Introduction
Testicular seminoma accounts for 40% of primary
testicular neoplasms, with 70–85% of patients presenting with
disease confined to the testis (Stage I), while 15–20% present with
infra-diaphragmatic lymphadenopathy (Stage II) (1). Prognostic factors of testicular seminoma
include age and pathology of the primary tumor: small vessel
invasion, tumor size, and invasion of rete testis (1). In the current study, a retrospective
analysis was conducted on the 3-, 5- and 10-year survival rates of
patients with testicular seminoma in 58 different cases. The aim of
the study was to determine factors that potentially affect
prognosis in order to provide a guideline for clinical treatment
and follow ups.
Materials and methods
Clinical materials
A total of 58 patients diagnosed with testicular
seminoma at the People's Hospital of Hebei between January 1999 and
January 2014 were enrolled in the current study. The age range of
the patients was 23–75 years (average age, 36 years). The tumors in
35 cases were located on the right side and the remaining 23 cases
were located on the left side. Pathological type was identified in
29 cases diagnosed with typical testicular seminoma, 13 cases with
testicular seminoma combined embryonal carcinoma, and 16 cases with
testicular seminoma combined embryonal carcinoma as well as
teratoma. Clinical stages were classified as: 17 cases in stage I,
32 cases in stage II, and 9 cases in stage III. Lactate
dehydrogenase (LDH), human chorionic gonadotropin (HCG) and
α-fetoprotein (AFP) were measured prior to the treatment. It was
found that 34 cases had high LDH (normal value: 109–245 IU/l),
accounting for 58.6% of the cases; 26 or 44.8% of the cases had
higher than normal HCG (normal value <2.6 mIU/ml); and 27 cases
or 46.6% had high AFP (normal value <7.0 ng/ml). All 58 cases
were treated with radical orchiectomy, with 35 cases undergoing
post-operation radiotherapy, 12 cases postoperative chemotherapy,
and 11 cases post-operation radiotherapy combined with
chemotherapy.
Methods used
Patient clinical data, including age, tumor
position, pathological type, clinical stage, AFP, HCG and LDH
levels, as well as postoperative treatment methods were identified
and classified (Table I).
Subsequently, the patients' conditions during the follow-up period
following surgery were recorded. The survival rate was also
calculated and prognosis was analyzed.
 | Table I.Log-rank single-factor analysis. |
Table I.
Log-rank single-factor analysis.
| Clinical factor | Case | χ2
test | P-value |
|---|
| Age, years |
| 0.785 | 0.940 |
|
20–29 | 6 |
|
|
|
30–39 | 20 |
|
|
|
40–49 | 16 |
|
|
|
50–59 | 9 |
|
|
|
>59 | 7 |
|
|
| Tumor side |
| 0.906 | 0.341 |
|
Right | 35 |
|
|
| Left | 23 |
|
|
| Pathological
side |
| 9.070 | 0.011 |
| Typical
testicular seminoma | 29 |
|
|
|
Testicular seminoma combined
embryonal carcinoma | 13 |
|
|
|
Testicular seminoma combined
embryonal carcinoma as well as teratoma | 16 |
|
|
| Clinical stage |
| 7.162 | 0.028 |
| I | 17 |
|
|
| II | 32 |
|
|
| III | 9 |
|
|
| LDH value |
| 5.285 | 0.022 |
|
Normal | 24 |
|
|
|
Increasing | 34 |
|
|
| HCG value |
| 3.677 | 0.055 |
|
Normal | 32 |
|
|
|
Increasing | 26 |
|
|
| AFP value |
| 4.783 | 0.029 |
|
Normal | 31 |
|
|
|
Increasing | 27 |
|
|
| Postoperative
treatment methods |
| 6.362 | 0.042 |
|
Postoperative
radiotherapy | 35 |
|
|
|
Postoperative
chemotherapy | 12 |
|
|
|
Postoperative
radiotherapy+chemotherapy | 11 |
|
Follow-ups
The follow-up process was performed by a variety of
means, including primarily phone calls, clinical visits and
reexaminations. Follow ups were mainly focused on patient
postoperative treatment, survival state, and survival time.
Survival time was calculated by months from the first time they
underwent therapy until the last follow-up. Death events were
defined as the end point. Patient survival or lost follow-up data
were processed as censored data. Deadline for the follow ups was
December 31, 2014.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
(SPSS, Inc., Chicago, IL, USA) statistical software. The
Kaplan-Meier survival curve was used to analyze the survival rate
and time. The log-rank single factor analysis was used to analyze
and examine the differences between the subgroups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical characteristics of testicular
seminoma
Association between testicular seminoma and
age
Of the 58 patients, 36 were aged 30–50 years,
accounting for 62.1% of all the cases. The incidence therefore was
relatively high (Table II).
 | Table II.Association between the age and the
occurrence of testicular seminoma. |
Table II.
Association between the age and the
occurrence of testicular seminoma.
| Age | Case | Proportion, % |
|---|
| 20–29 | 6 | 10.3 |
| 30–39 | 20 | 34.5 |
| 40–49 | 16 | 27.6 |
| 50–59 | 9 | 15.5 |
| >59 | 7 | 12.1 |
| Total | 58 | 100.0 |
Association between testicular seminoma and
testicular side
In 35 cases (60.3%) tumors were located on the right
side, and in 23 cases (39.7%) tumors were located on the left side.
The results showed the number of cases with right-side tumors was
superior to that of cases with left-side tumors (Table III).
 | Table III.Association between testicular
seminoma and testicle side. |
Table III.
Association between testicular
seminoma and testicle side.
| Tumor side % | Case | Proportion, |
|---|
| Left | 23 | 39.7 |
| Right | 35 | 60.3 |
| Total | 58 | 100.0 |
Association between testicular seminoma and
pathological types
Of the 58 cases, 29 cases were confirmed with
typical testicular seminoma, 13 cases with testicular seminoma
combined with embryonal carcinoma and 16 cases with testicular
seminoma combined with embryonal carcinoma and teratoma, accounting
for 50.0, 22.4 and 27.6%, respectively, of the testicular seminoma
patients. The proportion of typical testicular seminoma identified
was relatively higher (Table
IV).
 | Table IV.Association between testicular
seminoma and pathological types. |
Table IV.
Association between testicular
seminoma and pathological types.
| Pathological
type | Case | Proportion, % |
|---|
| Typical testicular
seminoma | 29 | 50.0 |
| Testicular seminoma
combined embryonal carcinoma | 13 | 22.4 |
| Testicular seminoma
combined embryonal carcinoma as well as teratoma | 16 | 27.6 |
| Total | 58 | 100.0 |
Clinical stage of testicular seminoma
Testicular seminoma was identified in the early
stages. In the present study, 49 cases were in stages I and II,
accounting for 84.5% (Table V).
 | Table V.Association between testicular
seminoma and clinical stages. |
Table V.
Association between testicular
seminoma and clinical stages.
| Clinical stage | Case | Proportion, % |
|---|
| I | 17 | 29.3 |
| II | 32 | 55.2 |
| III | 9 | 15.5 |
| Total | 58 | 100.0 |
Testicular seminoma treatment methods
Testicular seminoma is highly sensitive to
radiotherapy and its recurrence rate following radiotherapy is
extremely low. In the present study, 35 cases, accounting for 60.3%
of cases, were treated with radiotherapy. The postoperative
chemotherapy regimen administered was 3-cycle PEB regimen or
4-cycle EP regimen, and for patients in stage IIIC the 4-cycle PEB
regimen was administered (Table
VI).
 | Table VI.Association between testicular
seminoma and treatment methods. |
Table VI.
Association between testicular
seminoma and treatment methods.
| Treatment
methods | Case | Proportion, % |
|---|
| Postoperative
radiotherapy | 35 | 60.3 |
| Postoperative
chemotherapy | 12 | 20.7 |
| Postoperative
radiotherapy and chemotherapy | 11 | 19.0 |
| Total | 58 | 100.0 |
Kaplan-Meier survival rate
calculation
Analysis of the survival rate
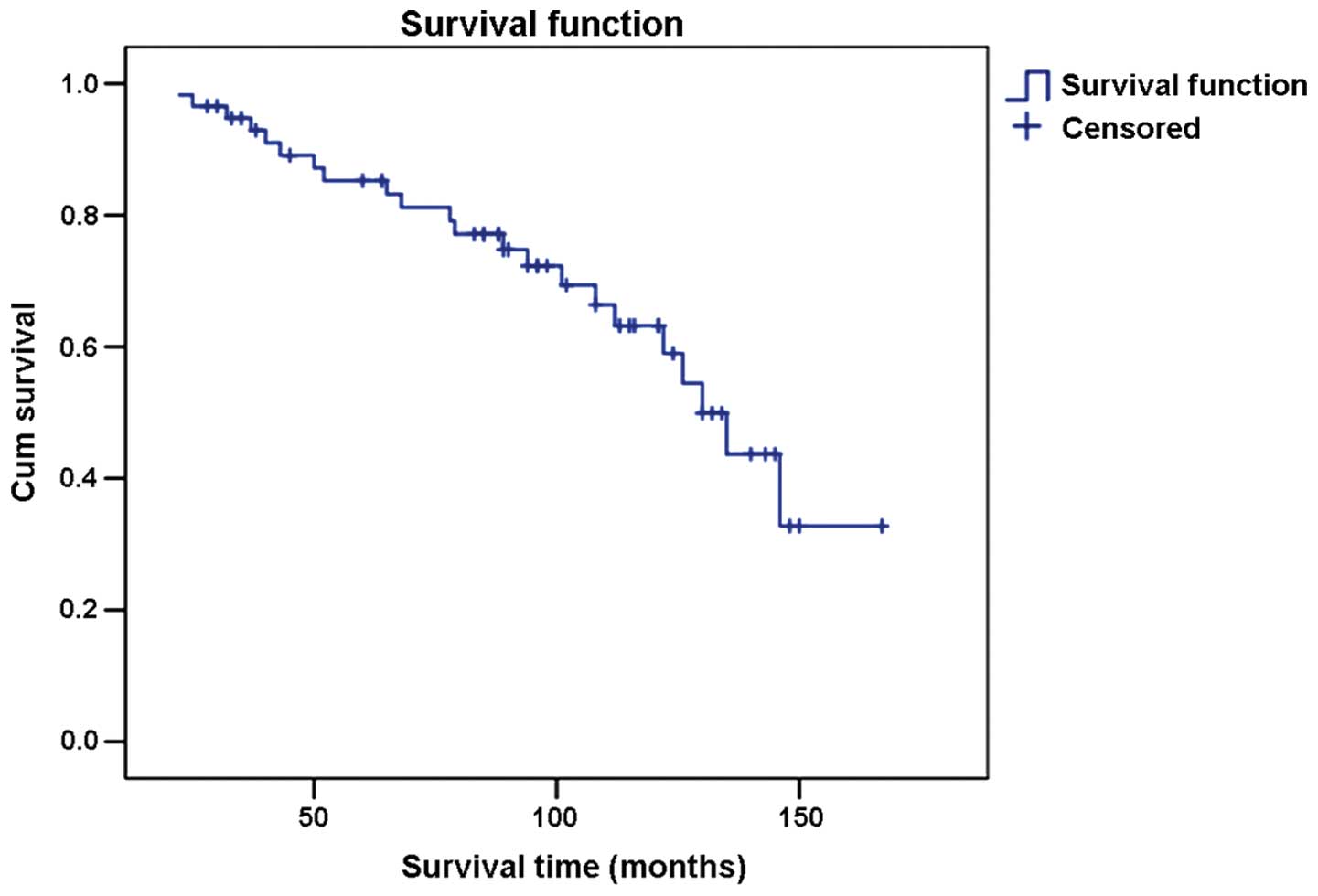
Results of the survival rate analyses revealed that
94.8% of the patients survived for 3 years after orchiectomy while
86.2% survived for 5 years and 70.7% for 10 years after orchiectomy
(Fig. 1).
Postoperative survival rate for different
pathological types
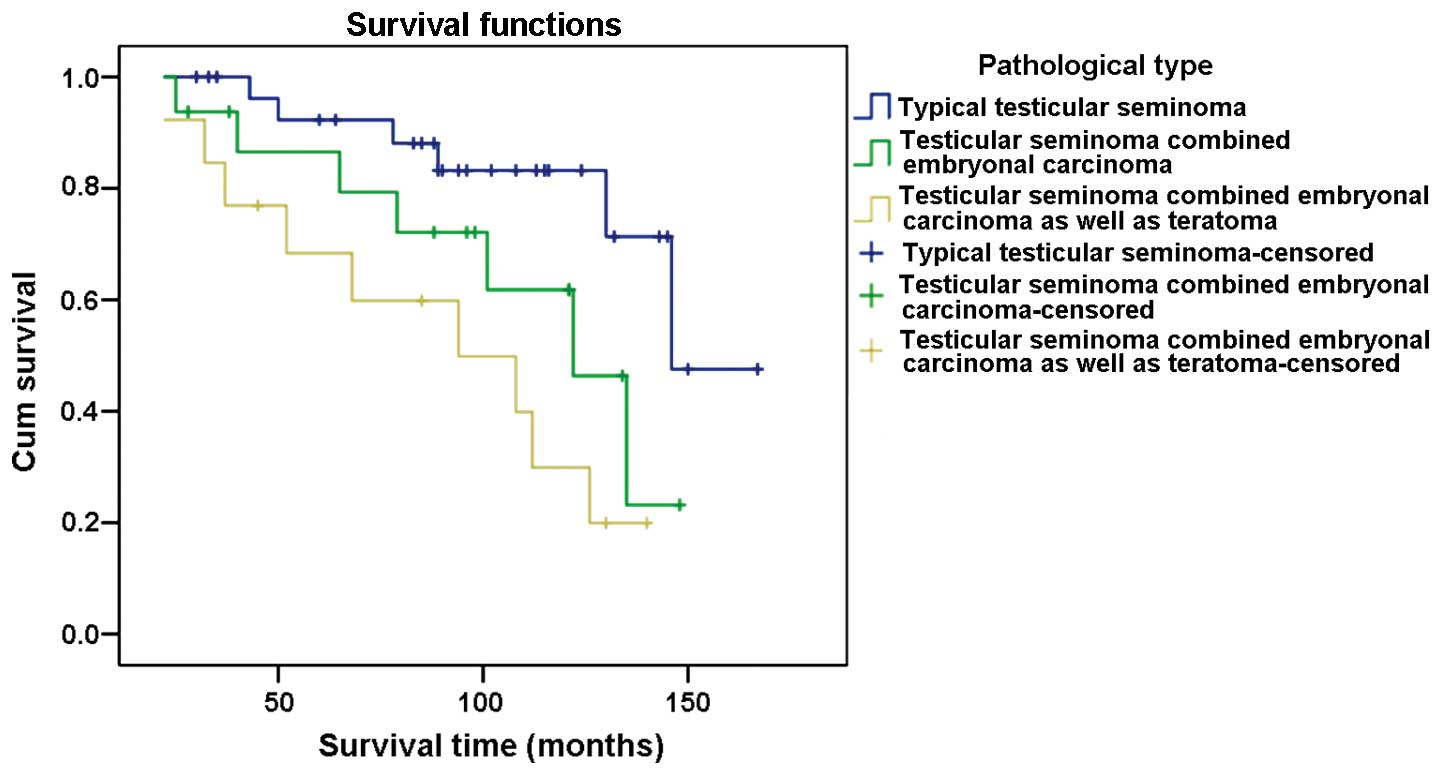
The 3-year survival rate for patients diagnosed with
typical testicular seminoma was 100%, 93.8% for cases with
testicular seminoma combined with embryonal carcinoma, and 84.6%
for those with testicular seminoma combined with embryonal
carcinoma and teratoma. The 5-year survival rate for patients
diagnosed with typical testicular seminoma was 93.1%, 87.5% for
cases with testicular seminoma combined with embryonal carcinoma,
and 69.2% for those with testicular seminoma combined with
embryonal carcinoma and teratoma. The 10-year survival rate for
patients diagnosed with typical testicular seminoma was 86.2%,
68.8% for cases with testicular seminoma combined with embryonal
carcinoma, and 38.5% for those with testicular seminoma combined
with embryonal carcinoma and teratoma (Fig. 2).
Postoperative survival rate of different clinical
stages
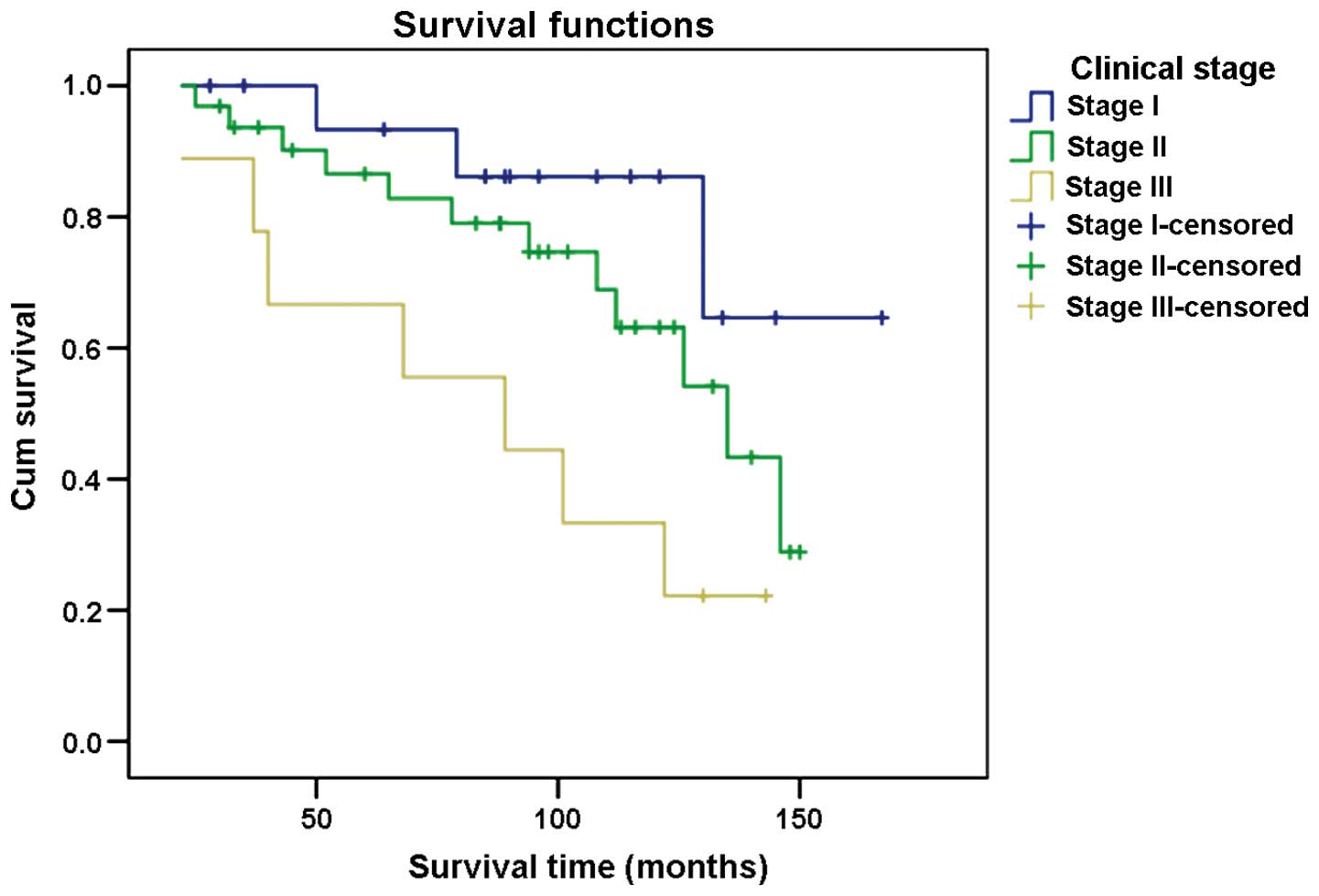
The 3-year survival rate for patients in different
clinical stages of cancer was: 100% for stage I, 93.8% for stage II
and 88.9% for stage III. The 5-year survival rate was 94.1, 87.5
and 66.7% for stages I, II and III, respectively. The 10-year
survival rate for stage I patients was 88.2% and for stages II and
III patients 71.9 and 33.3%, respectively (Fig. 3).
Survival rate for the different treatment
methods
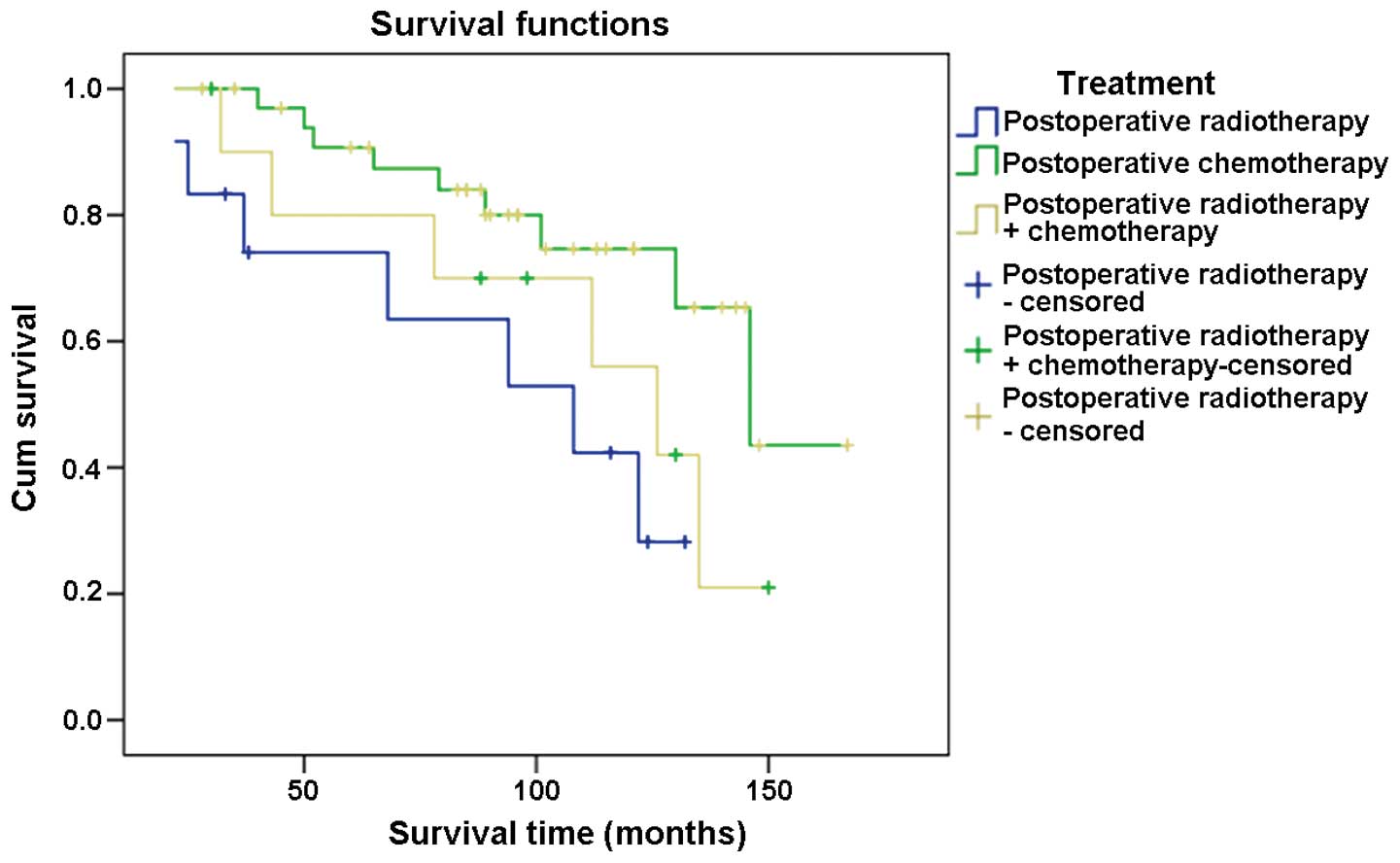
The 3-year survival rate for postoperative
radiotherapy, postoperative chemotherapy and postoperative
radiotherapy combined with chemotherapy was analyzed in the present
study. The results showed that all patients (100%) survived for 3
years following treatment with postoperative radiotherapy. For
patients who underwent postoperative chemotherapy an 83.3% 3-year
survival rate was observed. Ninety percent of our cases who were
treated with postoperative radiotherapy combined with chemotherapy
survived for 3 years following treatment.
The 5-year survival rate was analyzed for the
abovementioned set of treatments. The results showed that 91.4% of
cases survived for 5 years subsequent to their treatment by
postoperative radiotherapy. For patients who underwent
postoperative chemotherapy a 75.0% 5-year survival rate was
observed and 81.8% for those treated with postoperative
radiotherapy combined with chemotherapy (Fig. 4).
Results for the log-rank single factor
analysis
The log-rank single factor analysis results
indicated that the clinical stage, pathological type and
postoperative treatment methods were statistically significant with
regard to the prognosis of the patients with testicular seminoma
(P<0.05). Preoperative LDH and AFP levels also affected their
prognosis (P<0.05) (Table I).
Results of the multiple factor analysis
The results from the multiple factor analysis
indicated that the influence of clinical stage and postoperative
treatment methods was statistically significant on patient
prognosis (P<0.05).
Discussion
General
Testicular tumor is a relatively rare cancer, ranked
6th of the top common male tumors in the United States (2). Testicular seminoma is the most common
type of all testicular tumors. Most testicular seminoma are
identified in the early stages and patients have a good recovery
under the assistance of normative chemotherapy and radiotherapy
following orchiectomy. Therefore, testicular tumor is a typical
example of the treatable cancer types (3). The results of the present study showed
that the 3-, 5- and 10-year survival rates for testicular seminoma
patients were 94.8, 86.2 and 70.7%, respectively, indicating a
relatively high survival rate following orchiectomy.
Age characteristics of testicular
seminoma
The peak incidence of testicular tumor was primarily
evident in the 0–10, 20–40 and >60-year age groups. The
incidence prior to 18 years of age was relatively small. Previous
findings showed that patients aged 15–40 years had the highest
incidence of testicular germ cell tumors (4). As shown in Table II, none of the enrolled 58 patients
had seminoma prior to 20 years of age while the incidence for ages
30–50 was relatively high. The age distribution characteristic thus
may reflect the fact that testicular seminoma is associated with
sex hormones. Acquired characteristica (external factor of testis)
also play a major role in the occurrence of testicular seminoma
(5). Since the activation time of
these factors is relatively long, the onset of testicular seminoma
occurs at a late stage. Song and Huang (6) reported that of 55 testicular tumor
children included in their study, aged 2–12 years, there was only
one case with testicular seminoma, indicating that the onset of
testicular seminoma occurs at an advanced stage.
Characteristics of the side of
testicular seminoma
Current literature emphasizes that testicular germ
cell tumors often occur on the right side. However, recent
assessments suggested that there is no difference between the left
and right sides (6). In the present
study testicular germ cell tumors in 60.3% of patients (35 cases)
were on the right side while in 39.7% (23 cases) the tumors were on
the left side. The results showed that the incidence of right-side
tumors was higher than that of the left-side tumors. Nonetheless,
since there was a limited number of samples in the present study,
whether right-side tumors had a higher frequency than those on the
left side remains to be ascertained. A study with a larger sample
group is likely to yield more accurate epidemiological and
etiological results to determine and confirm whether the incidence
of testicular seminoma is associated the position of the tumor.
Association between clinical stage,
pathological types and the prognosis of testicular seminoma
Clinical stages of tumors were crucial in the
selection of therapy and in forming an accurate judgment concerning
prognosis. Shu et al (7)
performed a multiple factor analysis on 110 cases of testicular
germ cell tumors and suggested that the clinical stage of tumor was
a major factor affecting the prognosis of seminoma (7). In the present study, the 3-year survival
rate of clinical stages I, II and III was 100, 93.8 and 88.9%,
respectively; the 5-year survival rate was 94.1, 87.5 and 66.7%,
respectively; and the 10-year survival rate was 88.2, 71.9 and
33.3%, respectively. Differences on the 3-, 5- and 10-year survival
rates of the different stages were statistically significant
(P<0.05), which indicated that the survival rate was closely
associated with clinical stage. Therefore, early identification,
diagnosis and proper treatment are of great significance for the
prognosis of seminoma.
The prognosis of seminomas of different pathological
types varied. The 3-year survival rate of patients with typical
testicular seminoma, testicular seminoma combined embryonal
carcinoma, testicular seminoma combined embryonal carcinoma and
teratoma was 100, 93.8 and 84.6%, respectively; the 5-year survival
rate was 93.1, 87.5 and 69.2%, respectively; and the 10-year
survival rate was 86.2, 68.8 and 38.5%, respectively. The results
of the log-rank single factor analysis showed that differences on
the survival rate of different pathological types were
statistically significant (P<0.05).
Serum tumor markers
Currently, LDH, AFP, HCG are the most commonly used
serum tumor markers for identifying testicular germ cell tumors.
Patients (51%) with this type of cancer had increased levels of
these markers (7). LDH is a marker
for tissue destruction and its concentration is positively
associated with tumor size. A significant increase in the LDH level
is an indicative factor of a large tumor (7). On the other hand, LDH is widely
identified in various tissues and its specificity is low (7). Thus, a therapy regimen cannot be
determined solely on high LDH levels.
AFP and HCG are significant in determining the
characteristic of testicular mass prior to surgery. They are also
reliable in assessing the curative efficacy subsequent to surgery.
AFP of seminoma was usually within the normal limit. An increase in
AFP level indicates that seminoma contained mixed components, such
as embryonic carcinoma (7). Higher
AFP levels are associated with poorer prognosis. Nonetheless AFP is
not a specific tumor marker of testicular germ cell tumors. In
other malignant tumors, such as liver and gastric cancer an
increase in AFP levels has been observed (7). The increasing level of HCG is associated
with tumor size and prognosis. Almost 10–30% of seminoma cases
containing syncytiotrophoblast had high HCG levels (7). An increase in HCG indicates that poor
prognosis. However, the results of the single-factor analysis in
the present study indicated that HCG level variations did not
affect the prognosis of testicular seminoma (P=0.055) while LDH and
AFP levels were statistically significant with regard to prognosis
(P=0.022; P=0.029).
Postoperative treatment method and
prognosis
Radiotherapy has been the standard therapy for the
treatment of seminoma in stages I, IIA and IIB. Postoperative
adjuvant radiotherapy usually reduces the risk of local recurrence
(8). However, in recent years, the
importance of radiotherapy has been challenged. Zhang et al
(9) reported that systemic
chemotherapy was a safe and effective method for treating stage I
seminoma patients following radical orchiectomy. However, other
investigators have suggested radiotherapy increased the risk of
second primary tumors, and did not recommend use of routine
radiotherapy on patients in stage I (9).
Pure abdominal radiotherapy applied on testicular
seminoma in stages IIA and IIB may result in recurrence (10). Other studies revealed that in primary
treatment, chemotherapy or radiotherapy combined with chemotherapy
may produce improved curative effects than pure radiotherapy for
patients in stages IIA and IIB (10).
Patterson and associates (10)
performed chemotherapy for 4–6 weeks followed by radiotherapy on
their study group and reported that the 5-year survival rate
without recurrence of patients in stages IIA and IIB was
significantly higher than that of pure radiotherapy (11). In recent years, chemotherapy has been
widely used as the first choice of treatment for patients in stages
IIC and III of testicular seminoma, while chemotherapy combined
with local radiotherapy is the second choice. Results from a
previous study (12) revealed that
the 5-year survival rate of postoperative radiotherapy,
chemotherapy, and radiotherapy combined with chemotherapy was 100,
91.7 and 92.9%, respectively. Results from the current study showed
that the 5-year survival rate of the three classes of therapy was
91.4, 75.0 and 81.8%, respectively. Notably, the differences were
statistically significant.
We conclude that the 3-, 5- and 10-year survival
rate of patients with testicular seminoma following orchiectomy was
closely associated with the clinical stage, pathological type and
postoperative adjunctive therapy. For candidates of orchiectomy,
the clinical stage of the tumor must be assessed first and surgery
must be performed as early as possible. Subsequently, the
pathological types should be confirmed to determine the
postoperative adjunctive treatment method and improve the
postoperative survival rate.
References
|
1
|
Chung P, Daugaard G, Tyldesley S, Atenafu
EG and Panzarella T: Evaluation of a prognostic model for risk of
relapse in stage I seminoma surveillance. J Cancer Med. 4:155–160.
2015.(In Chinese). View
Article : Google Scholar
|
|
2
|
Travis LB, Beard C, Allan JM, Dahl AA,
Feldman DR, Oldenburg J, Daugaard G, Kelly JL, Dolan ME, Hannigan
R, et al: Testicular cancer survivorship: research strategies and
recommendations. J Natl Cancer Inst. 102:1114–1130. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Fu J, Lin H and Gong P: Clinical
pathology and diagnostic analysis on 15 cases of infantile
testicular germ cell tumors. Natl J Androl. 19:90–93. 2013.(In
Chinese).
|
|
4
|
Shanmugalingam T, Soultati A, Chowdhury S,
Rudman S and Van Hemelrijck M: Global incidence and outcome of
testicular cancer. Clin Epidemiol. 5:417–427. 2013.PubMed/NCBI
|
|
5
|
Zheng LW, Li FB, Liu RZ, Ji RG and Zhao
ZW: Clinical analysis of 87 cases of testicular tumor. Zhonghua Nan
Ke Xue. 11:445–447. 2005.(In Chinese). PubMed/NCBI
|
|
6
|
Song H and Huang C: Clinical analysis on
children testicular tumor (attached 55 case reports). Chin J Urol.
25:44–46. 2004.(In Chinese).
|
|
7
|
Shu B, Liu D, Huxila D, Du X and Chen P:
Multiple factor analysis on the prognosis of 110 cases with
testicular germ cell tumors. J Clin Urol. 26:528–531. 2011.(In
Chinese).
|
|
8
|
Ugwumba FO and Aghaji AE: Testicular
cancer: Management challenges in an African developing country. S
Afr Med J. 100:452–455. 2010.PubMed/NCBI
|
|
9
|
Zhang X, Liu Z, Zhou F, Han H, Qin Z and
Ye Y: A summary of 10-year experience in treating stage I
testicular seminoma. Cancer. 29:98–101. 2010.
|
|
10
|
Fucheng L: Clinical study on stage II
testicular seminoma. J Contemp Urol Reprod Oncol. 1:279–281.
2009.(In Chinese).
|
|
11
|
Mead GM, Fossa SD, Oliver RT, Joffe JK,
Huddart RA, Roberts JT, Pollock P, Gabe R and Stenning SP:
MRC/EORTC seminoma trial collaborators: Randomized trials in 2466
patients with stage I seminoma: patterns of relapse and follow-up.
J Natl Cancer Inst. 103:241–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang S and Wang C: Anlysis on the survival
rate after testicle partial excision for 1, 3, 5 years of patients
with testicular germ cell tumors. Chin J Hum Sex. 23:52–54.
2014.(In Chinese).
|