Introduction
The incidence of cutaneous melanoma continues to
rise in Canada. The estimated number of cases in Canada for 2014 is
6,500, accounting for ~3.5% of all cancer cases (1). The majority, up to 80% of cases, are
cured by surgery alone. Until recently, the prognosis in cases that
recurred has been poor, with a median overall survival (OS) time of
6 months in patients with metastatic disease. Molecularly targeted
agents and immune checkpoint inhibitors have significantly altered
this dismal prognosis (2).
Ipilimumab, an anti-cytotoxic
T-lymphocyte-associated protein 4 (CTLA-4) antibody was the first
agent to demonstrate a survival benefit in the treatment of
metastatic cutaneous melanoma, initially as a second-line and later
as a first-line treatment (3,4). Toxicities or adverse events reported in
these pivotal trials were immune-related adverse events (irAE), in
keeping with the drugs mechanism of action. A subsequent
meta-analysis of mature data from phase II and phase III trials, in
addition to >2,000 patients treated on the international
expanded access programme (EAP), has indicated a median OS time of
9.5 months, with durable responses beyond 3 years in 21% of
patients, the majority of whom were treated outside a clinical
trial population (5). Awareness and
management of irAE have also improved since ipilimumab approval,
and, whilst these side effects are manageable in the majority of
patients, they may result in significant long-term morbidity and
even, in rare cases, mortality (3,4). The
majority of irAE relate to cutaneous, hepatic or intestinal
inflammation or endocrinopathies. However rarer neurological
toxicity has also been documented in case reports (6). Newer checkpoint inhibitors targeting the
programmed cell death 1 (PD-1)/programmed death-ligand 1 (PD-L1)
axis are being evaluated in phase III trials, alone or in
combination with ipilimumab. These agents appear to have greater
activity and different patterns of immune toxicities (7,8).
Appreciation of potential irAE, when they may occur and their
management will be crucial to treating patients as these agents
become standard therapy, moving out of cancer centres and into
community practice. Checkpoint inhibitors are additionally being
evaluated as adjuvant treatments and early data has demonstrated a
delay in disease recurrence. If these agents are approved in the
adjuvant setting, a significantly higher number of patients will be
eligible for treatment with these agents (9), as is the case now with the recent
approval of adjuvant ipilimumab.
The Princess Margaret Cancer Centre (Toronto,
Canada) has the largest single centre experience of ipilimumab use
in metastatic melanoma in Canada. The aim of the present study was
to evaluate firstly the toxicity and secondly the outcomes of all
patients treated at this institution from 2008 to date.
Additionally, we sought to compare our practice with published
literature and, drawing additionally on our own experience, to
evaluate guidelines for the early detection and management of
ipilimumab-related toxicity in the Canadian context.
Patients and methods
Patients
This retrospective review was conducted at the
Princess Margaret cancer centre using a research ethics
board-approved protocol and in accordance with the Declaration of
Helsinki. Pharmacy records were searched to identify patients who
had received ipilimumab between 2008 and 2013, inclusively. These
patients records were evaluated to collect data on gender, age,
ECOG performance status, tumour burden, previous treatments,
mutation status of primary tumours, number of ipilimumab infusions,
response (by CT scan) at the end of treatment, toxicity (assessed
by CTCAE, version 3) incurred during and following treatment, time
to toxicity from the date of the first ipilimumab infusion, and
survival outcomes, allowing calculation of progression free
survival (PFS) and OS.
Statistical analysis
Associations between patient characteristics and
toxicity) or survival were tested using univariate and then
multivariate analysis (Chi square analysis and log rank test),
using SAS version 9.2 (SAS, Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient profiles
A total of 129 patients with metastatic cutaneous
melanoma were identified [for the purposes of this study, patients
with uveal (n=5), mucosal and acral melanomas (n=24) were
excluded]. All patients received ipilimumab as a second-line or
higher treatment. All patients received 3 mg/kg infusions every 3
weeks up to a planned 4 cycles. In addition, 7 patients also
received re-induction therapy, where a further 4 doses of
ipilimumab were administered after previous clinical benefit from
this treatment was demonstrated. Patient characteristics are shown
in Table I, and all patients received
≥1 ipilimumab infusion. The median and mean numbers of infusions
received were 4 and 3, respectively. Of the 129 patients, 9 had
M1a, 11 had M1b and 109 had M1c disease. Examination of sites of
metastases revealed that 33 patients (25.58%) had bone metastases,
88 (68.82%) had lung secondaries and 46 (35.66%) had liver disease.
In 112 patients (86.82%), metastases were detected at other sites,
including nodes, pancreas, spleen, adrenals or soft tissue masses.
Brain metastases were present in 31 patients prior to commencing
ipilimumab therapy, of which 13 subsequently progressed in the
brain and required further radiotherapy. New brain metastases
during or following ipilimumab treatment were detected in 18
patients.
 | Table I.Patient characteristics (n=129). |
Table I.
Patient characteristics (n=129).
| Characteristic | Value |
|---|
| Age, years |
|
|
Median | 57 |
|
Range | 24–83 |
| Gender, n |
|
|
Female | 48 |
| Male | 81 |
| Performance status,
n |
|
| 0 | 33 |
| 1 | 80 |
|
Unknown | 16 |
| LDH (at baseline;
U/l), n |
|
| ≤220 | 42 |
|
>220 | 83 |
|
Unknown |
4 |
| AJCC M stage, n |
|
| M1a |
9 |
| M1b | 11 |
| M1c | 109 |
| Ipilimumab - line of
treatment, n |
|
| Second
line | 80 |
| Third
line | 36 |
| Fourth
line |
7 |
| ≥Fifth
line |
6 |
| Treatments prior to
ipilimumab, n |
|
|
Chemotherapy | 105 |
| Targeted
agent/BRAF inhibitor | 29 |
|
Pembrolizumab (anti-PD1
antibody) |
7 |
| High dose
interleukin-2/other immunotherapy | 10 |
| Trial
(experimental agent) | 28 |
| BRAF mutation
statusa, n |
|
|
Positive | 33 |
|
Negative | 68 |
| NRAS mutation
statusa, n |
|
|
Positive | 13 |
|
Negative | 10 |
| KIT mutation
statusa, n |
|
|
Positive |
0 |
|
Negative |
5 |
Follow-up treatments
The majority of patients (n=91; 70.5%) did not
receive any active treatment following ipilimumab. Of those that
were subsequently treated, 25 (19.4%) received one further line of
treatment, 9 (7%) received two and 4 (3.1%) received more than two
further lines of treatment. These treatments included chemotherapy,
BRAF inhibitor or other targeted agent, re-induction with
ipilimumab, another immunotherapy (including anti-PD-1 antibodies),
adoptive cell transfer of lymphocytes with interleukin 2, or
treatment on a clinical trial with an experimental targeted therapy
or chemotherapy agent. Notably, 7 patients received anti-PD-1
antibody followed by ipilimumab treatment (of which 5 had
progressive disease, 1 had stable disease and 1 had a partial
response), whilst 10 patients received ipilimumab followed by
anti-PD-1 antibody (of which 6 had progressive disease, 2 stable
disease and 2 a partial response).
Toxicities
Treatment related toxicities included thyroid
dysfunction, diarrhoea and autoimmune colitis, hepatitis,
hypophysitis, rash or other autoimmune toxicities. All toxicities
are described in Table II, in
addition to the median time to onset, treatment thereof and outcome
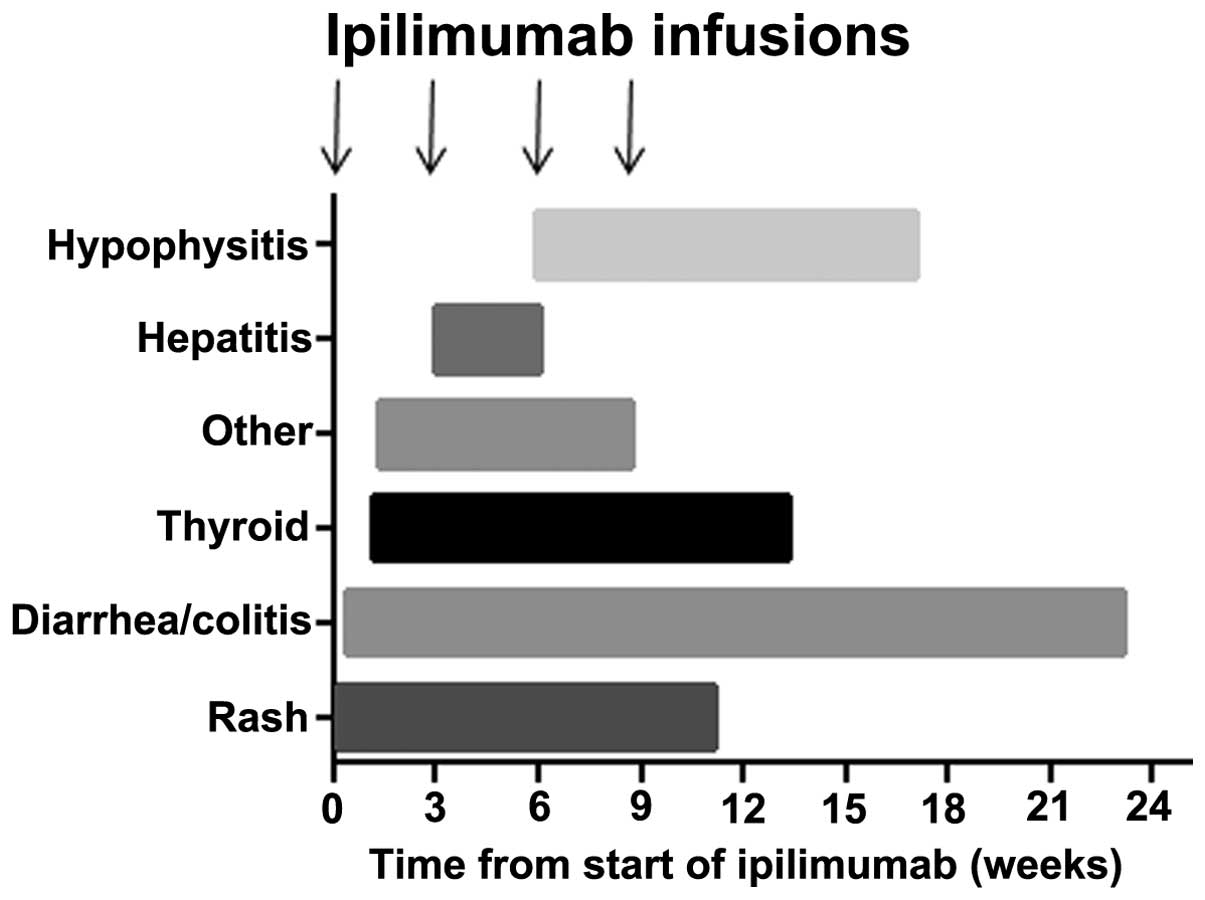
from treatment. Fig. 1 shows the
onset of toxicity in the current sample of patients relative to the
commencement of ipilimumab treatment. In 79 patients (61.2%), no
toxicities were experienced during or following treatment, whilst
27 patients (20.9%) experienced one toxicity, 20 patients (15.5%)
experienced two, and 3 patients (2%) experienced three toxicities.
The majority of toxicities resolved, with the exception of
endocrinopathies (hypophysitis or thyroid dysfunction) and bowel
toxicity, which required long-term treatment or, in the case of
autoimmune colitis, surgery in two patients. Colitis and
endocrinopathies were observed to occur even after treatment had
been completed, in contrast to other toxicities which occurred
within the 12-week period of treatment and resolved within 6 weeks
of the final dose of ipilimumab. Glucocorticoids were used in a
total of 34 (26%) patients to treat diarrhoea, hepatitis, rash,
hypophysitis, arthritis, myositis or suspected pneumonitis. The
median time to steroid treatment was 6.6 weeks (range, 2.7–27.3
weeks). Infliximab was required in 7 patients for refractory
diarrhoea despite steroids; 2 of these patients required repeated
infusions, 1 of whom subsequently underwent a resection with
ileostomy formation. The median time to infliximab treatment was
7.6 weeks (range, 4.7–34.7 weeks). Univariate analysis indicated
bone metastases (P=0.0373) to be associated with toxicity, whilst
NRAS mutation appeared to be associated with reduced toxicity
(P=0.024). Notably, the number of ipilimumab infusions received and
the number of previous treatments were not significantly associated
with toxicity. No factors were independently significantly
associated with toxicity on multivariate analysis.
 | Table II.Toxicities during ipilimumab
treatment, management thereof and outcome in 129 patients. |
Table II.
Toxicities during ipilimumab
treatment, management thereof and outcome in 129 patients.
| Toxicity | Patients, n (%) | Time to irAE onset,
days; median (range) | Treatment | Resolution of
toxicity |
|---|
| Diarrhoea | 34 (26.4) | 36 (3–162) | Steroids used for
grade ≥2 in 19 patients; inflixamab in 7 of these; Sur gery
required in 2 patients [total of 8 patients (6%)with severe
colitis] | Responded to
treatment apart from in 1 patient who required ileostomy; 1 patient
had a colectomy but failed to present beforehand with symptoms to
allow medical treatment |
| Rash | 18 (14.0) | 20 (1–78) | Prednisone required
in 7 patients for grade ≥2 rash | Resolved completely
post ipilimumab treatment |
| Thyroid
dysfunction | 8
(6.2) | 33 (8–93) | Thyroxine required in
5 patients | Thyroxine continued
post ipilimumab in all cases |
| Hypophysitis | 6
(4.7) | 63 (41–119) | Steroid replacement
in all 6 patients | Steroid replacement
needed long.term post ipilimumab treatment |
| Hepatitis | 4 (3.1) | 35 (21–42) | Prednisone used in 2
patients for grade 3 hepatitis | Completely
resolved |
| Other toxicity | 6
(4.7) | 41 (9–61) | Encephalopathy,
prednisone given, resolved within 24 h episcleritis, no treatment;
myositis, treated with prednisone; arthritis, treated with
prednisone; pulmonary inflammation, treated with prednisone. | All toxicities
completely resolved within 6 weeks from the last ipilimumab
treatment |
Response to treatment and survival
outcomes
Response was determined by CT scan 4 weeks after the
fourth cycle of ipilimumab. If stable disease or a response was
determined then a confirmatory scan was performed 4 weeks
thereafter, and patients followed up every three months with scans
unless the clinical picture dictated otherwise. Of the 129 treated
patients, 90 (70%) had progressive disease and 37 (28%) had either
a partial response (n=21, one of whom subsequently achieved a
complete response) or stable disease (n=16). In 2 patients who were
lost to follow-up, the response was unknown. Only 1 patient with
progressive disease at the end of treatment went on to have a
delayed response, which occurred 6 months later, and 1 patient with
stable disease exhibited disease progression at a solitary site,
which was resected with no further evidence of disease progression
to date. Univariate analysis indicated that the factors positively
associated with a response were the number of infusions received (4
infusions was superior to ≤3 P=0.0027), better baseline performance
status (0 vs. 1, P=0.0247), NRAS mutation (P=0.0022) and
development of any toxicity (P=0.0299). By contrast, patients with
bone metastases were less likely to respond to treatment
(P=0.0166). No significant effect was associated with line of
treatment, BRAF mutation, gender or age. Multivariate analysis
revealed <4 infusions (P=0.003) and male gender were associated
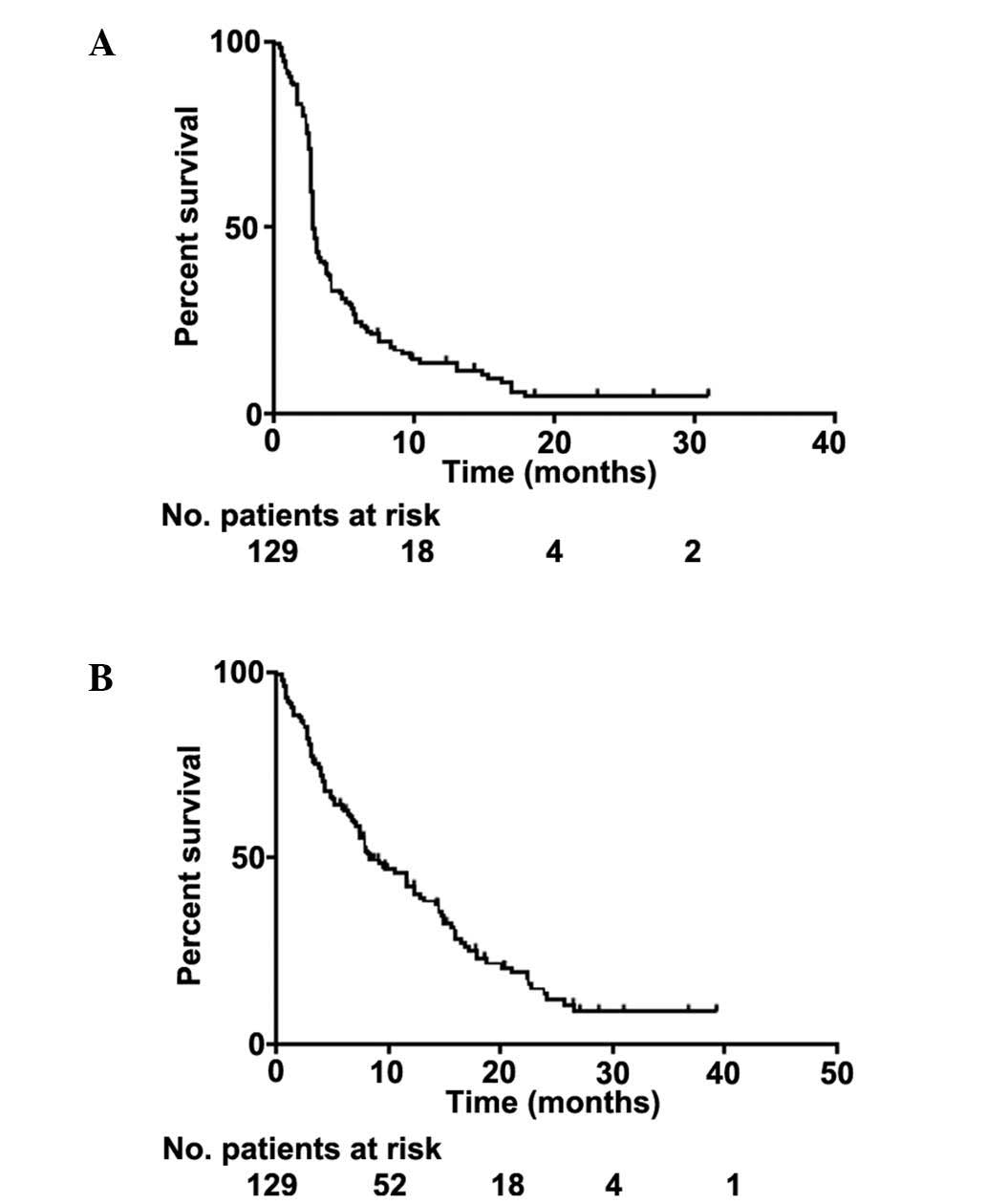
with progressive disease (P=0.0484). The median follow-up time was
8 months (range, 1–39 months). At the time of analysis, 101
patients were deceased and 28 were alive. The median PFS time was
2.83 months [95% confidence interval (CI), 2.76–3.32 months] and
the median OS time was 8.44 months (95% CI, 7.13–12.42 months)
(Fig. 2). The 1- and 2-year overall
survival rates were 42% and 13%, respectively, whilst survival for
≥3 years was observed in 8.6% of patients.
Factors associated with superior PFS by univariate
analysis, were low lactate dehydrogenase (LDH) level (<220 u/l;
P<0.0001), number of infusions (4 vs. ≤3, P=0.0002), toxicity
during treatment (P=0.0061) and female gender (P=0.0407). Patients
with liver (P=0.0006) or bone metastases (P=0.0092) had poorer PFS.
Similar characteristics were significantly associated with superior
OS: Low LDH (P<0.001), number of infusions (P<0.0001),
NRAS mutation (P=0.0241) and female gender (P=0.046). Bone
metastases and liver metastases were associated with poorer OS
(P=0.0004 and P=0.0015, respectively). Multivariate analysis
indicated that <4 infusions (P<0.0001 and P<0.0001) and
absence of toxicity (P=0.0002 and P=0.033) were prognostic factors
for poor PFS and OS, respectively; the presence of liver metastases
was a significant factor for inferior OS only (P=0.0048).
Discussion
The current study reports the real-world efficacy
and toxicity of the novel anti-CTLA-4 antibody ipilimumab in
patients treated primarily outside of clinical trials. The
toxicities reported in the current sample of patients were irAE, in
accordance with the published data on ipilimumab toxicity (6). These were, as expected, primarily
related to dermatological, endocrine or gastrointestinal
complications. In addition, a number of rarer toxicities, also
assumed to be irAE, were observed (Table
II).
A previous comprehensive review of 14 phase II–III
ipilimumab trials in metastatic melanoma determined the most
frequent grade ≥3 toxicities, in a pooled analysis of 1,498
patients, to be diarrhoea (7% of patients), colitis (4.9%),
perforation (0.3%), rash (2.5%), any liver dysfunction (2.1%),
hypothyroidism (0.1%) and hypopituitarism (2.1%) (6). Gastrointestinal toxicity of any grade
occurred in 33% of patients, and 3 succumbed to complications
associated with grade 3 or above gastrointestinal toxicity.
Dermatological toxicity accounted for the majority of irAEs, with
65% of patients reporting some grade of rash; however, <3% had
grade ≥3. Hepatic toxicity was more rare, occurring in <2% of
patients, and with 1% experiencing grade 3–4. The endocrinopathies
reported were related to thyroid dysfunction,
hypopituitarism/hypophysitis or adrenal insufficiency, with an
overall incidence of <5% and a grade ≥3 incidence of <3%
across all phase II–III trials. The time to onset of toxicity
varied according to the irAE; rash was the earliest toxicity,
followed by diarrhoea or colitis, then hepatic toxicity, and
finally hypophysitis. The majority of toxicities appeared and
resolved within the 12-week period (4 cycles) of ipilimumab
treatment, with the exception of hypophysitis and liver toxicity,
which in some cases could take longer to resolve or, in the case of
hypophysitis, fail to resolve completely (6). In contrast to the reviewed data, the
present study demonstrates that all of these toxicities have a
wider range of times to onset and may occur much earlier in the
course of treatment (Table II and
Fig. 1). One major difference to the
pooled data review described was the onset of diarrhoea; this
appeared to commence much later in some of the current patients and
extended well beyond the period of ipilimumab treatment.
There are no validated biomarkers for the prediction
of toxicity, response or outcome to ipilimumab. The current data
set is small and thus any associations are to be treated with
caution. Nevertheless, others have reported that response is
associated with NRAS mutation or autoimmune toxicity
(10,11). High LDH level is known to be a poor
prognostic factor in metastatic melanoma, and has been suggested as
a selection criterion for ipilimumab treatment (12). It most likely reflects overall disease
status in addition to performance status. The survival outcomes in
the present study are similar to those reported by other groups,
reflecting the poorer clinical status of patients treated outside
trials and particularly in the SAP and EAP, where ipilimumab may
have been used as a higher line of therapy (11,12).
The management of irAE is dependent upon vigilance
in patient monitoring and early detection. If detected early,
toxicities may be prevented from escalating, particularly in the
case of gastrointestinal, hepatic and dermatological irAE. A high
index of suspicion is required for the early detection of
endocrinopathies, as early symptoms are often non-specific. It is
unclear whether early immunosuppressive therapy can abrogate or
mitigate the extent of endocrinopathies once these begin. It is
essential that patients are made aware of the potential side
effects and the need to communicate any symptoms to their clinical
team. The average onset of different toxicities may guide
physicians; however, given the variability between patients in the
time to onset of toxicities (of any kind), this cannot be relied
upon. Extensive guidelines for the management of ipilimumab-related
toxicity are available as part of the Food and Drug Administration
(FDA)-approved YERVOY® (ipilimumab) Risk Evaluation and Mitigation
Strategy (REMS) (https://www.hcp.yervoy.com/pages/rems.aspx). These
have been expanded further for greater guidance (6). The REMS also includes a checklist of
symptoms and actions for healthcare workers to assess patients in
community clinics or emergency departments, and a patient wallet
card explaining potential irAEs and reminding patients to seek
urgent medical attention for specific irAEs. These are useful
adjuncts, particularly for general medical staff or emergency
departments where many patients will present. Our current practice
mandates a patient teaching session as to potential irAEs prior to
commencing ipilimumab. Patients are seen prior to every cycle of
treatment and every three months thereafter (depending on the
course of treatment), undergoing full thyroid function tests
(triiodothyronine, thyroxine and thyroid-stimulating hormone) and
random cortisol and adrenocorticotropic hormone (and testosterone)
evaluations, in addition to routine biochemical and haematological
blood tests.
Pembrolizumab, the anti-PD-1 antibody inhibitor, was
granted FDA approval in September 2014. In contrast to ipilimumab,
newer checkpoint inhibitors that target the PD-1/PD-L1 axis have a
different toxicity profile. Gastrointestinal toxicity and
hypophysitis are less common, whilst pneumonitis occurs in ~1% and
may be treatment limiting (8). The
kinetics of toxicity with these agents has not been fully defined
and, unlike ipilimumab where treatment consists of 4 cycles only,
these agents may be continued for up to 2 years. Our experience
with anti-PD-1 agents indicates that thyroid dysfunction is common
and early, detectable within the first 6 weeks. It tends firstly to
hyperthyroidism, which is rarely symptomatic, and then to
euthyroidism or hypothyroidism, requiring treatment. All other
toxicities appear to occur at any time during treatment and, in
certain cases, a year after the initiation of treatment
(unpublished data).
In conclusion, there is little doubt that antibodies
like ipilimumab that act as immune checkpoint inhibitors have
significantly altered treatment paradigms in metastatic melanoma
(2). It is likely that greater
toxicity will be reported as these agents are more widely used;
however, certain irAEs, particularly neurological toxicities, will
be rare and idiosyncratic. Careful observation and early
intervention via a multidisciplinary approach will therefore be
required to optimally manage these patients. In addition the use of
checkpoint inhibitors is likely to expand to other tumour types,
and it will be necessary for non-oncology physicians to be familiar
with their potential toxicities and their management.
Acknowledgements
Dr Leila Khoja was supported by grants from the
Canadian Institutes of Health Research and the Guiletti Family
Fellowship fund. Dr Craig Gedye was supported by a Canadian
Institutes of Health Research/Kidney Cancer Canada Fellowship, a
Royal Australasian College of Physicians CSL Fellowship, is
supported by a National Health and Medical Research Council
Overseas Postdoctoral Fellowship, and is a Hunter Cancer Research
Alliance Clinical Research Fellow.
References
|
1
|
Canadian Cancer Society: Canadian Cancer
Statistics. http://www.cancer.ca/en/cancer-information/cancer-101/canadian-cancer-statistics-publication/?region=skAccessed.
August 01–2014
|
|
2
|
Gedye C, Hogg D, Butler M and Joshua AM:
New treatments for metastatic melanoma. CMAJ. 186:754–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hodi FS, ODay SJ, McDermott DF, et al:
Improved survival with ipilimumab in patients with metastatic
melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robert C, Thomas L, Bondarenko I, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schadendorf D, Robert C, Weber JS, et al:
Pooled analysis of long-term survival data from phase II and phase
III trials of ipilimumab in metastatic or locally advanced,
unresectable melanoma. J Clin Oncol. 33:1889–1894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tarhini A: Immune-mediated adverse events
associated with ipilimumab ctla-4 blockade therapy: The underlying
mechanisms and clinical management. Scientifica (Cairo).
2013:8575192013.PubMed/NCBI
|
|
7
|
Topalian SL, Sznol M, McDermott DF, et al:
Survival, durable tumor remission, and long-term safety in patients
with advanced melanoma receiving nivolumab. J Clin Oncol.
32:1020–1030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robert C, Ribas A, Wolchok JD, et al:
Anti-programmed-death-receptor-1 treatment with pembrolizumab in
ipilimumab-refractory advanced melanoma: A randomised
dose-comparison cohort of a phase 1 trial. Lancet. 384:1109–1117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eggermont AM, Chiarion-Sileni V, Grob JJ,
et al: Ipilimumab versus placebo after complete resection of stage
III melanoma: Initial efficacy and safety results from the EORTC
18071 phase III trial. ASCO Meeting Abstracts. 32:LBA90082014.
|
|
10
|
Johnson DB, Lovly CM, Flavin M, et al:
NRAS mutation: A potential biomarker of clinical response to
immune-based therapies in metastatic melanoma (MM). ASCO Meeting
Abstracts. 31:90192013.
|
|
11
|
Zimmerman ZF, Storer B, Godara A, et al:
Outcomes and clinical markers associated with benefit from
ipilimumab (Ipi) in patients with advanced melanoma: A
retrospective single-institution study. ASCO Meeting Abstracts.
31:e200482013.
|
|
12
|
Kelderman S, Heemskerk B, van Tinteren H,
et al: Lactate dehydrogenase as a selection criterion for
ipilimumab treatment in metastatic melanoma. Cancer Immunol
Immunother. 63:449–458. 2014.PubMed/NCBI
|