilateral occipital and posterior
parietal lobes of the brain (1).
Involvement of other areas of the brain, including the frontal
lobes, cerebellum, basal ganglia and brain stem, has also been
reported.
RPLS is primarily associated with hypertension,
eclampsia, renal impairment, cytotoxic drugs, immunosuppressants
and molecular targeted agents. The list of common antineoplastic
drugs that predispose patients to RPLS is expanding, and includes
cisplatin, L-asparaginase, thalidomide, vinflunine, methotrexate,
vincristine and cytarabine (2–8). Certain
combination regimens have also been associated with RPLS, including
a combination treatment of ziv-aflibercept with cisplatin and
pemetrexed, the cyclophosphamide, hydroxydaunorubicin, Oncovin and
Prednisone regimen, intrathecal methotrexate and intravenous
ifosfamide, idarubicine and etoposide, bevacizumab/folinic acid,
fluorouracil and irinotecan, and capecitabine and cyclophosphamide
(9–13).
The mechanisms underlying RPLS have been postulated
to be either severe hypertension leading to failed auto-regulation
and endothelial injury/vasogenic edema, or vasoconstriction leading
to brain ischemia and subsequent vasogenic edema. However, the
mechanism by which cytotoxic agents cause RPLS in a normotensive
environment is not fully understood, but the disruption of the
blood-brain barrier is suspected to be a major contributory factor
(14).
The present report describes a normotensive patient
who had received cisplatin/pemetrexed for treatment of non-small
cell lung cancer (NSCLC) and who subsequently developed RPLS, but
was able to recover following treatment. Written informed consent
was obtained from the patient's family.
Case report
The current report describes the case of a
65-year-old female patient that presented to the Leo W. Jenkins
Cancer Center (Greenville, NC, USA) in July 2014 with stage IIA
NSCLC [tumor-node-metastasis staging score, T1N1M0 (15)] on the left upper lobe, with an
enlarged left hilar lymph node. The patient received
cisplatin/pemetrexed (75 and 50 mg/m2, respectively) by
intravenous administration, as neoadjuvant chemotherapy, every 3
weeks. However, 3 days after the third cycle of this therapy, the
patient was referred to the Emergency Department of Vidant Medical
Center (Greenville, NC, USA)presenting with progressive confusion,
followed by tonic-clonic seizures, a fever, abdominal pain and a
headache. Neurological examination indicated a limited attention
span, disorientation, generalized hyperreflexia and paraparesis
with extensor posturing of the bilateral lower extremities and
reduced dorsiflexion capability. The blood pressure of the patient
was 137/89 mmHg (normal range, 100–140/60–90 mmHg). Biological
investigations at admission revealed a normal white blood cell
count and platelet count, borderline hyponatremia (134 mmol/l;
normal range, 135–145 mmol/l) and mildly elevated ammonia levels
(45 mmol/l; normal range, 11–35 mmol/l). Electroencephalography and
a lumbar puncture gave normal results, with an opening pressure of
140 mm H2O, and no organisms were cultured. Cranial
computed tomography (CT) scans revealed attenuation abnormalities
on the bilateral parietal region and left occipital lobe, with
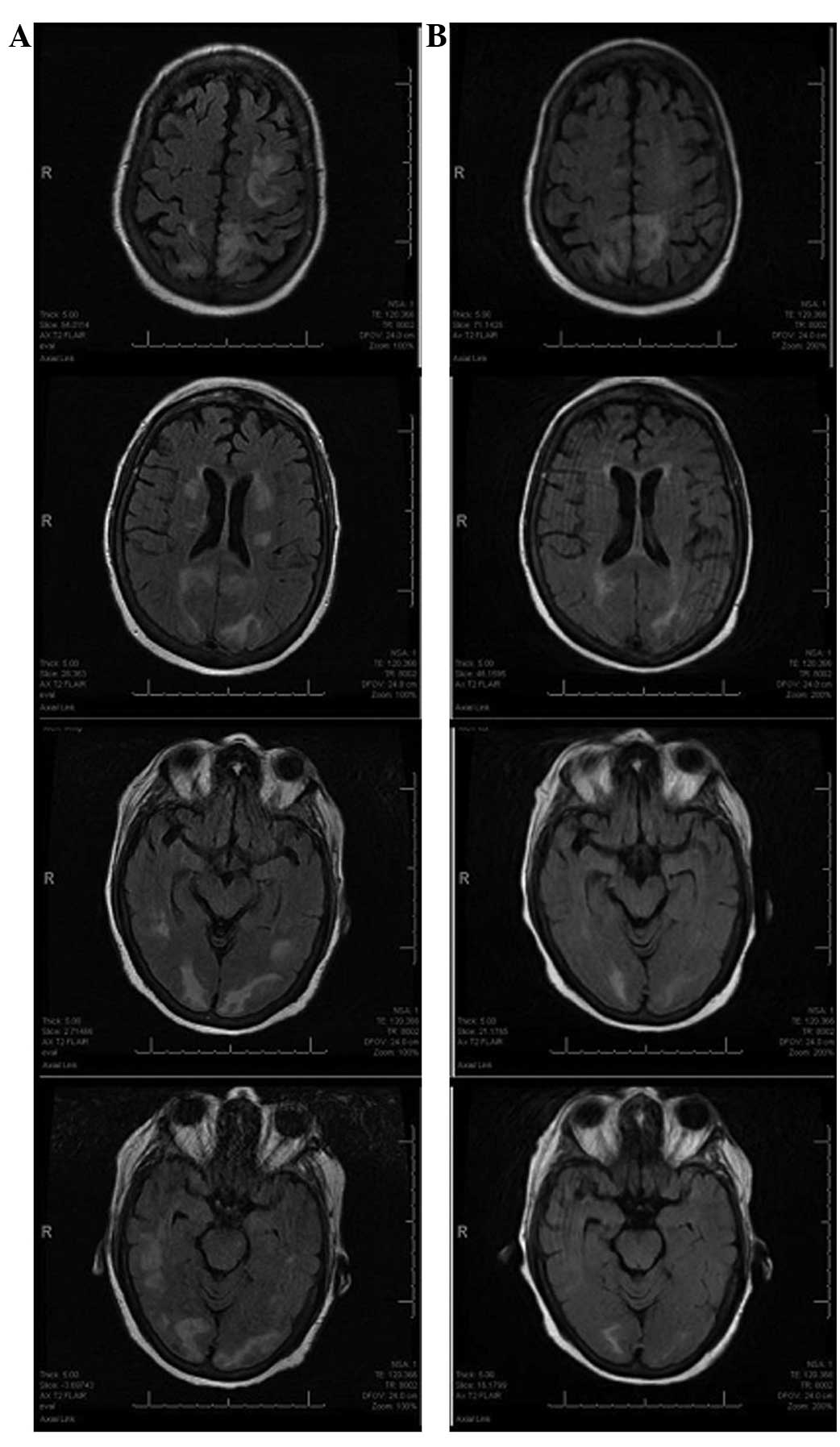
suspected metastasis. Cranial T2-weighted magnetic resonance
imaging (MRI) revealed bilateral areas of increased signal
intensity in the occipital, temporal and periventricular white
matter (Fig. 1A).
The patient experienced multiple generalized
seizures following admission, which were resolved by lorazepam
treatment (2 mg, every 2 h as required). Anticonvulsants
(levetiracetam; 1,500 mg every 12 h), dexamethasone (4 mg, every 6
h) and antihypertensive agents (amlodipine, 5 mg daily; metoprolol,
25 mg twice daily) were then administered as required in order to
treat the remaining symptoms. The patient's mental condition
gradually recovered and full consciousness was regained, but the
patient reported residual weakness with an ECOG score of 3,
reflective of poor ability to function with regard to daily
activity and self-care. A brain MRI scan performed 10 days later
indicated partial alleviation of the subcortical edema (Fig. 1B). The patient was discharged on the
day 15 post-admission, and a follow-up cranial CT examination 1
month later demonstrated improved, but not complete, resolution of
the abnormalities. The patient succumbed to the disease in
September 2014.
Discussion
The present study was unable to confirm whether
cisplatin or pemetrexed was responsible for the onset of RPLS, but
there are multiple previous studies of cisplatin-associated RPLS,
whether alone or in combinatorial treatments alongside other
cytotoxic drugs (5,16–23).
However, to the best of our knowledge, no studies have indicated
that pemetrexed alone can induce RPLS; it is therefore conceivable
that cisplatin is predominantly responsible for the RPLS reported
in the present study.
RPLS is a rare neurological syndrome of the brain
that was first defined by Hinchey et al in 1996 (1). The condition is reported in numerous
patients with eclampsia, acute hypertensive encephalopathy
associated with renal disease and those receiving immunosuppressive
therapy or interferon. An association with cisplatin administration
in cancer patients has been widely reported in previous studies
(Table I) (5,16–23), predominantly occurring within the
first 3 cycles of chemotherapy, with the exception of one
late-onset case in the 7th cycle (16). In the majority of cases, patients
develop hypertension, and additionally present with headaches,
seizures, altered mental status and visual disturbances, whilst
normotension is only observed in a few cases (5,16–18). Cranial CT/MRI usually indicates
cortical/subcortical edema in the bilateral occipital and parietal
lobes; uncommon areas in which to observe legions include the
thalamus, the cerebellum, the periventricular regions, and the
frontal and temporal lobes. Symptoms typically improve rapidly upon
gaining control of the patient's blood pressure, upon treatment
with anticonvulsants and/or upon withdrawal of cytotoxic drugs. It
is notable that this syndrome has serious and potentially
life-threatening adverse effects if left untreated.
 | Table I.Characteristics of reported cases of
patients with cancer with cisplatin-associated RPLS. |
Table I.
Characteristics of reported cases of
patients with cancer with cisplatin-associated RPLS.
| First author
(ref.) | Age,
years/gender | Diagnosis | Drug treatment | Cycle | Time after
chemotherapy | Clinical
presentation | Highest BP, mmHg | Location of lesions
from CT/MRI data | Recovery time |
|---|
| Ito et al
(5) | 70/M | Osteo-sarcoma | DDP, 50 mg, injected
intraarterially | 1st | 26 days | Generalized
convulsions; lethargy; hyperreflexia; mild weakness in lower left
extremities; hallucination at later stages; mild
hypomagnesemia | 180/100 | Bilateral occipital
lobes; white matter of the parietal and frontal lobes | Symptoms subsided
after 3 days; CR confirmed by MRI in 6 months |
| Dersch et al
(16) | 41/F | NSCLC | DDP, gemcitabine,
bevacizumab | 7th | 4 weeks | Focal seizures;
headaches; ataxia; hallucinations | 245/140 | Bilateral frontal and
parietal cortical/subcortical regions, right thalamus; right
temporal cortical/subcortical regions | Symptoms subsided
following a reduction in BP. PR confirmed by MRI in 5 weeks |
| Zahir et al
(17) | 23/M | Germ cell | DDP, 20
mg/m2; etoposide 100 mg/m2, (days 1.5) | 1st | A few hours | Tonic-clonic
seizures; blurring of vision; hyperreflexia of the lower limbs | 170/110 | Bilateral
periventricular, posterior parietal and occipital regions | Symptoms subsided in
48 h. Repeated DDP administration without dose reduction; no
neurological complications occurred |
| Kwon et al
(18) | 58/F | Gallbladder
cancer | Gemcitabine, 1,200
mg/m2, days 1 and 8; DDP, 60 mg/m2, days
1.5 | 3rd | 2 weeks | Headache; dizziness;
tonic-clonic seizures | 170/90 | Patchy
cortical/subcortical lesions on occipital and parietal lobes | CR confirmed by MRI
after 10 days |
| Maeda et al
(19) | 50/M | Bladder cancer | Gemcitabine, 1,200
mg/m2, days 1 and 8; DDP, 60 mg/m2, days 1.5 | 2nd | 5 weeks | Semicomatose;
hypercalcemia | Almost normal | Subcortical lesions
on the posterior occipital lobes and thalamus | Symptoms improved
after a few days; PR confirmed by MRI in 4 weeks. Succumbed to
respiratory failure 6 weeks after RPLS treatment |
| Rangi et al
(20) | 49/F | Gestational
trophoblastic disease | Etoposide and DDP for
2 months, then gemcitabine, carboplatin and Taxol for 2 months | 4th | 2 months | Headache; confusion;
tonic-clonic seizures; bilateral visual disturbance | Normal | Subcortical white
matter of the posterior cerebellar hemispheres and occipital and
parietal lobes | Symptoms subsided in
48 h. CR confirmed by MRI in 6 weeks |
| Onujiogu et al
(21) | 64/F | Fallopian tube
cancer | Paclitaxel, 135
mg/m2; DDP, 100 mg/m2, injected
intraperitoneally | 1st | 5 days | Lethargy; aphasia;
leucopenia; hyponatremia | 160/93 with
hypertension history | White matter
throughout the frontal, parietal, occipital and temporal lobes | Symptoms subsided in
4 days. Almost CR confirmed by MRI after 12 days |
| Paul et al
(22) | 37/n/a | Gastric cancer | DDP, 50
mg/m2 and 5-fluorouracil, 2,000 mg/m2 | 1st | During
chemotherapy | Tonic-clonic
seizures | U | White matter of the
parietal and occipital lobes | Multiple
recurrences |
| Nomura et al
(23) | 61/F | Ovarian
carcinoma | DDP and
etoposide | 2nd | Following second
course | Headache; fever;
partial seizure; confusion; mild right hemiparesis; cortical
blindness after 10 days | U | Subcortical white
matter of the occipital cortex; gracile fasciculus; dorsal root
ganglia | Symptoms subsided
after 1 month. Succumbed to aspiration pneumonia on the 43rd day
following treatment |
| Present study | 65/F | NSCLC | DDP, 75
mg/m2 and pemetrexed, 50 mg/m2 | 3rd | 3 days | Altered mental
status; generalized seizures; fever; headache; hyperreflexia;
paraparesis of the lower extremities | Almost normal | White matter of the
occipital, temporal and periventricular regions | Symptoms subsided
after 10 days. PR confirmed by MRI after 10 days |
The precise pathophysiology of RPLS is incompletely
understood. As hypertension presents in the majority of patients
with RPLS, hypertensive encephalopathy is a probable mechanism of
its development. Sudden elevations in systemic blood pressure
disrupt the blood-brain barrier, causing the local exchange of
fluids. The cerebral white matter is composed of myelinated fiber
tracts in an extracellular matrix containing glial cells,
arterioles and capillaries, and is susceptible to vasogenic edema
(1). The carotid vessels are supplied
with a greater number of sympathetic adrenergic innervations than
those of the vertebral-basilar system; this inherent deficiency of
sympathetic adrenergic innervation may inhibit the vasoconstriction
of posterior cerebral vessels, making them prone to RPLS
development (24). In normotensive
patients with RPLS, including the present case, another possible
hypothesis is that damage to the endothelial cells of cerebral
vessels by cytotoxic drugs destroys the blood-brain barrier, as
proposed in a rat model (14).
Numerous RPLS patients have demonstrated metabolic abnormalities,
including fever, leukocytosis, hyponatremia, hypocalcemia and
hypomagnesemia (2–8), implying that metabolic abnormalities may
disturb the integrity of the blood-brain barrier and lead to
cerebral edema. In a previous study, a postmortem examination of a
patient with cisplatin-associated RPLS indicated severe nerve cell
loss, gliosis and spongy changes to the bilateral occipital cortex,
including the visual field; furthermore, mild to moderate
demyelination in the subcortical white matter of the occipital
cortex, gracile fasciculus and dorsal root ganglia was observed.
Notably, platinum was detected in the bilateral occipital cortex,
spinal cord and cauda equina, suggesting that platinum may
contribute to the damaging effects of RPLS (23).
The use of an intravenous cisplatin/pemetrexed
regimen can be associated with RPLS; although typically reversible,
this syndrome is serious and can be fatal if left untreated. Early
recognition is vital in order to control the blood pressure or to
withdraw cytotoxic drugs in a timely manner for the reversal of
RPLS. The precise underlying mechanism of RPLS is not fully
understood and is posited to be multifactorial, but it is likely to
be associated with the integrity of the blood-brain barrier.
Glossary
Abbreviations
Abbreviations:
|
RPLS
|
reversible posterior
leukoencephalopathy syndrome
|
|
NSCLC
|
non-small cell lung cancer
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
References
|
1
|
Hinchey J, Chaves C, Appignani B, Breen J,
Pao L, Wang A, Pessin MS, Lamy C, Mas JL and Caplan LR: A
reversible posterior leukoencephalopathy syndrome. N Engl J Med.
334:494–500. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chow S, Cheung CS, Lee DH, Howson-Jan K
and Xenocostas A: Posterior reversible encephalopathy syndrome in a
patient with multiple myeloma treated with thalidomide. Leuk
Lymphoma. 53:1003–1005. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Helissey C, Chargari C, Lahutte M, Ricard
D, Vedrine L, Ceccaldi B and Le Moulec S: First case of posterior
reversible encephalopathy syndrome associated with vinflunine.
Invest New Drugs. 30:2032–2034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olascoaga Hualde J, Castiella Molins T,
Hernández Souto S, Moreno Becerril F, Petri Yoldi ME, de Sagaseta
Ilurdoz M and Molina Garicano J: Reversible posterior
leukoencephalopathy: Report of two cases after vincristine
treatment. An Pediatr (Barc). 68:282–285. 2008.(In Spanish).
PubMed/NCBI
|
|
5
|
Ito Y, Arahata Y, Goto Y, Hirayama M,
Nagamutsu M, Yasuda T, Yanagi T and Sobue G: Cisplatin
neurotoxicity presenting as reversible posterior
leukoencephalopathy syndrome. AJNR Am J Neuroradiol. 19:415–417.
1998.PubMed/NCBI
|
|
6
|
Patel A, Ayto R and MacDonald DH:
Posterior reversible encephalopathy after intrathecal methotrexate
therapy in diffuse large B-cell lymphoma. Br J Haematol.
161:6072013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rathi B, Azad RK, Vasudha N, Hissaria P,
Sawlani V and Gupta RK: L-asparaginase-induced reversible posterior
leukoencephalopathy syndrome in a child with acute lymphoblastic
leukemia. Pediatr Neurosurg. 37:203–205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saito B, Nakamaki T, Nakashima H, Usui T,
Hattori N, Kawakami K and Tomoyasu S: Reversible posterior
leukoencephalopathy syndrome after repeat intermediate-dose
cytarabine chemotherapy in a patient with acute myeloid leukemia.
Am J Hematol. 82:304–306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abali H, Eren OO, Dizdar O, Karadağ O,
Erman M, Yilmaz A, Uluç K, Erdem I and Türker A: Posterior
leukoencephalopathy after combination chemotherapy in a patient
with lymphoma. Leuk Lymphoma. 46:1825–1828. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allen JA, Adlakha A and Bergethon PR:
Reversible posterior leukoencephalopathy syndrome after
bevacizumab/FOLFIRI regimen for metastatic colon cancer. Arch
Neurol. 63:1475–1478. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen H, Modiano MR, Neal JW, Brahmer JR,
Rigas JR, Jotte RM, Leighl NB, Riess JW, Kuo CJ, Liu L, et al: A
phase II multicentre study of ziv-aflibercept in combination with
cisplatin and pemetrexed in patients with previously untreated
advanced/metastatic non-squamous non-small cell lung cancer. Br J
Cancer. 10:602–608. 2014. View Article : Google Scholar
|
|
12
|
Edwards MJ, Walker R, Vinnicombe S, Barlow
C, MacCallum P and Foran JM: Reversible posterior
leukoencephalopathy syndrome following CHOP chemotherapy for
diffuse large B-cell lymphoma. Ann Oncol. 12:1327–1329. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yasaki S, Tukamoto Y, Yuasa N, Ishikawa T
and Yoshii F: Late-onset leukoencephalopathy induced by long-term
chemotherapy with capecitabine and cyclophosphamide for liver
metastasis from breast cancer. Rinsho Shinkeigaku. 52:251–256.
2012.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sugimoto S, Yamamoto YL, Nagahiro S and
Diksic M: Permeability change and brain tissue damage after
intracarotid administration of cisplatin studied by double-tracer
autoradiography in rats. J Neurooncol. 24:229–240. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edge SB, Byrd DR, Compton CC, et al: Lung.
AJCC Cancer Staging Manual (7th). (New York, NY). Springer.
253–270. 2010.
|
|
16
|
Dersch R, Stich O, Goller K, Meckel S,
Dechent F, Doostkam S, Weiller C and Bardutzky J: Atypical
posterior reversible encephalopathy syndrome associated with
chemotherapy with Bevacizumab, Gemcitabine and Cisplatin. J Neurol.
260:1406–1407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zahir MN, Masood N and Shabbir-Moosajee M:
Cisplatin-induced posterior reversible encephalopathy syndrome and
successful re-treatment in a patient with non-seminomatous germ
cell tumor: A case report. J Med Case Rep. 6:4092012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwon EJ, Kim SW, Kim KK, Seo HS and Kim Do
Y: A case of gemcitabine and cisplatin associated posterior
reversible encephalopathy syndrome. Cancer Res Treat. 41:53–55.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maeda T, Kikuchi E, Matsumoto K, Yazawa S,
Hagiuda J, Miyajima A, Nakagawa K, Fujiwara H, Hoshino H and Oya M:
Gemcitabine and cisplatin chemotherapy induced reversible posterior
leukoencephalopathy syndrome in a bladder cancer patient. Int J
Clin Oncol. 15:508–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rangi PS, Partridge WJ, Newlands ES and
Waldman AD: Posterior reversible encephalopathy syndrome: A
possible late interaction between cytotoxic agents and general
anaesthesia. Neuroradiology. 47:586–590. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Onujiogu N, Lengyel E and Yamada SD:
Reversible posterior leukoencephalopathy syndrome following
intravenous paclitaxel and intraperitoneal cisplatin chemotherapy
for fallopian tube cancer. Gynecol Oncol. 111:537–539. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paul F, Aktas O, Dieste FJ, Kreitsch P,
Vogel HP and Zipp F: Relapsing reversible posterior
leukoencephalopathy after chemotherapy with cisplatin and
5-fluorouracil. Nervenarzt. 77:706–710. 2006.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nomura K, Ohno R, Hamaguchi K, Hata T,
Hatanaka H and Matsuyama H: Clinicopathological report of cisplatin
encephalopathy. Rinsho Shinkeigaku. 35:64–69. 1995.(In Japanese).
PubMed/NCBI
|
|
24
|
Perloff D: Hypertension and
pregnancy-related hypertension. Cardiol Clin. 16:79–101. 1998.
View Article : Google Scholar : PubMed/NCBI
|