Introduction
Bone morphogenetic protein 9 (BMP9) also known as
growth differentiation factor 2, plays a key role in the promotion
of osteosarcoma growth (1,2). Findings of previous studies suggest that
the Notch signaling pathway plays a central role in mediating the
signal activity of BMP9 (3,4). The Hey1 gene significantly
increases during pluripotent stem cell differentiation induced by
BMP9 (5). The Hey1 gene is
highly conserved and the classical target gene of Notch signaling
pathway is involved in the regulation of cell proliferation,
differentiation and apoptosis (6).
Additionally, there is an interaction between the Notch signaling
pathway and the TGF-β/BMP signaling pathway (7). It has also been reported that the
abnormal Notch signaling pathway resulted in the abnormality of
cell proliferation and differentiation (8,9).
The Notch signaling pathway is a mechanism whereby
adjacent cells communicate with each other, conveying spatial
information and genetic instructions for cells (9). The abnormal Notch signaling pathway
occurred in various common types of human malignant tumors, such as
T-cell leukemia, lung cancer, colon cancer, prostatic cancer, and
breast cancer. Based on these findings, this study verified that
the mechanism of BMP9 promotes the growth of osteosarcoma mediated
by the Notch signaling pathway in vitro.
Materials and methods
Materials
Osteosarcoma cell lines 143B and MG63 were purchased
from the American Type Culture Collection (Manassas, VA, USA) and
saved by the Bone and Molecular Oncology Research Center of the
University of Chicago (Chicago, IL, USA). The adenovirus vector
BMP9 adenovirus (AdBMP9) was constructed and saved by the Bone and
Molecular Oncology Research Center of the University of Chicago.
Compound E (γ-secretase inhibitor XXI) blocker of the Notch
signaling pathway was purchased from Enzo Life Sciences, Inc.
(Farmingdale, NY, USA), dissolved in DMSO and kept in the freezer
at −80°C.
Experimental grouping
Control and experimental groups used in this study
were as follows: i) AdBMP9, ii) a recombinant adenovirus expressing
the dominant-negative mutant of Notch1 (AdR-dnNotch1), iii)
AdBMP9+AdR-dnNotch1 and iv) AdBMP9+compound E. These were used to
process the 143B and MG63 cell lines, and to observe how they
affect cell proliferation, migration and transformation of the cell
cycle.
Construction of AdR-dnNotch
Polymerase chain reaction (PCR) fragments containing
target genes were constructed. A nucleotide sequence for the Notch1
receptor gene was obtained from Genebank, and the Notch1 receptor
protein was analyzed using the Swiss-Prot database, to determine
the extracellular structure domain, transmembrane segment and
intracellular domain. The primers (5′-CCTGAGGGCTTCAAAGTGTC-3′,
5′-CGGAACTTCTTGGTCTCCAG-3′), which only amplified the fragments
containing the extracellular structure domain and transmembrane
segment of Notch1 receptor, were designed using Primer3 software
(PrimerPremier & Oligo, Whitehead Institute, Cambridge, CA,
USA). The coding regions containing the extracellular domain and
transmembrane segment of Notch1 receptor were used for high
fidelity PCR amplification. PCR products were purified and cloned
into plasmid vector pAdTrace-TOX4. The cloning steps were as
follows: PCR product and vector pAdTrace-TOX4 were digested with
corresponding restriction enzyme. Digested fragments were purified
and ligated with T4 ligase to the pAdTrace-TOX4 plasmid. Then, the
product was diluted to 200 µl with ddH2O and mixed with
700 µl ethanol, and centrifuged for 5 min at 13,000 × g and ethanol
precipitation was conducted. The precipitate was subsequently
dissolved in 30 µl dH2O. Subsequently,
electrotransformation was conducted followed by the selection of
positive clones and identification.
Detection methods
The cell proliferation ability was detected by
cresyl violet-stained (Beyotime, Shanghai, China) and quantitative
analysis. Cell migration ability was detected using the scratch
test. Cell cycle transition was tested using flow cytometry (BD-LSR
II, BD Biosciences, San Jose, CA, USA). The expression of receptors
and Notch ligands induced by BMP9 and the nucleocytoplasmic traffic
level of Notch intracellular domain (NICD) were examined using
RT-PCR and immunofluorescence staining.
Statistical analysis
SPSS 19.0 (SPSS, Inc., Chicago, IL, USA)was used for
the statistical analysis. Measurement data were presented by mean ±
standard deviation. ANOVA was used for comparisons between groups.
Counting data were presented as (%), and χ2 test was
used for comparisons among groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Ligand and Notch receptor
expression
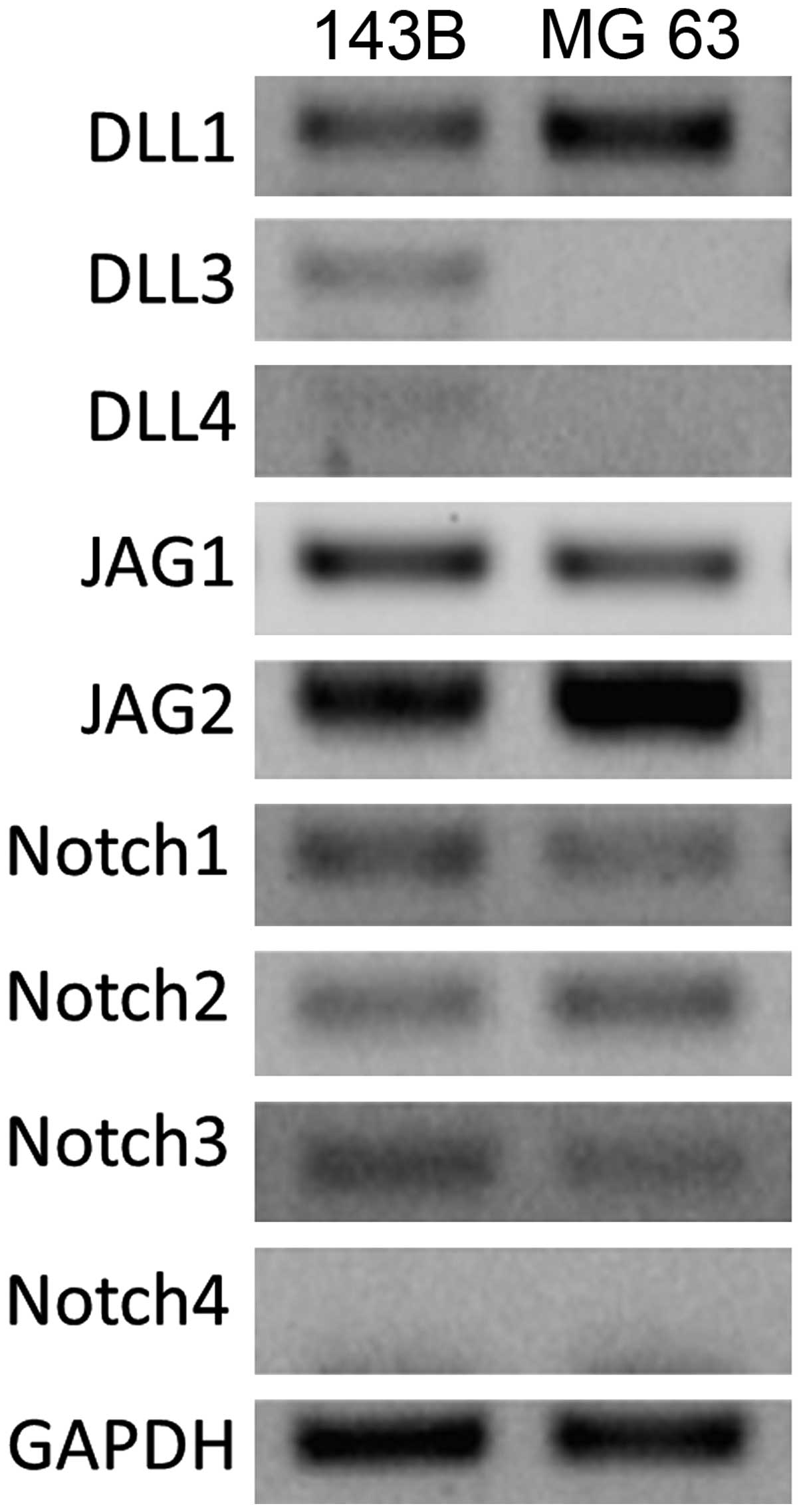
We detected high levels of expression for Notch
ligands DLL1, JAG1 and JAG2, as well as Notch receptors Notch1,
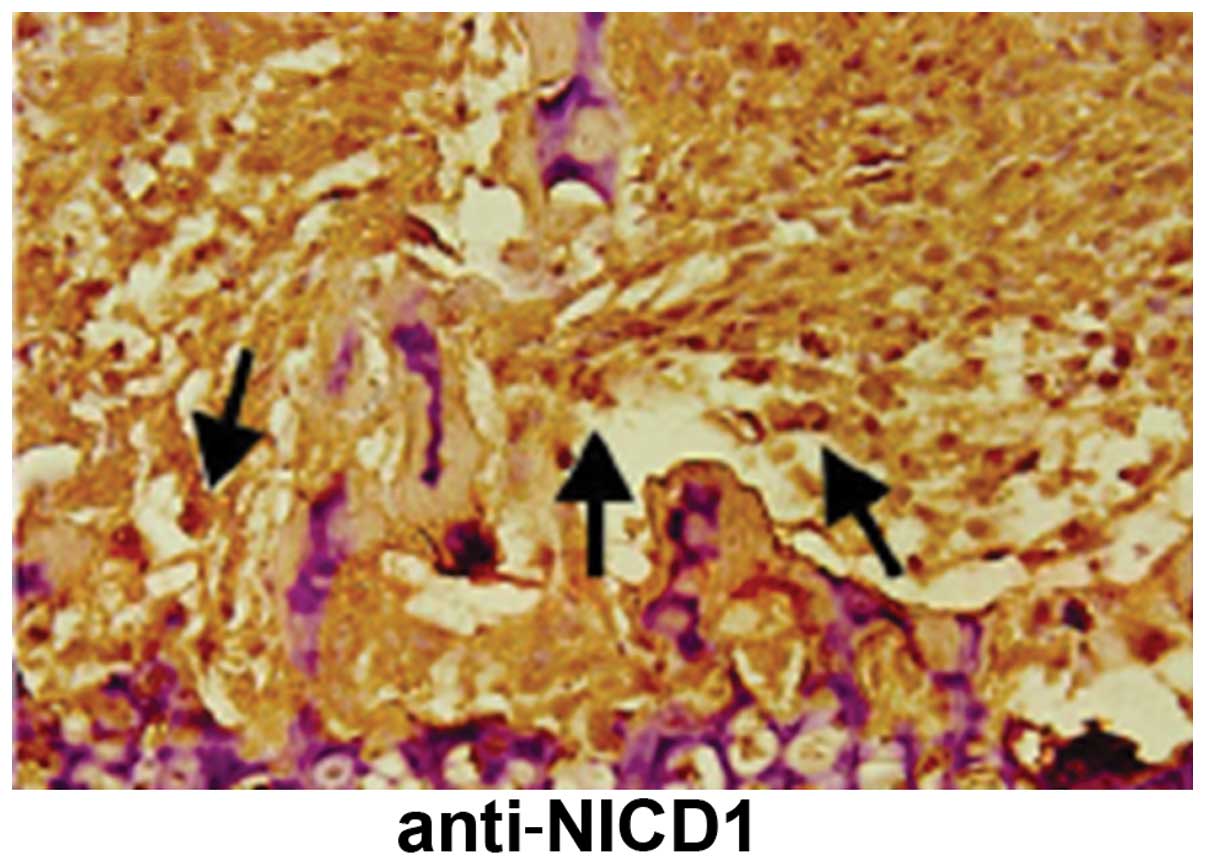
Notch2 and Notch3 in the two cell lines. NICD1, an intracellular
reactive molecule released by Notch, which was expressed in the
cells, appeared brown and uniformly distributed, suggesting a
positive expression of Notch1 receptors in situ in
osteosarcomas (Figs. 1 and 2).
Comparison of the ability of cell
proliferation and migration
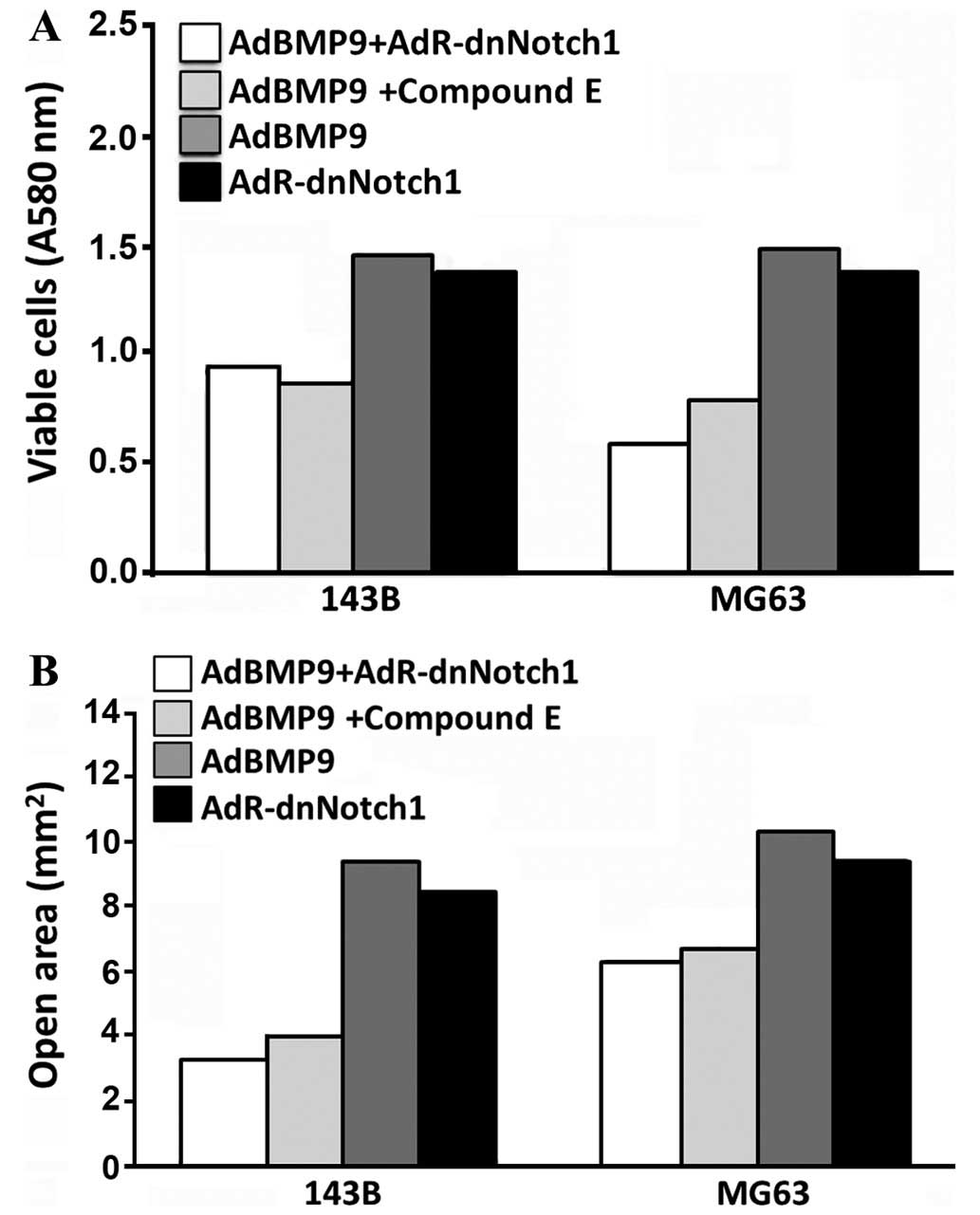
Cell proliferation ability and migration increased
in the AdBMP9 group and this increase was more prominent than that
in the AdBMP9+AdR-dnNotch1 and AdBMP9+compound E groups (Fig. 3). The differences were statistically
significant (P<0.05). However, the ability of cell proliferation
and migration in the AdR-dnNothc1 group was lower than that in the
AdBMP9 group. Differences had not statistically significant
(P>0.05).
Comparison of the cell cycle
ratio
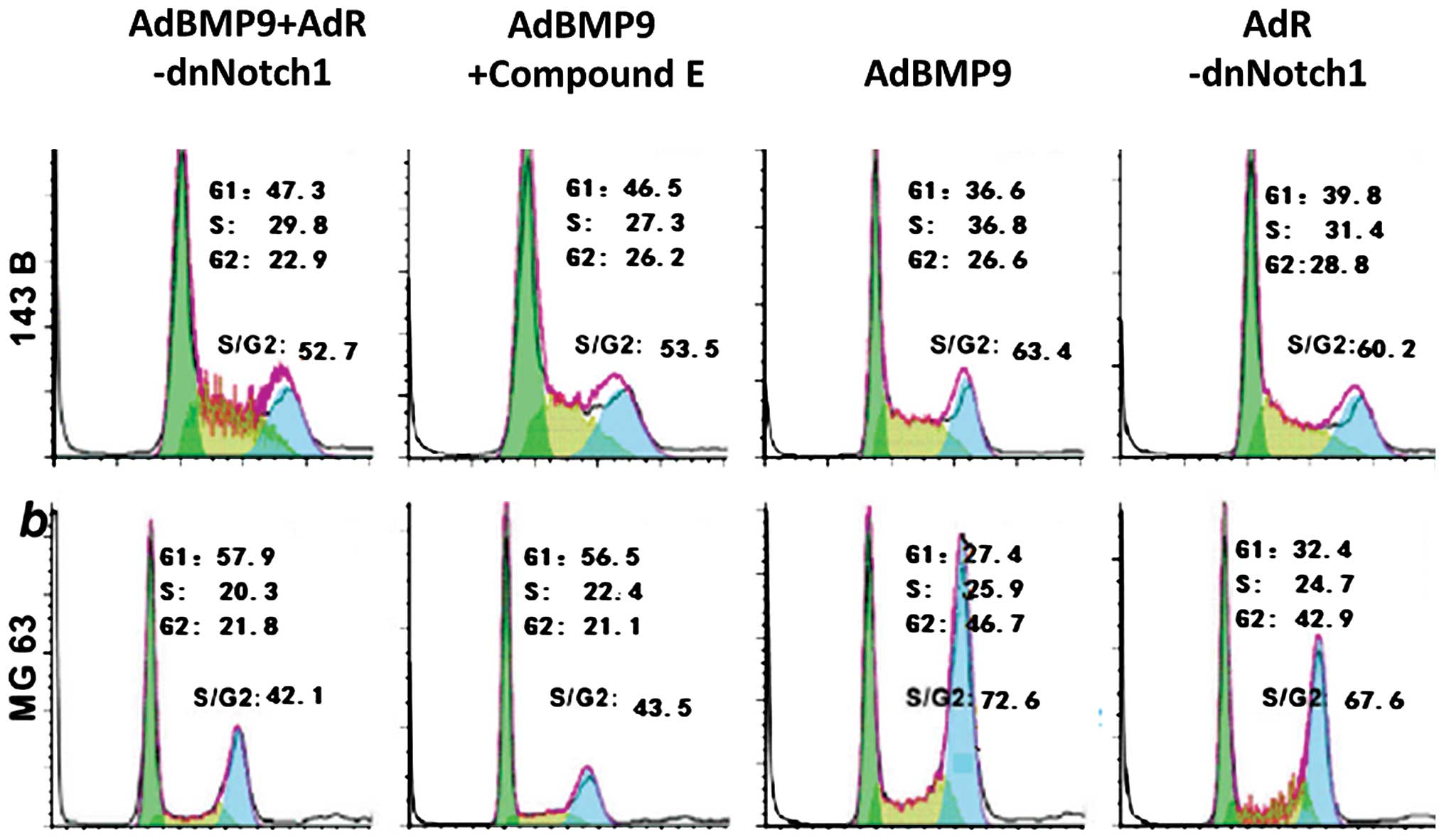
The cell cycle ratio in the S/G2 phase increased
considerably in the AdBMP9 group and the increase was more intense
than that in the AdBMP9+AdR-dnNotch1 and AdBMP9+compound E groups
(Fig. 4). The differences were
statistically significant (P<0.05). Nevertheless, the cell cycle
ratio in the S/G2 phase in the AdR-dnNotch1 group was lower than
that in the AdBMP9 group, although the differences were not
statistically significant (P>0.05).
Discussion
Notch signaling is an unusual signaling pathway,
whose activity does not depend on secondary messengers for
amplification. Instead, Notch signaling is modulated by
glycosylation, differential intracellular trafficking, and
ubiquitin-dependent degradation. The Notch signaling pathway
consists of various Notch receptors including Notch1, Notch2,
Notch3 and Notch4; Notch ligands including Jag1, Jag2, Dll1, Dll3
and Dll4; positive and negative regulators and transcription
factors (10). The majority of Notch
ligands are type I transmembrane proteins. Some signaling pathways
such as the transforming growth factor (TGF)-β/Smad signaling
pathway, mitogen-activated protein kinase (MAPK)-mediated signaling
pathway, Wnt signaling pathway and Notch signaling pathway are
involved in the regulation of the mechanism of osteogenesis and
osteosarcoma process in the bone marrow mesenchymal stem cells
(11,12). Previous studies have demonstrated that
early target gene regulation in BMP9 such as in Hey1 gene
was among the characteristic targets for the Notch pathway
(13). Knockout mice with
Notch gene showed severe skeletal abnormalities. The
Dll3 mutation caused spinal ribs hypoplasia. The
overexpression of Notch ligands and Notch receptors damaged the
differentiation of osteoblasts and osteoclasts of progenitor
cells.
The Notch receptors are composed of the
extracellular, transmembrane and intracellular domains. The
extracellular domain has several epidermal growth factor receptor
(EGFR) (EGF-like repeats) and three Lin/Notch repeats. The
intracellular domain of Notch, containing the RAM (recombination
binding protein-J associated molecular) structural domain, Notch
cytokine response region, six Ankyrin repeats (also known as
CDC10), and proline-glutamate-serine-threonine rich region, is
responsible for transferring extracellular signals to the nucleus
(14,15).
With the Notch ligands binding to Notch receptors,
the receptor conformation is modified and the S2 and S3 sites
within the extracellular domain are cut by a disintegrin and
metalloprotease 10 and γ-secretase (2). NICD is then released and transported
into the nucleus and interacts with transcription factors such as
CSL/RBPJk and the mastermind-like (MAML) protein, replacing the
co-repressor complex to activate CSL and collect co-activators,
such as MAML and p300, and subsequently initiating transcription
from the downstream target genes (2–4). The main
target genes for Notch are hairy/enhancer of split (HES) family,
and HES-relate repressor. Other factors involved in Notch
regulation include cell cycle regulatory factors, such as p21,
cyclin D1, c-Myc, nuclear factor-κB2 and factors regulating
apoptosis (16,17).
The Notch proteins were shown to fulfill various
functions in tumor development and the expression patterns for
Notch ligands and receptors were different in the same type of
tumors (18,19). The Notch signaling pathway is
dysregulated in many human malignancies (12). Notch1 is a typical cancer gene and
carcinogenesis may be achieved by interacting with other signaling
pathways, including the Ras/MAPK, TGF-β, vascular endothelial
growth factor, human epidermal growth factor 2 signaling pathways
(20). The results of the present
study show that the ability of cell proliferation and migration in
the AdBMP9 group markedly increased and this increase was more
intense compared to that observed in the AdBMP9+AdR-dnNotch1 and
AdBMP9+compound E groups. Nevertheless, the ability of cell
proliferation and migration in the AdR-dnNothc1 group was lower
than that in the AdBMP9 group, although the differences were not
statistically significant (P>0.05). The cell cycle ratio in the
S/G2 phase increased significantly in the AdBMP9 group and was
higher than that in the AdBMP9+AdR-dnNotch1 and AdBMP9+compound E
groups. Thus, the Notch signaling pathway potentially plays an
important role in mediating the growth of osteosarcoma promoted by
BMP9.
References
|
1
|
Tang N, Song WX, Luo J, Haydon RC and He
TC: Osteosarcoma development and stem cell differentiation. Clin
Orthop Relat Res. 466:2114–2130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharff KA, Song WX, Luo X, Tang N, Luo J,
Chen J, Bi Y, He BC, Huang J, Li X, et al: Hey1 basic
helix-loop-helix protein plays an important role in mediating
BMP9-induced osteogenic differentiation of mesenchymal progenitor
cells. J Biol Chem. 284:649–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andersson ER, Sandberg R and Lendahl U:
Notch signaling: Simplicity in design, versatility in function.
Development. 138:3593–3612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Louvi A and Artavanis-Tsakonas S: Notch
and disease: a growing field. Semin Cell Dev Biol. 23:473–480.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharff KA, Song WX and Luo X: Hey 1 basic
helix-loop-helix protein plays an important role in mediating
BMP9-induced differentiation of mesenchynal progenitor cells. J
Biol Chem. 284:649–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kadesch T: Notch signaling the demise of
elegant simplicity. Curr Opin Genet Dev. 14:506–512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walsh DW, Roxburgh SA and McGettigan P:
Co-regulation of Gremlin and Notch signaling in diabetic
nephropathy. Biochin Biophys Acta. 1782:10–21. 2008. View Article : Google Scholar
|
|
8
|
Tao J, Chen S and Lee B: Alteration of
Notch signaling in skeletal development and disease. Ann N Y Acad
Sci. 1192:257–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Penton AL, Leonard LD and Spinner NB:
Notch signaling in human development and disease. Semin Cell Dev
Biol. 23:450–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bolós V, Grego-Bessa J and de la Pompa JL:
Notch signaling in development and cancer. Endocr Rev. 28:339–363.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Engin F, Bertin T, Ma O, Jiang MM, Wang L,
Sutton RE, Donehower LA and Lee B: Notch signaling contributes to
the pathogenesis of human osteosarcomas. Hum Mol Genet.
18:1464–1470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanaka M, Setoguchi T, Hirotsu M, Gao H,
Sasaki H, Matsunoshita Y and Komiya S: Inhibition of Notch pathway
prevents osteosarcoma growth by cell cycle regulation. Br J Cancer.
100:1957–1965. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang P, Yang Y, Nolo R, Zweidler-McKay PA
and Hughes DP: Regulation of NOTCH signaling by reciprocal
inhibition of HES1 and Deltex 1 and its role in osteosarcoma
invasiveness. Oncogene. 29:2916–2926. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang P, Yang Y, Zweidler-McKay PA and
Hughes DP: Critical role of notch signaling in osteosarcoma
invasion and metastasis. Clin Cancer Res. 14:2962–2969. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li B: Bone morphogenetic protein-Smad
pathway as drug targets for osteoporosis and cancer therapy. Endocr
Metab Immune Disord Drug Targets. 8:208–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kubota T, Michigami T and Ozono K: Wnt
signaling in bone metabolism. J Bone Miner Metab. 27:265–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guruharsha KG, Kankel MW and
Artavanis-Tsakonas S: The Notch signalling system: recent insights
into the complexity of a conserved pathway. Nat Rev Genet.
13:654–666. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tien AC, Rajan A and Bellen HJ: A Notch
updated. J Cell Biol. 184:621–629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin L, Velazquez OC and Liu ZJ: Notch
signaling: Emerging molecular targets for cancer therapy. Biochem
Pharmacol. 80:690–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roti G, Carlton A, Ross KN, Markstein M,
Pajcini K, Su AH, Perrimon N, Pear WS, Kung AL, Blacklow SC, et al:
Complementary genomic screens identify SERCA as a therapeutic
target in NOTCH1 mutated cancer. Cancer Cell. 23:390–405. 2013.
View Article : Google Scholar : PubMed/NCBI
|