Introduction
Uterine cervical cancer represents the second most
common type of cancer, in terms of incidence and mortality rates
(15.2 and 7.8 cases per 100,000 individuals, respectively), in
women worldwide, with an increased incidence (17.8 cases per
100,000 individuals) in low resource countries (1). Almost all cases of cervical cancer are
caused by persistent infections with high-risk-human papilloma
virus (HR-HPV), which may cause cervical intraepithelial neoplasia
(CIN) and squamous cell carcinoma (SCC) (2).
Cervical biopsy, which is used in conjunction with
Papanicolaou cytology testing, HPV DNA testing and colposcopy, has
an important role in the evaluation and management of women with
cervical dysplastic lesions. Thus, cervical biopsy is crucial for
the prevention and early detection of cervical cancer (3). However, misinterpretation of
histological changes may occur for various reasons, such as the
presence of atrophy, immature metaplasia, transitional metaplasia,
reparative or inflammatory atypia, inadequate sample size and
tissue artifacts. These factors may cause inter-observer variation
and poor intra-observer reproducibility (4–6).
A number of biomarkers have been evaluated for their
potential to improve the diagnostic consistency and accuracy of
cervical biopsy interpretation (4–6). Many of
these studies (7–9) and other such studies (10,11)
endorse the use of p16 and Ki-67 immunostains, and more recently
the ProExC immunostain (11–13), as useful adjunct techniques to confirm
a diagnosis of high-grade squamous intraepithelial lesion (HSIL)
and to distinguish it from its mimics. p16 is a cyclin-dependent
kinase inhibitor and a surrogate marker for HPV E7-mediated
degradation of retinoblastoma protein (pRb) (14,15). There
is evidence to suggest that a negative feedback mechanism
controlling p16 levels in normal cells is disrupted by a reduction
of pRb activity in proliferating squamous epithelial cells
expressing HR-HPV E7 (16). With
regard to p16, it has been demonstrated that almost 100% of cases
of HSIL and SCC associated with HR-HPV express high levels of p16,
whereas non-dysplastic cervical epithelium or low-grade SIL (LSIL)
associated with low-risk (LR) or negative HPV types do not express
p16 (17). Ki-67 is a nuclear protein
and is detected by mindbomb E3 ubiquitin protein ligase-1 (MIB-1),
a monoclonal antibody that is associated with RNA transcription and
cell cycle progression (18). To
improve diagnostic accuracy, other markers, including Ki-67, have
been used in conjunction with p16 in the histological assessment of
SIL and SCC of the uterine cervix (5,19). Similar
to p16, Ki-67 is overexpressed in HSIL and SCC (20).
Considering the aforementioned knowledge, the
present study aimed to investigate the efficacy of p16 and Ki-67
immunohistochemical stains in the pathological assessment of
uterine cervical biopsy samples, and identify any viral and
histopathological correlations.
Materials and methods
Materials
The following terms were used to search in the
pathology data program of Chosun University Hospital, (Gwangju,
Republic of Korea) for the final reports of patients who had
undergone HPV DNA testing of cervical biopsy specimens obtained
between January 2012 and March 2013: ‘Squamous metaplasia’, ‘LSIL’
and ‘HSIL’. According to the 2014 WHO classification of tumors of
the female genital tract (21), a
total of 103 specimens were identified with the following
pathological diagnoses: Squamous metaplasia, 1 case; LSIL with HPV,
30 cases; LSIL with CIN 1, 22 cases; HSIL with CIN 2, 21 cases; and
HSIL with CIN 3, 29 cases. By reviewing the medical records and
pathological reports of the selected patients, details of the
patients' age, underlying disease, other tumor history,
cervico-vaginal cytological diagnosis and HPV infection status were
identified. The present study was approved by the Institutional
Review Board (IRB) of the Chosun University Hospital (Gwangju,
Korea) (CHOSUN 2014-03-002). IRB approval included a waiver of
informed consent.
HPV DNA testing and genotyping of HPV DNA for HR-HPV
were achieved by polymerase chain reaction-based HPV genotyping
assay using a HPV 9G DNA chip (Fammed Co., Ltd., Seongnam, Republic
of Korea), according to the manufacturer's instructions. The DNA
chip was able to detect 28 HPV genotypes, including 14 HR-HPVs (16,
18, 31, 33, 39, 45, 51, 52, 54, 56, 58, 59, 66 and 68) and 7
LR-HPVs (6, 11, 34, 40, 42, 43 and 44). The HPV genotypes were
classified as HR-HPV or LR-HPV according to the scheme proposed by
Dunne et al (22).
Immunohistochemical analysis
Prior to immunohistochemical analysis, 3-µm thick
serial sections were prepared from representative formalin-fixed,
paraffin-embedded tissue blocks and mounted on positively charged
glass slides. Immunostaining was performed using a BenchMark XT
autostainer (Ventana Medical Systems, Tucson, AZ, USA). The primary
antibodies were anti-human p16 (mouse monoclonal antibody, clone
E6H4; cat no. 725–4713; 1:100 dilution; Ventana Medical Systems)
and Ki-67 (mouse monoclonal antibody, clone MM1; cat no. ACK02;
1:50 dilution; Leica Biosystems, Ltd., Newcastle, UK). Briefly,
antigen retrieval was performed using peroxidase and alkaline
phosphatase blocking reagent (Dako Korea LLC, Seoul, Republic of
Korea) for 10 min at 95–99°C in a water bath. After inactivation of
endogenous peroxidases, the slides were incubated for 30 min at
room temperature with primary antibodies or negative control
antibody. The slides were then incubated with polyclonal anti-mouse
secondary antibody (cat. no. E0433; 1:250 dilution) for 30 min at
room temperature. Primary antibodies and alkaline phosphatase
enzyme complexes (cat. no. D0651; Dako Korea LLC) were incubated
for 30 min at room temperature, then visualized following
incubation with 3,3′-diaminobenzidine (Dako Korea LLC) for 20 min
at room temperature. Next, the slides were counterstained with
Mayer's hematoxylin (Merck Millipore, Darmstadt, Germany) for 5 min
at room temperature. The slides were visualized under a microscope
(BX50; Olympus Corporation, Tokyo, Japan).
Immunohistochemical
interpretation
p16 staining was interpreted as positive when
nuclear or nuclear and cytoplasmic, strong and diffuse block
staining beginning from the basal cell layer of the epithelium was
observed. By contrast, non-specific focal or patch nuclear staining
was considered to indicate negative p16 staining, with cytoplasmic
only, wispy, blob-like, puddled, scattered, single cells and a
complete lack of staining also defined as negative p16 expression
(23). The expression of Ki-67 was
categorized into four groups based on the distribution and
proportions of cells with positive nuclear staining, as follows:
Score 0, <10% of the cells, restricted to the parabasal cell
layers; score 1, 10–29% of the cells, restricted to the lower third
of the epithelium; score 2, 30–69% of the cells, reaching the upper
third of the epithelium; score 3, ≥70% epithelial cells, including
full thickness expression of Ki-67 (24).
Histological evaluation
Hematoxylin and eosin staining was performed
according to standard protocols (25). Two independent observers evaluated the
hematoxylin and eosin (H&E)-stained slides, and then determined
initial diagnoses based on the histological features according to
the 2014 WHO Classification (21).
The whole slides were reviewed independently by two observers who
were blinded to all clinicopathological information. These
diagnoses included benign lesions, such as squamous metaplasia or
LSIL, and precancerous lesions, such as HSIL. The observers used a
two-tier system of terminology, according to The Lower Anogenital
Squamous Terminology Standardization Project for HPV-Associated
Lesions (23). Then, each observer
evaluated the H&E slides a second time, and analyzed p16 and
Ki-67 staining. The final diagnosis was determined by revising all
diagnoses against each other and by a final comparison of all
diagnoses by a third observer. The two pathologists' diagnoses were
concordant with the diagnosis of the third pathologist in all 103
cases. Finally, consensus diagnoses were reached.
Statistical analysis
SPSS software (version 22.0; SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. The χ2 test
was performed between the final diagnoses of p16 and Ki-67 status.
These values were then compared with the following HPV infection
statuses: HR-HPV, LR-HPV and HPV-negative. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of p16 and Ki-67
HR-HPV group
The HPV genotyping assay detected HR-HPV genotypes
in 68.93% (71/103) of cases. Of the 28 cases of benign lesion (NILM
and LSIL), 27 cases were p16 negative, with only 1 case of LSIL
exhibiting p16 positivity. Excluding 1 case of negative p16
expression, almost all of the 43 cases of HSIL exhibited positive
expression for p16 (Table I).
 | Table I.Comparison of p16 result and final
diagnosis according to HPV infection status. |
Table I.
Comparison of p16 result and final
diagnosis according to HPV infection status.
|
| Final diagnosis, n
(%) |
|
|---|
|
|
|
|
|---|
| HPV status | p16 | HSIL | LSIL | NILM | P-value |
|---|
| HR (n=71) | + | 42 (59.1) | 1 (1.4) | 0 (0.0) | <0.001 |
|
| − | 1 (1.4) | 26 (36.6) | 1 (1.4) |
|
| LR (n=6) | + | 1 (16.6) | 0 (0.0) | 0 (0.0) | 0.014 |
|
| − | 0 (0.0) | 5 (83.3) | 0 (0.0) |
|
| N (n=26) | + | 5 (19.2) | 0 (0.0) | 0 (0.0) | 0.001 |
|
| − | 3 (11.5) | 16 (61.5) | 2 (7.7) |
|
| Total (n=103) | + | 48 (46.6) | 1 (0.9) | 0 (0.0) |
|
|
| − | 4 (3.9) | 47 (45.6) | 3 (2.9) |
|
There were 17 cases HR-HPV with Ki-67 negativity,
including 16 cases of LSIL and 1 case of NILM. All Ki-67 staining
scores of 3 in the HR-HPV group were HSIL. Twelve cases showed
score of 1 in Ki-67 staining that of 3 cases of HSIL and 9 cases of
LSIL. A Ki-67 score of 2 was observed in 16 cases, including 14
cases of HSIL and 2 cases of LSIL (Table
II). In HR-HPV patients, p16 positivity (Table I) and Ki-67 score (Table II) were found to correlate with the
severity of dysplasia (both P<0.001).
 | Table II.Comparison of Ki-67 result and final
diagnosis according to HPV infection status. |
Table II.
Comparison of Ki-67 result and final
diagnosis according to HPV infection status.
|
|
| Final diagnosis, n
(%) |
|---|
|
|
|
|
|
|---|
| HPV status | Ki-67 score | HSIL | LSIL | NILM | P-value |
|---|
| HR (n=71) | 0 | 0 (0.0) | 16 (22.5) | 1 (1.4) | <0.001 |
|
| 1 | 3 (4.2) | 9 (12.6) | 0 (0.0) |
|
|
| 2 | 14 (19.7) | 2 (2.8) | 0 (0.0) |
|
|
| 3 | 26 (36.6) | 0 (0.0) | 0 (0.0) |
|
| LR (n=6) | 0 | 0 (0.0) | 2 (33.3) | 0 (0.0) | <0.001 |
|
| 1 | 1 (16.6) | 1 (16.6) | 0 (0.0) |
|
|
| 2 | 0 (0.0) | 2 (33.3) | 0 (0.0) |
|
|
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| N (n=26) | 0 | 0 (0.0) | 10 (38.4) | 2 (7.7) | <0.001 |
|
| 1 | 0 (0.0) | 2 (7.7) | 0 (0.0) |
|
|
| 2 | 7 (26.9) | 1 (3.8) | 0 (0.0) |
|
|
| 3 | 1 (3.8) | 1 (3.8) | 0 (0.0) |
|
| Total (n=103) | 0 | 0 (0.0) | 28 (27.2) | 3 (2.9) |
|
|
| 1 | 4 (3.9) | 14 (13.6) | 0 (0.0) |
|
|
| 2 | 21 (20.3) | 5 (4.8) | 0 (0.0) |
|
|
| 3 | 27 (26.2) | 1 (0.9) | 0 (0.0) |
|
LR-HPV group
The HPV genotyping assay detected 5.82% (6/103)
cases with LR-HPV genotypes. In the 5 cases of LSIL, all were p16
negative. Furthermore, there was only 1 case of HSIL with p16
positivity (Table I) and 2/5 cases
(33.3%) of LSIL exhibited a Ki-67 score of 0. In addition, two and
one cases of LSIL had Ki-67 scores of 2 and 1, respectively. There
was 1 case of HSIL with a Ki-67 score of 1 (Table II). Furthermore, in the LR-HPV group,
p16 positivity (Table I) and Ki-67
score (Table II) were significantly
associated with malignant potential (P=0.014 and P<0.001,
respectively).
HPV-negative group
The HPV genotyping assay detected 25.24% (26/103)
cases with negative HPV genotypes. All 18 cases of benign lesion
(NILM and LSIL) were p16 negative. Excluding 3 cases, 5/8 cases
(19.2%) of HSIL showed positive expression for p16 (Table I). A Ki-67 score of 0 was exhibited by
12/18 cases of benign lesion, including of 2 cases of NILM and 10
cases of LSIL. Furthermore, 4/16 cases of LSIL showed a Ki-67
expression score of 1, while only 1/16 cases of LSIL exhibited a
Ki-67 expression score of 2 and 3. There were 8 cases of HSIL, with
a score of 3 in 1 case and a score of 2 in 7 cases (Table II). In the HPV-negative group, p16
positivity (Table I) and Ki-67 score
(Table II) were significantly
associated with the degree of dysplasia (P=0.001 and P<0.001,
respectively).
Discordance between p16 and Ki-67 expression, and
the HPV genotype
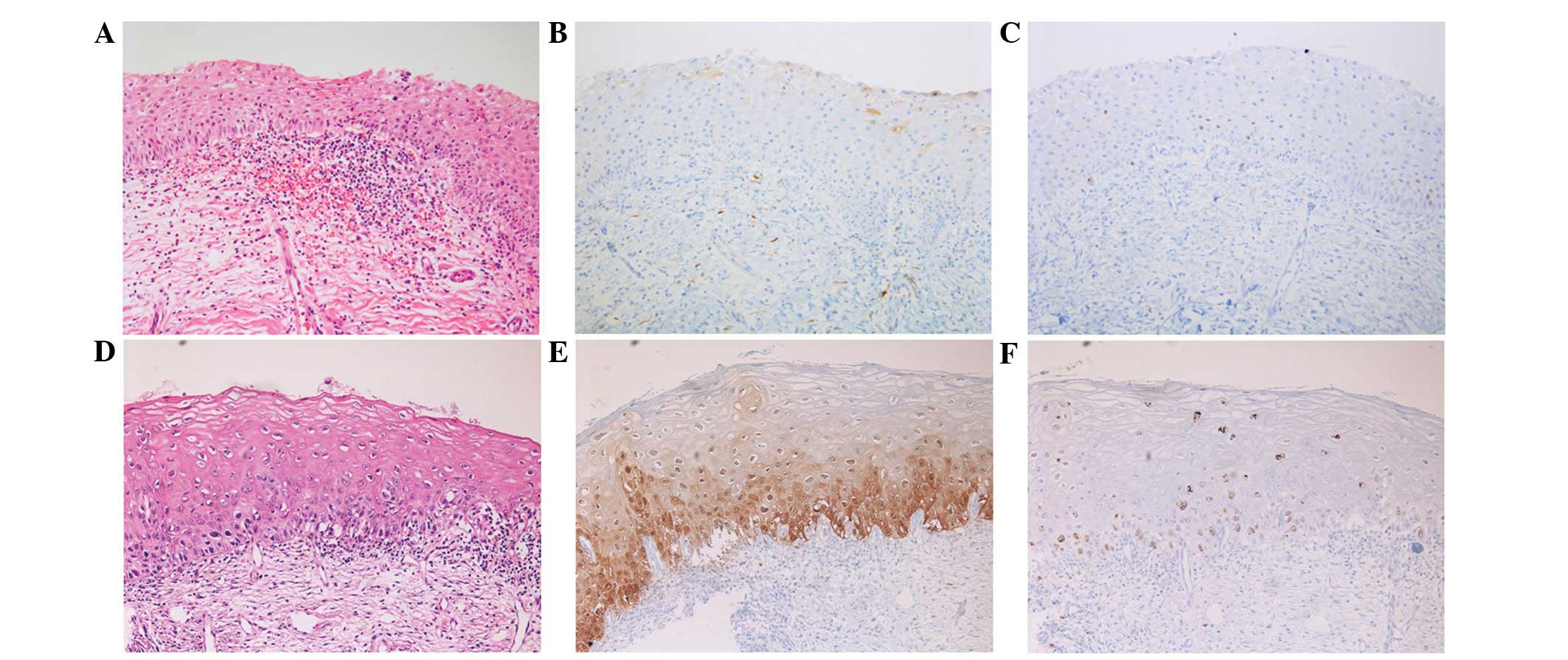
Analysis of the H&E slides only revealed 2 cases
of immature squamous metaplasia with an initial diagnosis of LSIL
(n=1) and HSIL (n=1). These cases exhibited immunohistochemical
negativity for p16 and a low Ki-67 index (Fig. 1). However, discordance was identified
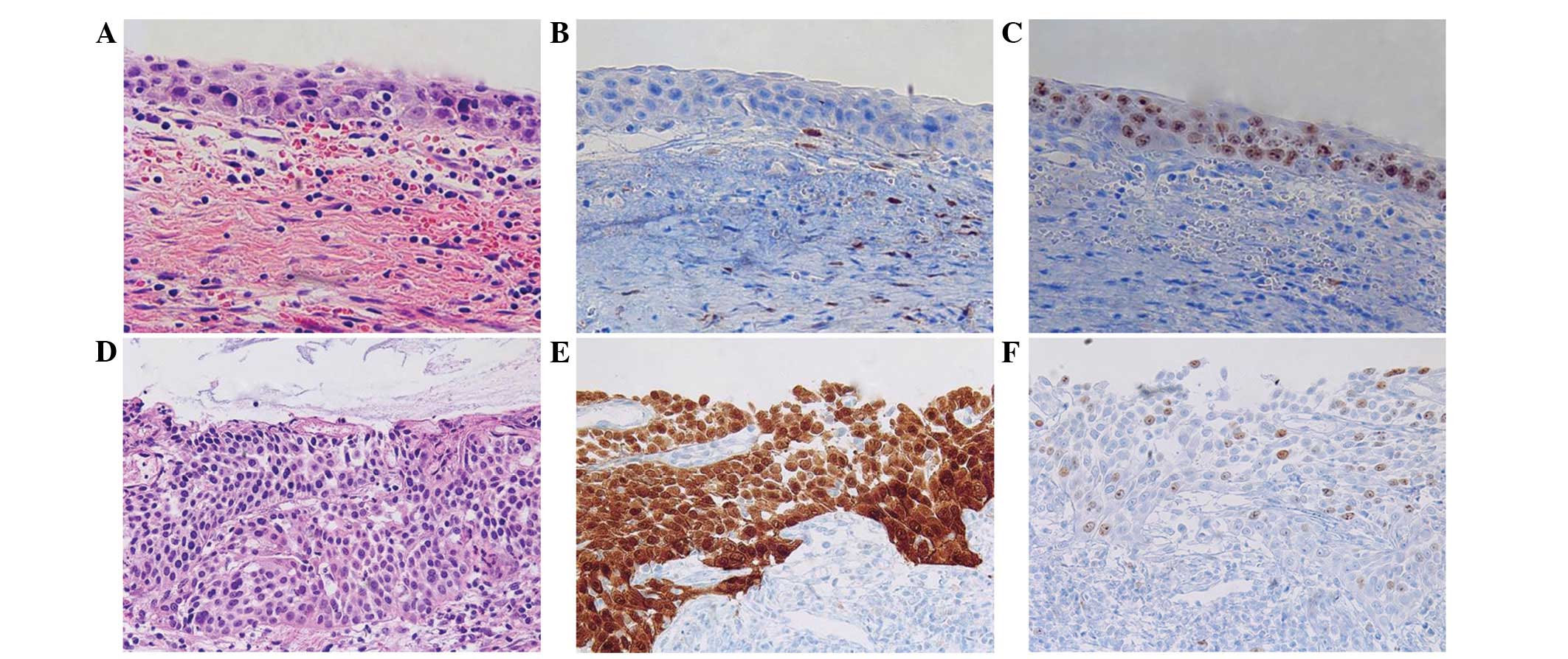
in the HPV-negative group (Table I).
In the HPV-negative group, 3/26 cases exhibited completely negative
expression for p16; thus, the discordance rate was 11.54%. However,
these cases were consistent with a pathological diagnosis of HSIL,
according to the H&E slides and the high Ki-67 index (score, 2)
(Fig. 2A–C). Therefore, these cases
were finally diagnosed based on histological findings and the Ki-67
result (Table III). In the LR-HPV
and HR-HPV groups, expression of p16 and Ki-67 exhibited a strong
correlation with malignant potential of the lesion (Fig. 2D–F). Only one case of HSIL diagnosis
was negative in p16 result and one case of LSIL was positive for
p16 positive in the total HR-HPV group (n=71), thus, the discordant
rate was 2.82% (Table I).
 | Table III.Cases of discordance between HPV N
group and result of HPV genotyping. |
Table III.
Cases of discordance between HPV N
group and result of HPV genotyping.
| Case | Initial
diagnosis | Final
diagnosis | p16 | Ki-67 score | HPV viral Pap | Genotype |
|---|
| 1 | HSIL | HSIL | − | 2 | N | N |
| 2 | HSIL | HSIL | − | 2 | N | N |
| 3 | HSIL | HSIL | − | 2 | N | N |
Discussion
Diagnostic interpretation of dysplasia in the
uterine cervix typically includes analysis of hypercellularity,
significant atypia, mitotic figures and disorientation from the
parabasal to upper layers (21).
However, unusual histological features, including mildly increased
cellularity, the absence of mitotic figures and questionable
atypia, may be observed in the lesion (9). In daily practice, the accurate diagnosis
of cervical dysplastic lesions is subject to inter-observer and
intra-observer variability by these factors (6).
Immunostaining may improve the diagnostic
reproducibility and accuracy of the CIN lesion. Previous studies
have demonstrated that p16 and Ki-67 are co-expressed in almost
100% of cases of high-grade squamous and glandular lesions, and
these markers are rarely co-expressed in normal or benign lesions
of cervical epithelial lesion (14,26–29).
Immunohistochemical staining for p16 has been investigated in
cervical pathology as a marker for HPV-transformed lesions, and
various studies have demonstrated diffuse and continuous staining
of p16, beginning in the basal and parabasal cell layers, which is
defined as block positive staining (7,23,30). Based on the concept that HPV-mediated
transformation is triggered by dysregulated expression of the viral
oncogenes E6 and E7 in basal and parabasal cells, p16
immunohistochemistry was hypothesized to distinguish between
transforming and nontransforming HPV infections, and only the block
positive p16 expression pattern was defined as a hallmark of
HPV-dependent transformation and thus considered as p16 positive
(27,31,32).
Ki-67 is detected by the MIB-1 monoclonal antibody
and is a nuclear protein that is associated with RNA transcription
and cell cycle progression (18).
Similar to p16, Ki-67 is overexpressed in CIN 2/3, SCC,
adenocarcinoma in situ and adenocarcinoma (20). However, in contrast to p16, Ki-67 is
also overexpressed in the basal cells of normal squamous mucosa and
in benign proliferative lesions, including basal cell hyperplasia
of the squamous mucosa (26).
Therefore, a combination of p16 and Ki-67 immunostaining is
recommended for specificity in distinguishing LSIL versus HSIL from
its mimickers, as opposed to using each immunostaining marker alone
(11).
By conducting a review of previous studies, it was
found that these immunostaining markers could be applied in
selective HR-HPV groups and high-grade dysplastic lesions (29,30). Thus,
the present study investigated the efficacy of p16 and Ki-67
immunostaining markers in the accurate interpretation of cervical
biopsies, and comparing the results of HR-HPV, LR-HPV and
HPV-negative groups. Diffuse and strong staining for p16 and Ki-67
revealed HSIL in the HR-HPV and LR-HPV groups. Almost all HSIL
cases in the HR-HPV group were p16 positive. Obvious expression of
p16 has been associated HR-HPV infection (5); this was also found in the present study.
In addition, 2 cases of immature squamous metaplasia with previous
LSIL (n=1)/HSIL (n=1) were identified by H&E staining alone;
furthermore, these cases demonstrated negativity for p16 and a low
Ki-67 index in the HR-HPV and HPV-negative viral test groups.
However, numerous cases in the HPV-negative group exhibited
discordancy between the H&E and immunohistochemical findings.
Three unusual cases that showed an unexpected p16 immunostaining
result were identified out of the total 26 HPV-negative patients
(Table III). Representatively, 2
cases were implicated pathological significance. Case 3 (Table III) demonstrated p16 negativity and
a Ki-67 score of 3, and exhibited histopathological features
consistent with HSIL. Thus, a final diagnosis of HSIL was
determined. However, the patient's clinical stage was advanced;
therefore, the therapeutic options were limited. The patient
underwent radiation therapy and chemotherapeutic regimen two times
but succumbed due to pancytopenia and multiple lymph node
metastasis 9 months later. Case 2 (Table III) was initially diagnosed with
HSIL, although the patient's immunohistochemical profile of p16 was
not compatible with HSIL. However, H&E staining and Ki-67 index
results indicated HSIL and thus, the final diagnosis was confirmed
by conization specimens resected with a clear resection margin 1
month later. Case 1 (Table III) had
an initial diagnosis of HSIL with negative p16 expression and a
Ki-67 score of 2; in this case the first diagnosis was not revised.
The diagnosis was finally confirmed by analysis of a hysterectomy
specimen 3 weeks later. Three months after hysterectomy, case 1
exhibited no evidence of local recurrence or metastasis. The
aforementioned 3 cases of the negative-HPV group were diagnosed via
different diagnostic methods, due to the unusual p16 pathology
results. Therefore further treatments, including conization and
hysterectomy were performed to allow for additional pathological
examinations, that did not rely on the atypical p16 scores. In HPV
negative patients, we hypothesize that HSIL with a negative p16
score must be considered when interpreting cervical biopsies to
prevent underdiagnosis to LSIL or NILM. For case 1, the negative
p16 result did not correspond with the clinicopathological setting,
and thus H&E staining and Ki-67 index were applicable. It is
currently unknown what causes true-negative p16 immunostaining in
HSIL or SCC in the HPV-negative group. The positivity of p16
expression and the Ki-67 score increased with the severity of the
cervical lesion in the HR-HPV and LR-HPV groups. However,
correlation between p16 score and final diagnosis was stronger in
the HR-HPV group compared with the HPV-negative group. Thus, Ki-67
score is a more useful marker than p16 score in HPV-negative
cases.
In conclusion, simultaneously positive p16
expression and high Ki-67 index are implicated in diagnosis of HSIL
for HR-HPV and LR-HPV precancerous lesions. The two markers are
efficient in advancing the diagnostic accuracy of cervical biopsies
in cases of HR-HPV and LR-HPV, however, application in discrete
daily sign-out processing of immunohistochemical findings should be
considered in the HPV-negative group. This is a pilot study with a
small number of cases, however, pathologists should be aware that
unusual immunostaining results in HPV-negative patients, such as
negative p16 staining in HSIL, may imply factors other than HR-HPV
and LR-HPV infection. In the future, novel immunostaining markers
or other methods that may be applicable for HPV-negative patients
should be assessed for the reproducible diagnosis of cervical
disease.
Acknowledgements
The current study was supported by a National
Research Foundation of Korea Grant funded by the Ministry of
Education, Science and Technology (MEST) through the Research
Center for Resistant Cells (grant no. R13-2003-009).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schiffman M, Castle PE, Jeronimo J,
Rodriguez AC and Wacholder S: Human papillomavirus and cervical
cancer. Lancet. 370:890–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wright TC Jr, Massad LS, Dunton CJ,
Spitzer M, Wilkinson EJ and Solomon D: 2006 American Society for
Colposcopy and Cervical Pathology-sponsored Consensus Conference:
2006 consensus guidelines for the management of women with cervical
intraepithelial neoplasia or adenocarcinoma in situ. J Low
Genit Tract Dis. 11:223–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wright TC Jr, Cox JT, Massad LS, Twiggs LB
and Wilkinson EJ: ASCCP-Sponsored Consensus Conference: 2001
Consensus guidelines for the management of women with cervical
cytological abnormalities. JAMA. 287:2120–2129. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalof AN and Cooper K: p16INK4a
immunoexpression: surrogate marker of high-risk HPV and high-grade
cervical intraepithelial neoplasia. Adv Anat Pathol. 13:190–194.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klaes R, Benner A, Friedrich T, Ridder R,
Herrington S, Jenkins D, et al: p16INK4a immunohistochemistry
improves interobserver agreement in the diagnosis of cervical
intraepithelial neoplasia. Am J Surg Pathol. 26:1389–1399. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horn LC, Reichert A, Oster A, Arndal SF,
Trunk MJ, Ridder R, et al: Immunostaining for p16INK4a used as a
conjunctive tool improves interobserver agreement of the histologic
diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol.
32:502–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma L, Fisk JM, Zhang RR, Ulukus EC, Crum
CP and Zheng W: Eosinophilic dysplasia of the cervix: a newly
recognized variant of cervical squamous intraepithelial neoplasia.
Am J Surg Pathol. 28:1474–1484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aoyama C, Liu P, Ostrzega N and
Holschneider CH: Histologic and immunohistochemical characteristic
of neoplastic and nonneoplastic subgroups of atypical squamous
lesions of the uterine cervix. Am J Clin Pathol. 123:699–706. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benevolo M, Mottolese M, Marandino F,
Vocaturo G, Sindico R, Piperno G, et al: Immunohistochemical
expression of p16INK4A is predictive of HR-HPV infection in
cervical low-grade lesions. Mod Pathol. 19:384–391. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pinto AP, Schlecht NF, Woo TY, Crum CP and
Cibas ES: Biomarker (ProExC, p16INK4A, and MiB-1) distinction of
high-grade squamous intraepithelial lesion from its mimics. Mod
Pathol. 21:1067–1074. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sanati S, Huettner P and Ylagan LR: Role
of ProExC: a novel immunoperoxidase marker in the evaluation of
dysplastic squamous and glandular lesions in cervical specimens.
Int J Gynecol Pathol. 29:79–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walts AE and Bose S: p16, Ki-67, and BD
ProExC immunostaining: a practical approach for diagnosis of
cervical intraepithelial neoplasia. Hum Pathol. 40:957–964. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cameron RI, Maxwell P, Jenkins D and
McCluggage WG: Immunohistochemical staining with MIB1, bcl2 and p16
assists in the distinction of cervical glandular intraepithelial
neoplasia from tubo-endometrial metaplasia, endometriosis and
microglandular hyperplasia. Histopathology. 41:313–321. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doorbar J, Quint W, Banks L, Bravo IG,
Stoler M, Broker TR, et al: The biology and life-cycle of human
papillomaviruses. Vaccine. 30(Suppl 5): F55–F70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khleif SN, DeGregori J, Yee CL, Otterson
GA, Kaye FJ, Nevins JR and Howley PM: Inhibition of cyclin
D-CDK4/CDK6 activity is associated with an E2F-mediated induction
of cyclin kinase inhibitor activity. Proc Natl Acad Sci USA.
93:4350–4354. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo M, Baruch AC, Silva EG, Jan YJ, Lin E,
Sneige N and Deavers MT: Efficacy of p16 and ProExC immunostaining
in the detection of high-grade cervical intraepithelial neoplasia
and cervical carcinoma. Am J Clin Pathol. 135:212–220. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hitchcock CL: Ki-67 staining as a means to
simplify analysis of tumor cell proliferation. Am J Clin Pathol.
96:444–446. 1991.PubMed/NCBI
|
|
19
|
McCluggage WG: Immunohistochemistry as a
diagnostic aid in cervical pathology. Pathology. 39:97–111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cina SJ, Richardson MS, Austin RM and
Kurman RJ: Immunohistochemical staining for Ki-67 antigen,
carcinoembryonic antigen, and p53 in the differential diagnosis of
glandular lesions of the cervix. Mod Pathol. 10:176–180.
1997.PubMed/NCBI
|
|
21
|
Reich O, Regauer S, Marth C, Schmidt D,
Horn LC, Dannecker C, Menton M and Beckmann MW: Precancerous
lesions of the cervix, vulva and vagina according to the 2014 WHO
classification of tumors of the female genital tract. Geburtshilfe
Frauenheilkd. 75:1018–1020. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dunne EF, Unger ER, Sternberg M, McQuillan
G, Swan DC, Patel SS and Markowitz LE: Prevalence of HPV infection
among females in the United States. JAMA. 297:813–819. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Darragh TM, Colgan TJ, Cox JT, Heller DS,
Henry MR, Luff RD, et al: The Lower Anogenital Squamous Terminology
Standardization Project for HPV-Associated Lesions: Background and
consensus recommendations from the College of American Pathologists
and the American Society for Colposcopy and Cervical Pathology. J
Low Genit Tract Dis. 16:205–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reuschenbach M, Seiz M, von Knebel
Doeberitz C, Vinokurova S, Duwe A, Ridder R, et al: Evaluation of
cervical cone biopsies for coexpression of p16INK4a and Ki-67 in
epithelial cells. Int J Cancer. 130:388–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kiernan JA: Histological and Histochemical
Methods: Theory and Practice. 12:(3rd). Oxford, UK:
Butterworth-Heinemann. 1999.
|
|
26
|
Agoff SN, Lin P, Morihara J, Mao C, Kiviat
NB and Koutsky LA: p16INK4a expression correlates with degree of
cervical neoplasia: a comparison with Ki-67 expression and
detection of high-risk HPV types. Mod Pathol. 16:665–673. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang J, Mittal KR, Wei JJ, Yee H,
Chiriboga L and Shukla P: Utility of p16INK4a, CEA, Ki67, P53 and
ER/PR in the differential diagnosis of benign, premalignant, and
malignant glandular lesions of the uterine cervix and their
relationship with Silverberg scoring system for endocervical
glandular lesions. Int J Gynecol Pathol. 26:71–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pavlakis K, Messini I, Athanassiadou S,
Kyrodimou E, Pandazopoulou A, Vrekoussis T and Stathopoulos EN:
Endocervical glandular lesions: a diagnostic approach combining a
semiquantitative scoring method to the expression of CEA, MIB-1 and
p16. Gynecol Oncol. 103:971–976. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiao X, Bhuiya TA and Spitzer M:
Differentiating high-grade cervical intraepithelial lesion from
atrophy in postmenopausal women using Ki-67, cyclin E, and p16
immunohistochemical analysis. Low Genit Tract Dis. 9:100–107. 2005.
View Article : Google Scholar
|
|
30
|
Bergeron C, Ordi J, Schmidt D, Trunk MJ,
Keller T and Ridder R: European CINtec Histology Study Group:
Conjunctive p16INK4a testing significantly increases accuracy in
diagnosing high-grade cervical intraepithelial neoplasia. Am J Clin
Pathol. 133:395–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Doeberitz MV and Vinokurova S: Host
factors in HPV-related carcinogenesis: cellular mechanisms
controlling HPV infections. Arch Med Res. 40:435–442. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
von Knebel Doeberitz M: New markers for
cervical dysplasia to visualise the genomic created by aberrant
oncogenic papillomavirus infections. Eur J Cancer. 38:2229–2242.
2002. View Article : Google Scholar : PubMed/NCBI
|