Introduction
Renal cell carcinoma (RCC) originates in the renal
cortex, and is a highly metastatic urinary tumor that accounts for
~2% of adult malignancies and 3% of childhood malignancies
(1–3).
The incidence of RCC increases with age, and is higher in males
than females, with a male-to-female ratio of 2:1 (4,5). In
addition, the incidence of RCC is significantly higher in European
and American countries compared with Asian countries, and the
incidence is particularly low in Japan and India (6). Due to the resistance to chemotherapy and
radical nephrectomy demonstrated by RCC, the use of microRNAs
(miRNAs) as potential biomarkers for the diagnosis, prognosis and
therapeutic treatment of RCC has increased in recent years
(7–10).
miRNAs are a group of small RNAs ~22 nucleotides in
length that negatively regulate gene expression by binding to the
3′-untranslated region (3′-UTR) of the target mRNAs (11,12).
miRNAs are involved in the regulation of cell proliferation,
development, differentiation and apoptosis (13–15). It is
estimated that ~30% of human genes are regulated by miRNAs, and
there is increasing evidence supporting the participation of miRNAs
in human cancer (16,17).
Eukaryotic elongation factor-2 kinase (eEF2K) is a
calmodulin (CaM)-dependent protein kinase that phosphorylates and
inhibits eEF2, thus preventing the elongation phase of protein
translation (18). eEF2K consists of
several domains, including an N-terminal catalytic domain, a
C-terminal α-helical region and a linker containing several
regulatory phosphorylation sites. Previous studies have identified
high expression levels of eEF2K in several types of cancer, and
suggested that eEF2K may promote cell proliferation during nutrient
starvation in these tumors (19–21). eEF2K
has been considered as a potential cancer-therapeutic target, and
the use of miRNA delivery agents for the treatment of different
types of cancer has been investigated in previous clinical trials
(1,22–25).
Therefore, the identification of novel anticancer miRNAs capable of
targeting eEF2K is of importance.
Previous studies have reported that miRNA-877 acts
as a tumor suppressor in human cancer (26). However, the role of miRNA-877 in RCC
has not been investigated at present. In the present study, the
expression levels of miRNA-877 were observed to be downregulated in
the serum and tissues derived from patients with RCC. Furthermore,
miRNA-877 was demonstrated to be directly bound to eEF2K, thus
inhibiting its transcription and preventing the proliferation of
RCC cells. In summary, the results of the present study suggest
that miRNA-877 may be a potential biomarker for the diagnosis and
therapeutic treatment of RCC.
Materials and methods
Human blood and tissues
Human plasma and surgical specimens, consisting of
100 RCC tissues and 100 paired adjacent normal tissues, were
collected from The Third Affiliated Hospital of Soochow University
(Changzhou, Jiangsu, China) between January 2012 and December 2014.
The total cohort included 55 men and 45 women, with an age range of
45–73 years. The tissues were freshly frozen in liquid nitrogen and
subsequently stored at −80°C. Written informed consent for
participation in the present study was obtained from all patients
and the study was approved by the ethics committee of the Third
Affiliated Hospital of Soochow University.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
miRNA was extracted from the tissue specimens using
the miRNeasy Mini kit (Qiagen GmbH, Hilden, Germany), according to
the manufacturer's instructions. The miScript II RT kit (Qiagen
GmbH) was used for the reverse transcription of miRNA and mRNA in
these tissues. The levels of miRNA-877 in the samples were
quantified by RT-qPCR, using the miScript SYBR Green PCR kit
(Guangzhou Funeng Gene Co., Ltd., Guangzhou, Guangdong, China),
according to the manufacturer's instructions. The primer for U6,
used as an internal control, was provided in the kit. The relative
expression levels of miRNA-877 in the samples were calculated by
the 2−∆∆Ct method, and normalized to the levels of
U6.
Cell culture and transfection
The human RCC ACHN, Caki-2, 769-p, 786-O and HEK293
cell lines were purchased from the American Type Culture Collection
(Manassas, VA, USA), and cultured in Dulbecco's modified Eagle
medium containing 5% or 10% (HEK293 cells) heat-inactivated fetal
bovine serum (FBS; Thermo Fisher Scientific Inc., Waltham, MA,
USA), at 37°C in a 5% CO2 tissue culture incubator. The
cells were then subcultured at 90% confluency, and maintained in
DMEM supplemented with 10% FBS, at 37°C in a 5% CO2
tissue culture incubator. The human RCC cells (5×105
cells/well) were seeded into 6-well plates for in vitro
wound healing assays and transfection. The HEK293 cells
(1×104 cells/well) were seeded into 24-well plates for
the luciferase assay. In order to investigate the role of miR-877
in RCC, miR-877 mimic or inhibitor (Guangzhou Funeng Gene Co.,
Ltd.) were transfected into the cells using Lipofectamine 2000
(Thermo Fisher Scientific Inc.), according to the manufacturer's
protocol.
Western blot analysis
Total protein content was extracted from the tissue
specimens using 250 µl RIPA lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) and 5 mM phenylmethanesulfonyl
fluoride (Beyotime Institute of Biotechnology), according to the
manufacturer's instructions. Total protein was quantified by
Bradford assay (27), and 30–50 µg
protein was subsequently used for western blot assay. The proteins
were subjected to 8–12.5% gel electrophoresis, and transferred onto
nitrocellulose membranes (EMD Millipore, Boston, MA, USA). The
membranes were treated for 1 h with Tris-buffered saline containing
0.1% Tween 20 (TBST) and 5% skim milk (Amresco LCC, Solon, OH,
USA), and then incubated overnight at 4°C with primary polyclonal
or monoclonal antibodies as follows: Anti-eEF2K (polyclonal rabbit
anti-human; cat no. 3692; 1:1,000); anti-phosphorylated (p) EF2
(Thr56) (polyclonal rabbit anti-human; cat no. 2331; 1:1,000);
anti-pAkt (Ser473) (monoclonal rabbit anti-human; cat no. 4060;
1:1,200); and anti-cyclin D1 (monoclonal rabbit anti-human; cat no.
2922; 1:1,000; Cell Signaling Technology, Inc., Danvers, USA).
Next, the membranes were washed three times with TBST for 6 min
each, and incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG (cat no. 7074; 1:2,000; Cell Signaling Technology,
Inc.) secondary antibody for 2 h at room temperature. Proteins were
visualized by enhanced chemiluminescence. The expression levels of
the target proteins were normalized to those of GAPDH.
In vitro wound healing assay
Cells were transfected with miR-877 mimic or
scramble control miRNA and cultured in 60-mm tissue culture plates
under standard conditions (25). At
24 h post-transfection, the cells were incubated with mitomycin C
(10 µg/ml; Sigma-Aldrich, St. Louis, MO, USA) for 2 h to prevent
any potential cell migration due to cell proliferation.
Subsequently, mitomycin C was removed from the medium, and the
cells were washed 3 times with 1X phosphate-buffered saline (PBS;
Nanjing Sunshine Biotechnology Co., Ltd., Nanjing, China), prior to
being scratched with a Axygen P200 pipette tip (Corning, Inc.,
Corning, NY, USA), in order to create an artificial wound (width,
~0.5 mm; length, 2 cm). The cells were then washed 3 times with PBS
to remove any cellular debris. Next, fresh medium was added to the
cells, which were cultured for additional 24 h. To monitor the
healing process, the wounds were photographed at 0 and 24 h after
the cells had been scratched.
Luciferase assay and constructs
The 3′-UTRs of the wild-type and mutant eEF2K
sequences were cloned into the PsiCHECK-2 Vector (Promega
Corporation, Madison, WI, USA), as previously described (12). A Fast Mutagenesis System mutation kit
(TransGen Biotech, Inc., Beijing, China) was used for mutant
construction, and DNA sequences were confirmed by DNA sequencing. A
total of 2×105 RCC cells were seeded into 48-well plates
and incubated for 24 h prior to transfection. Then, 40 ng of the
PsiCHECK-2-eEF2K-3′-UTR plasmid and 10 pmol scramble control miRNA
or miR-877 mimic were added to 50 µl Opti-MEM (Thermo Fisher
Scientific Inc.), and co-transfection was subsequently performed
using 0.5 µl Lipofectamine 2000/50 µl Opti-MEM. The luciferase
activity of the cell extracts was measured at 24 h
post-transfection, using the Dual-Luciferase Reporter Assay System
(Promega Corporation). The transfection efficiency was normalized
by detecting the activity of Renilla luciferase, according to the
Dual-Luciferase Reporter Assay System manufacturer's
instructions.
Bioinformatics
The binding sites for miR-877 on the 3′-UTR of the
potential target genes were predicted by miRanda software
(available from http://www.microrna.org).
Statistical analysis
The RT-qPCR results were analyzed using the
2−∆∆Ct method. Multiple regression analysis was used to
evaluate the association between the expression levels of miR-877
and the clinical features of RCC. P<0.05 was considered to
indicate a statistically significant difference.
Results
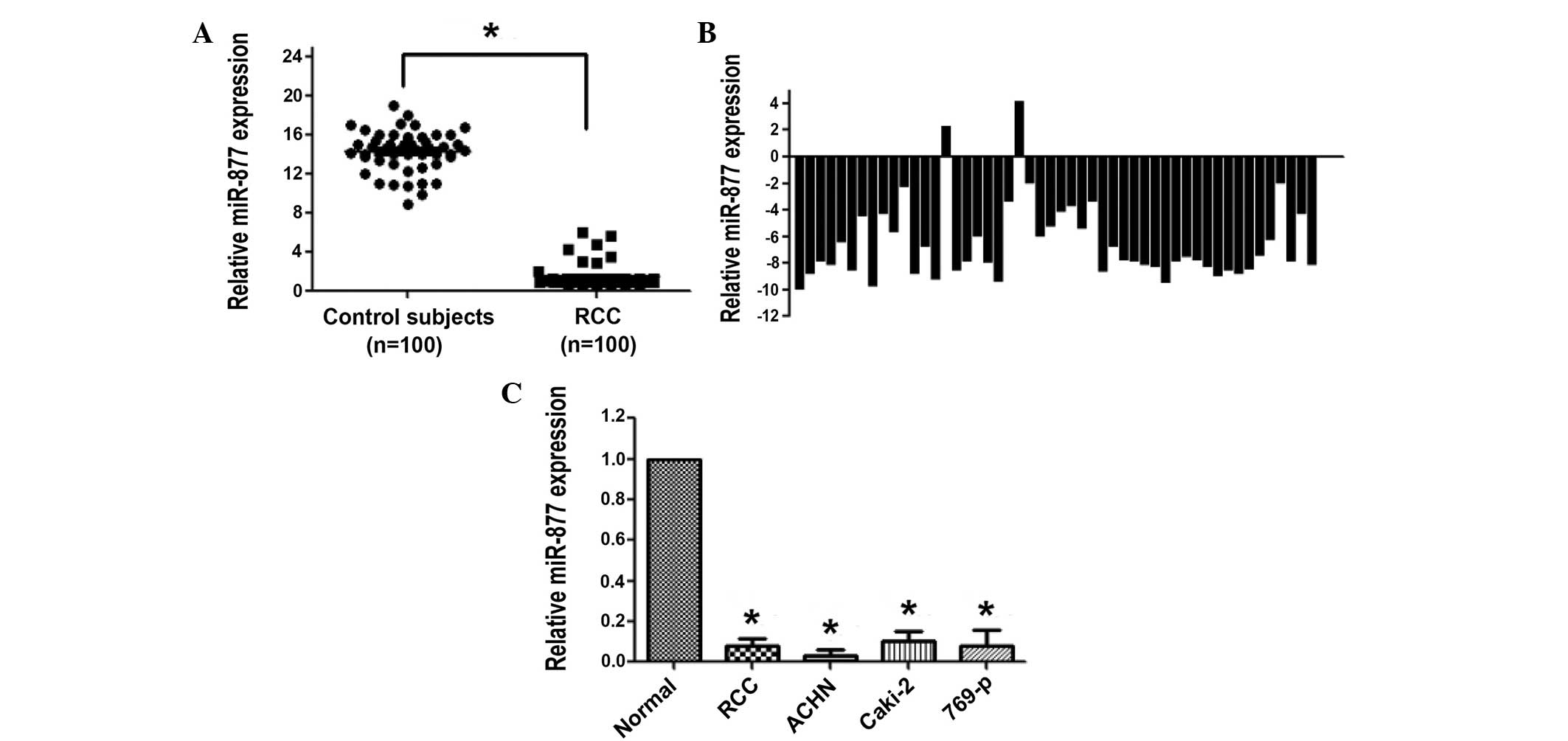
Downregulation of miR-877 expression
in plasma and RCC tissues
The plasma levels of miR-877 in 100 patients with
RCC and 100 healthy control individuals were measured by RT-qPCR.
The data indicated that the concentration of miR-877 in the plasma
was markedly reduced in patients with RCC compared with the healthy
control individuals (P<0.001; Fig.
1A). The clinical significance of miR-877 was further validated
by analyzing the expression levels in 50 RCC and paired tissues via
RT-qPCR. In agreement with the results obtained in the plasma,
RT-qPCR revealed that miR-877 was significantly downregulated in
96% of the RCC samples (P<0.001; Fig.
1B). Notably, miR-877 was barely expressed in the RCC ACHN,
Caki-2 and 769-p cell lines (Fig.
1C).
Overexpression of miR-877 suppressed
RCC cell proliferation
To explore the biological significance of miR-877,
miR-877 mimic and control miRNA (Guangzhou Funeng Gene Co., Ltd.)
were transfected into the RCC ACHN, Caki-2 and 769-p cell lines.
The efficiency of the transfection was assessed by RT-qPCR. The
expression of miR-877 was observed to demonstrate a 400–2,400-fold
increase in the ACHN, Caki-2 and 769-p cells. There were no evident
morphological changes in the cells overexpressing miR-877, but
cellular proliferation analyses demonstrated that the
overexpression of miR-877 suppressed cell proliferation in the 3
RCC cell lines (P<0.05; Fig.
2).
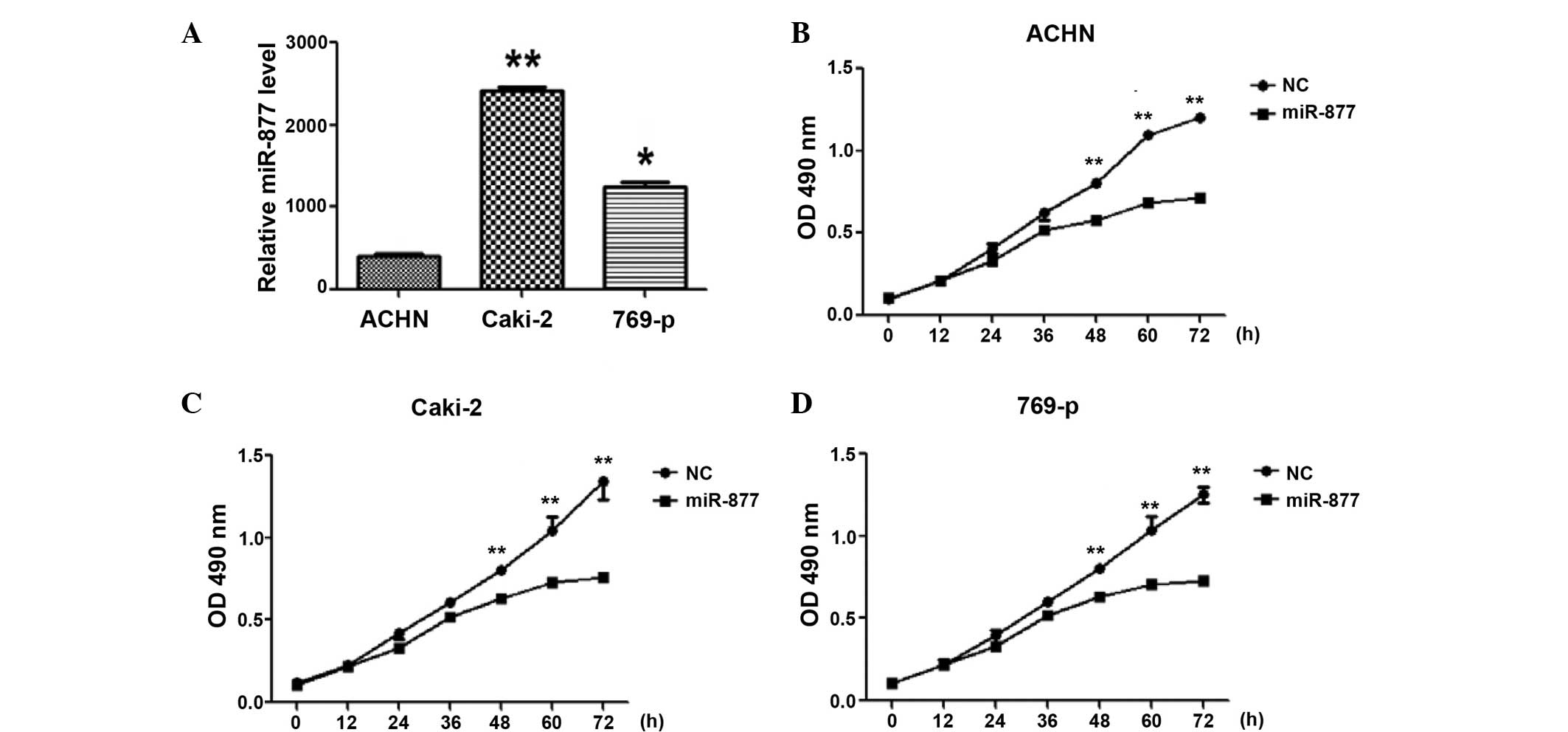 | Figure 2.Effect of the overexpression of the
miRNA miR-877 on the proliferation ability of RCC cells. (A)
RT-qPCR analysis was used to evaluate the efficiency of the
transfection of miR-877 into different RCC cell lines, including
ACHN, Caki-2 and 769-p. *P<0.01, **P<0.001 vs. ACHN. RCC cell
lines (B) ACHN, (C) Caki-2 and (D) 769-p, were transfected with
miR-877 or control miRNA, and their proliferation ability was
investigated by MTT assay.**P<0.01 vs. control. miRNA, microRNA;
RCC, renal cell carcinoma, RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; OD, optical
density; NC, negative control. |
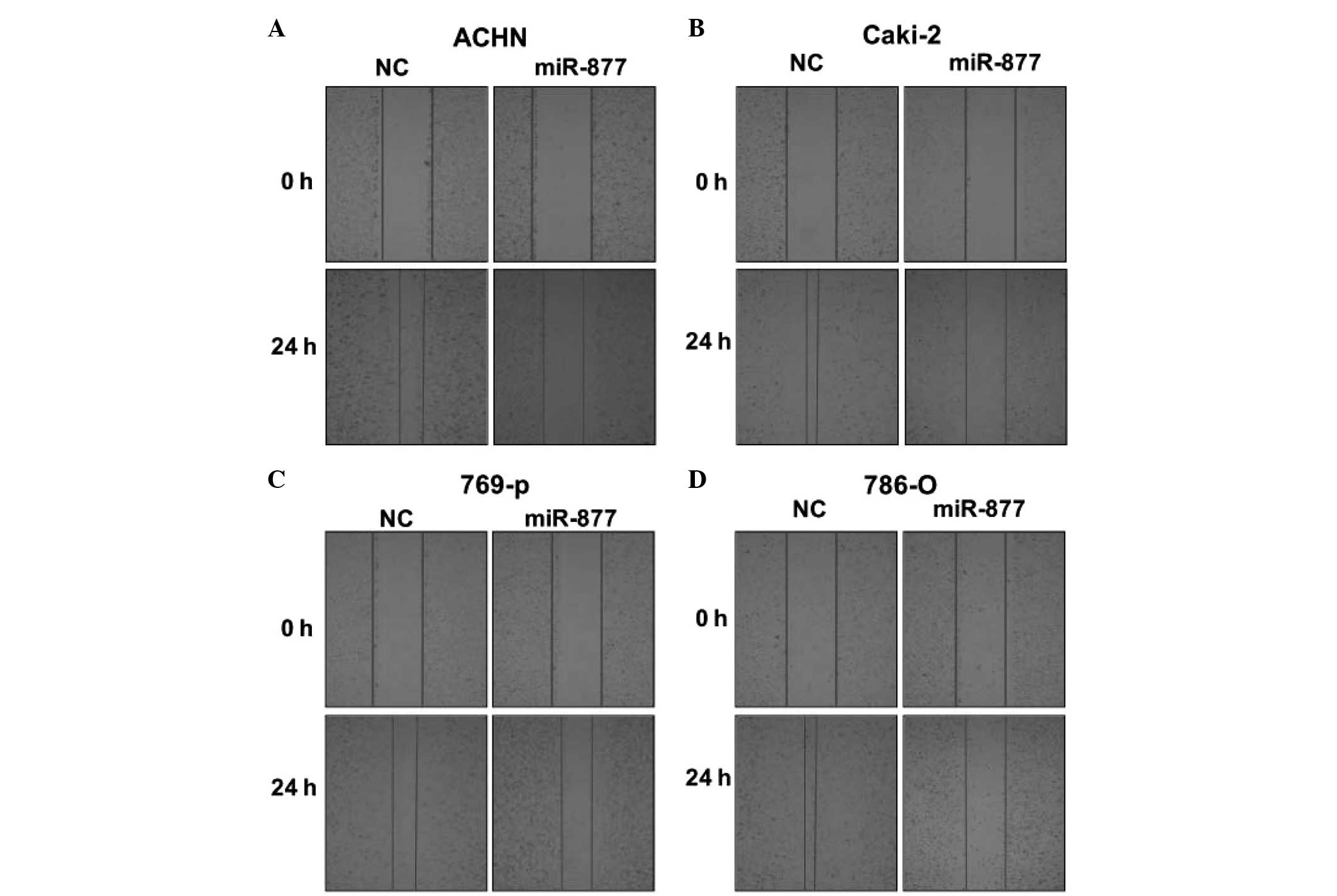
Overexpression of miR-877 reduced RCC
cell motility during wound healing
To investigate the effect of miR-877 on the
migration ability of RCC cells, a scratch wound healing assay was
performed. For this purpose, 4 different RCC cell lines, consisting
of the ACHN, Caki-2, 769-p and 786-O cell lines, were transfected
with miR-877 mimic or scramble control miRNA, and the migration
distance was measured at 0 and 24 h after the cells had been
scratched. Compared with the scramble control miRNA, all the RCC
cell lines that were transfected with miR-877 mimic exhibited
enhanced migration at 24 h (P<0.01; Fig. 3).
miR-877 directly targeted eEF2K by
affecting the eEF2K/EF2 signaling pathway
Bioinformatics analysis predicted the 3′-UTR of
eEF2K to contain complementary binding sites for miR-877 (Fig. 4A). To test whether eFF2K was a
functional target of miR-877, dual luciferase reporter assays were
performed, and to assess whether eEF2K was a functional target of
miR-877, dual luciferase reporter assays were performed. Therefore,
HEK293 cells were transfected with a wild-type or mutant
PsiCHECK-2-eEF2K-3′-UTR plasmid, and the cells were co-transfected
with miR-877 mimic or scramble control miRNA. Compared with the
scramble control miRNA, the miR-877 mimic significantly reduced the
luciferase activity of wild-type PsiCHECK-2-eEF2K-3′-UTR, but did
not alter the activity of the mutant plasmid (Fig. 4B). In addition, the mRNA and protein
levels of eEF2K were significantly reduced in Caki-2 and 786-O
cells transfected with miR-877 mimic or scramble control miRNA
(Fig. 4C and D). These results
suggest that eEF2K mRNA was a direct target of miRNA-877. In order
to assess whether miR-877 affects the eEF2K/EF2 signaling pathway,
the levels of downstream proteins in the cascade, including Akt,
cyclin D1 and pEF2 were measured by western blot analysis. As
presented in Fig. 5, the protein
levels of cyclin D1, Akt, pAkt and pEF2 were reduced in the
miR-877-transfected RCC cells.
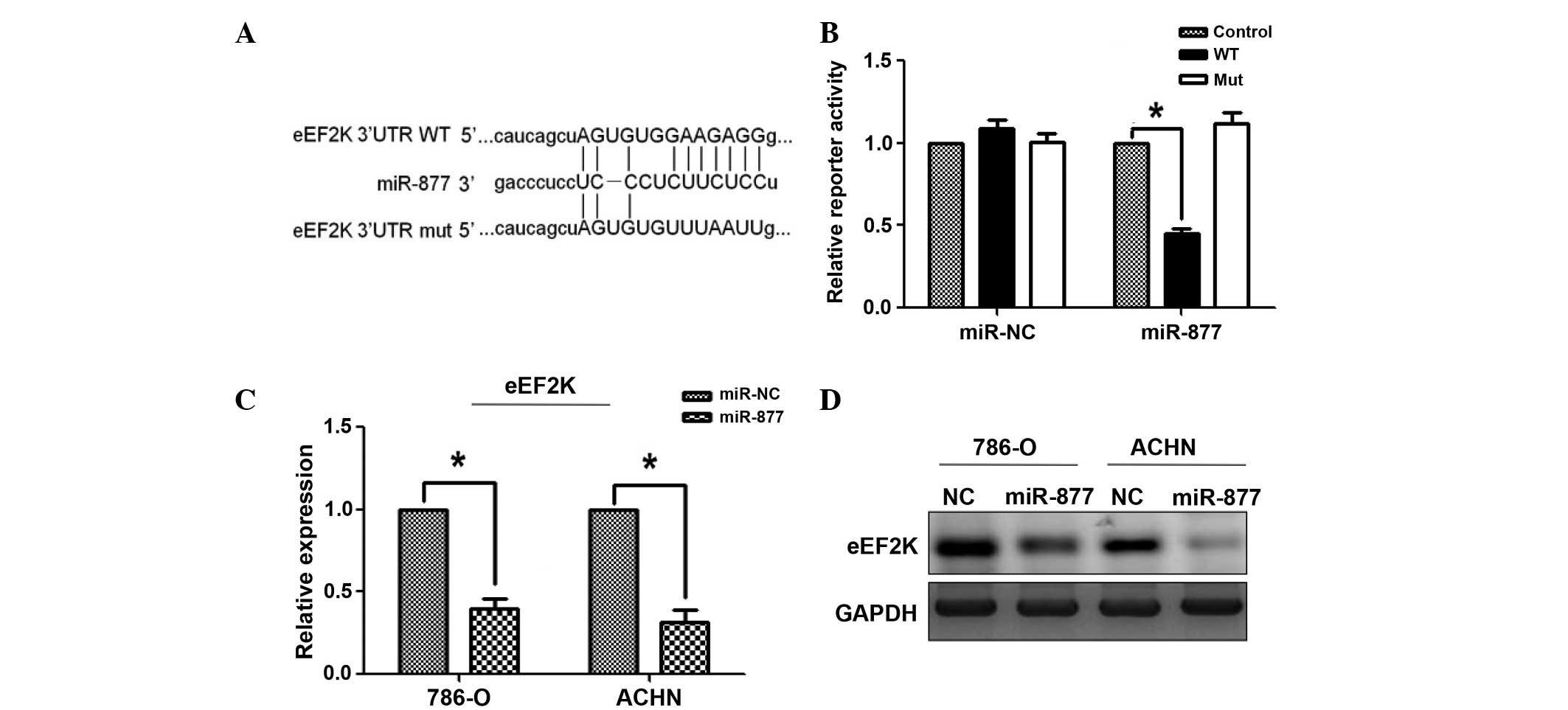 | Figure 4.The miRNA miR-877 downregulated the
expression of eEF2K by targeting the 3′-UTR of eEF2K. (A) Sequence
alignment of the wild-type and mutant eEF2K 3′-UTR, indicating the
potential binding sites for miR-877. (B) Luciferase reporter assays
revealed a reduction in reporter activity following the
transfection of the wild-type eEF2K 3′-UTR reporter construct into
the 786-O and ACHN RCC cells overexpressing miR-877. The eEF2K
3′-UTR mutant and control constructs did not exhibit any effect on
reporter activity. A Renilla luciferase construct was
co-transfected into the cells as an internal control. The
normalized luciferase activity of the control construct in each
experiment was set to 1. The data represent the mean ± standard
error. *P<0.01 vs. control. The (C) mRNA and (D) protein levels
of eEF2K were examined by RT-qPCR and western blot analysis,
respectively, in RCC 786-O and ACHN cells overexpressing miR-877.
The mRNA data were normalized to the levels of GAPDH, which was
used as a loading control in western blot analysis. *P<0.01 vs.
control miRNA. miR, microRNA; eEF2K, eukaryotic elongation factor-2
kinase; UTR, untranslated region; RT-qPCR, reverse
transcription-polymerase quantitative chain reaction; NC, negative
control; WT, wild-type; mut, mutant. |
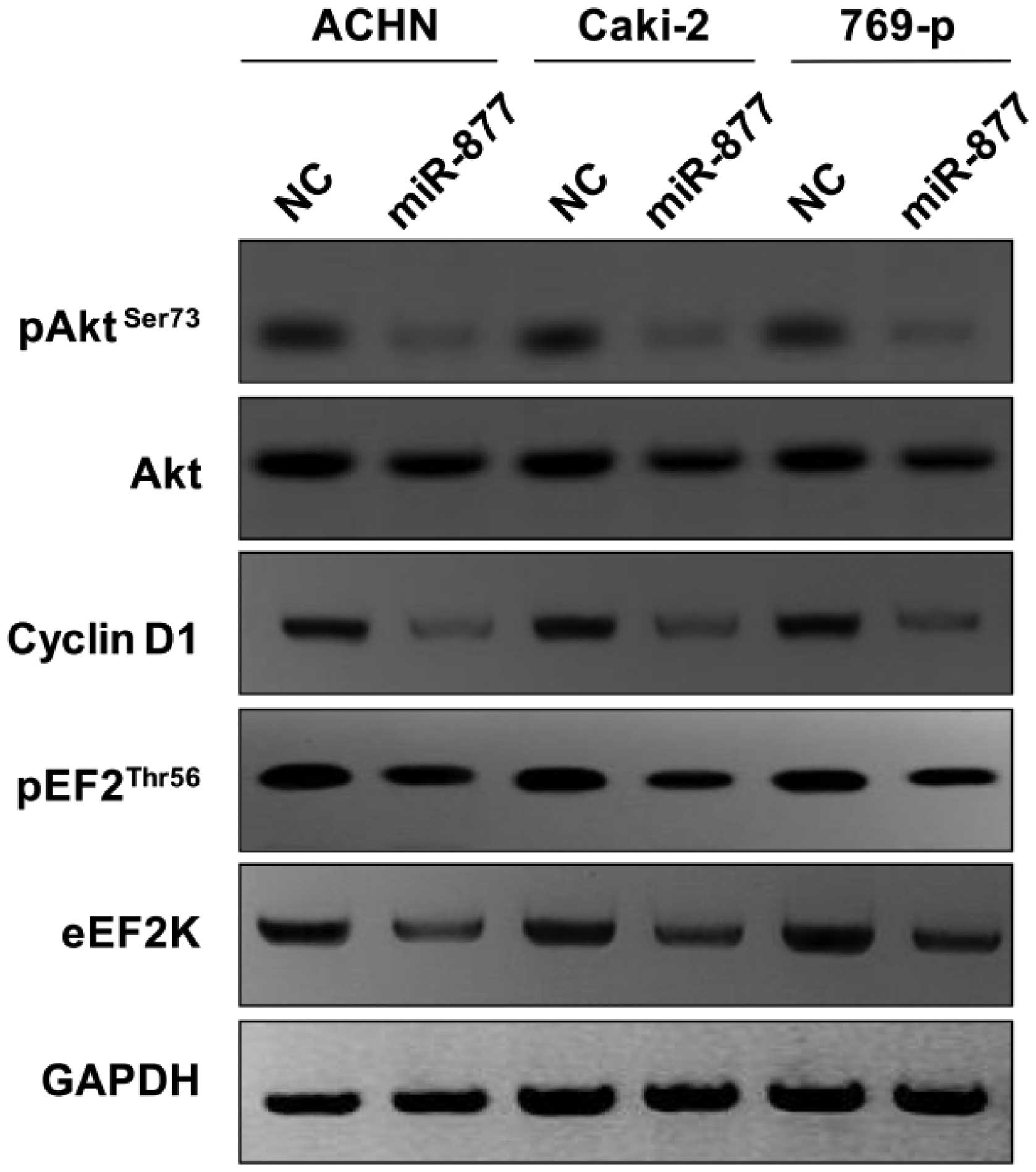 | Figure 5.Downstream molecular effects of the
overexpression of mimic of the miRNA miR-877 in the RCC ACHN,
Caki-2 and 769-p cells. The RCC cells were transfected with miR-877
mimic, and the cell lysates were subjected to western blot
analysis. GAPDH was used as a control. Overexpression of miR-877
mimic reduced the protein levels of eEF2K, pEF2, cyclin D1, Akt and
pAkt. miRNA, microRNA; RCC, renal cell carcinoma; eEF2K, eukaryotic
elongation factor-2 kinase; p, phosphorylated; Akt, protein kinase
B; NC, negative control. |
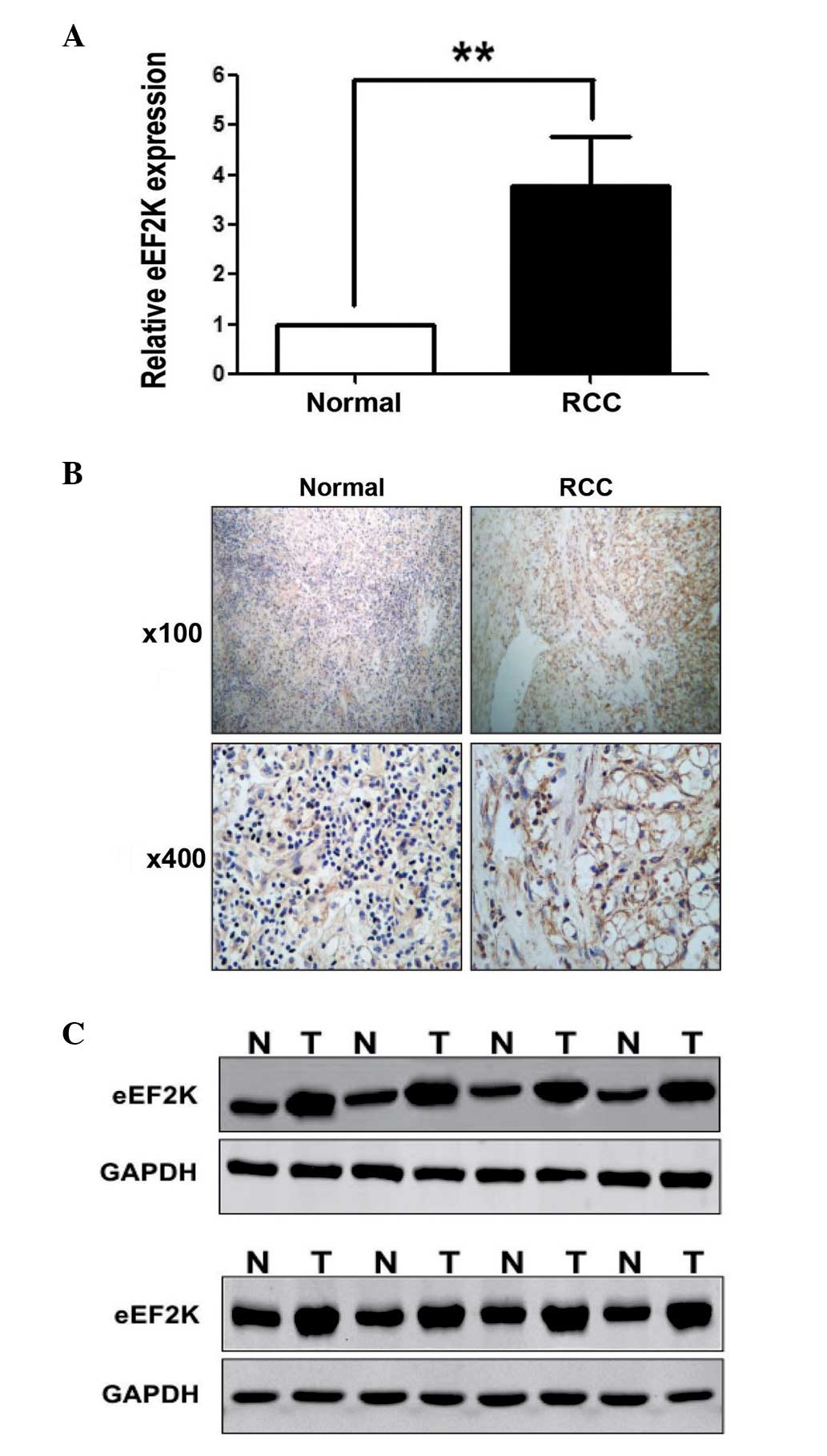
eEF2K protein levels are upregulated
in RCC tissues
To investigate whether miR-877 was involved in the
pathogenesis of human RCC, the protein levels of eEF2K in RCC
tissues were evaluated. The mRNA and protein expression levels of
eEF2K in 16 pairs of RCC and normal tissues were analyzed by
RT-qPCR, western blotting and immunohistochemistry (IHC). The 16
pairs of samples exhibited a significant increase in the levels of
eEF2K in the RCC tissues, compared with the control tissues
(Fig. 6A). In addition, low levels of
miR-877 were associated with increased expression levels of eEF2K
mRNA, and vice versa. Notably, compared with the normal tissues,
marked overexpression of the eEF2K protein was detected in the 16
RCC tissue specimens by IHC and western blot analysis (Fig. 6B and C, respectively), further
supporting that eEF2K is upregulated in RCC tissues.
Discussion
RCC is one of the most common neoplasms of the
kidney in adults (28). Metastatic
RCC is challenging to treat, and the 5-year survival rate is
<10% (3). Therefore, early
diagnosis of RCC is of importance. miRNAs are small non-coding RNA
molecules containing ~22 nucleotides. miRNAs are well conserved in
plants and animals, and are considered to be a vital and
evolutionarily ancient component of genetic regulation (13). In animals, miRNAs are able to
recognize target mRNAs through specific binding sites located at
the 3′ end of the target RNA molecule (14). The binding of the miRNA to its target
mRNA induces the cleavage of the targeted mRNAs, which constitutes
a post-transcriptional regulatory mechanism of gene expression
(14).
Previously, miRNAs have been revealed to perform an
important role in cancer metastasis, and miRNAs are considered to
be prognostic biomarkers in cancer (13). It has been demonstrated that miRNAs
are stable and resistant to extreme pH and temperature in body
fluids (29,30), and serum miRNAs have been reported as
the best biomarker for cancer diagnosis (31,32). Thus,
the use of serum as a diagnostic tool for patients with RCC is of
relevance. In the present study, a miRNA signature was identified
in plasma and tissues of patients with RCC. The expression levels
of miR-877 were observed to be downregulated in the RCC specimens,
whereas the expression levels of its target gene eEF2K were
upregulated in these samples. This trend has also been previously
reported in other types of cancer, which suggests that miRNAs may
be involved in the development of metastatic RCC (33,34).
The results of the present study indicated that
miR-877 was downregulated in blood and tissues of patients with
RCC. These findings suggest that miR-877 may be a potential
biomarker for RCC. During the process of tumor development, miRNAs
may act as oncogenes or tumor suppressors, depending on the target
genes (35). Therefore, gene
regulation by miRNAs is a complex process, since miRNAs may possess
direct and indirect targets (36).
The direct targets of miRNAs may be affected at the mRNA level by
mRNA degradation, or at the protein level by translation inhibition
(37). The indirect targets of miRNAs
may be downstream pathway molecules of the miRNA target genes
(13).
In the present study, miR-877 was predicted to
target sites on the 3′-UTR of eEF2K, according to bioinformatics
analysis. eEF2K is a CaM-dependent protein kinase III that is
activated by Ca2+/CaM. The activation of eEF2K triggers
the phosphorylation of eEF2, which leads to an increase in nuclear
transcription (20). It has
previously been demonstrated that the activity of eEF2K was
associated with proliferation of tumor cells, and high expression
levels of eEF2K have been detected in several types of malignancies
(18). eEF2K has been found to
modulate the activity of certain apoptotic proteins, which resulted
in the inhibition of cancer apoptosis (20). eEF2K has also been reported to perform
a regulatory role in autophagy (21).
eEF2K has been previously been considered a potential cancer
target, but therapeutic interventions based on eEF2K have not been
developed at present (38).
In the present study, eEF2K was upregulated in RCC
tissues. In order to investigate the role of miR-877 in RCC, the
RCC cells were transfected with miR-877 mimic and western blot
analysis was performed. The results demonstrated a significant
downregulation of eEF2K in the cells transfected with miR-877 mimic
compared with the control group. In addition, certain cellular
phenotypes, including cell proliferation and migration, were
demonstrated to be regulated by miR-877 via the eEF2K/eEF2
signaling pathway.
In conclusion, the results of the present study may
aid the understanding of the mechanism behind RCC metastasis.
Furthermore, the specific expression pattern of miR-877 observed in
the present study and the effects exerted by miR-877 on multiple
signaling pathways in RCC cells suggest that miR-877 may be a
potential therapeutic intervention for the treatment of cancer.
References
|
1
|
Tong AW and Nemunaitis J: Modulation of
miRNA activity in human cancer: A new paradigm for cancer gene
therapy? Cancer Gene Ther. 15:341–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu Z, Jian Z, Shen SH, Purisima E and Wang
E: Global analysis of microRNA target gene expression reveals that
miRNA targets are lower expressed in mature mouse and
Drosophila tissues than in the embryos. Nucleic Acids Res.
35:152–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steffens S, Roos FC, Janssen M, Becker F,
Steinestel J, Abbas M, Steinestel K, Wegener G, Siemer S, Thüroff
JW, et al: German Renal Cell Cancer Network: Clinical behavior of
chromophobe renal cell carcinoma is less aggressive than that of
clear cell renal cell carcinoma, independent of Fuhrman grade or
tumor size. Virchows Arch. 465:439–444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Doehn C, Witzsch U and Siebels M: Active
surveillance for renal cell carcinoma. Aktuelle Urol. 43:243–249.
2012.(In German). PubMed/NCBI
|
|
5
|
Chen T, Fallah M, Sundquist K, Liu H and
Hemminki K: Risk of subsequent cancers in renal cell carcinoma
survivors with a family history. Eur J Cancer. 50:2108–2118. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hew MN, Zonneveld R, Kümmerlin IP, Opondo
D, de la Rosette JJ and Laguna MP: Age and gender related
differences in renal cell carcinoma in a European cohort. J Urol.
188:33–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cairns P: Renal cell carcinoma. Cancer
Biomark. 9:461–473. 2010.PubMed/NCBI
|
|
8
|
Olshan AF, Kuo TM, Meyer AM, Nielsen ME,
Purdue MP and Rathmell WK: Racial difference in histologic subtype
of renal cell carcinoma. Cancer Med. 2:744–749. 2013.PubMed/NCBI
|
|
9
|
Banyra O, Tarchynets M and Shulyak A:
Renal cell carcinoma: How to hit the targets? Cent European J Urol.
66:394–404. 2014.PubMed/NCBI
|
|
10
|
Ngo TC, Wood CG and Karam JA: Biomarkers
of renal cell carcinoma. Urol Oncol. 32:243–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
White NM, Khella HW, Grigull J, Adzovic S,
Youssef YM, Honey RJ, Stewart R, Pace KT, Bjarnason GA, Jewett MA,
et al: miRNA profiling in metastatic renal cell carcinoma reveals a
tumor-suppressor effect for miR-215. Br J Cancer. 105:1741–1749.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beresneva EV, Rykov SV, Hodyrev DS,
Pronina IV, Ermilova VD, Kazubskaia TP, Braga EA and Loginov VI:
Methylation profile of group of miRNA genes in clear cell renal
cell carcinoma; involvement in cancer progression. Genetika.
49:366–375. 2013.(In Russian). PubMed/NCBI
|
|
13
|
Mei Q, Li X, Guo M, Fu X and Han W: The
miRNA network: Micro-regulator of cell signaling in cancer. Expert
Rev Anticancer Ther. 14:1515–1527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Filip A: MiRNA - new mechanisms of gene
expression control. Postepy Biochem. 53:413–419. 2007.(In Polish).
PubMed/NCBI
|
|
15
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giannakakis A, Coukos G, Hatzigeorgiou A,
Sandaltzopoulos R and Zhang L: miRNA genetic alterations in human
cancers. Expert Opin Biol Ther. 7:1375–1386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mezzanzanica D, Canevari S, Cecco LD and
Bagnoli M: miRNA control of apoptotic programs: Focus on ovarian
cancer. Expert Rev Mol Diagn. 11:277–286. 2011.PubMed/NCBI
|
|
18
|
Rose AJ, Alsted TJ, Jensen TE, Kobberø JB,
Maarbjerg SJ, Jensen J and Richter EA: A
Ca(2+)-calmodulin-eEF2K-eEF2 signalling cascade, but not
AMPK, contributes to the suppression of skeletal muscle protein
synthesis during contractions. J Physiol. 587:1547–1563. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu LL, Xie T, Zhang SY and Liu B:
Eukaryotic elongation factor-2 kinase (eEF2K): A potential
therapeutic target in cancer. Apoptosis. 19:1527–1531. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arora S, Yang JM, Kinzy TG, Utsumi R,
Okamoto T, Kitayama T, Ortiz PA and Hait WN: Identification and
characterization of an inhibitor of eukaryotic elongation factor 2
kinase against human cancer cell lines. Cancer Res. 63:6894–6899.
2003.PubMed/NCBI
|
|
21
|
Xie CM, Liu XY, Sham KW, Lai JM and Cheng
CH: Silencing of EEF2K (eukaryotic elongation factor-2 kinase)
reveals AMPK-ULK1-dependent autophagy in colon cancer cells.
Autophagy. 10:1495–1508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu H, Yang X, Liu J, Zhou L, Zhang C, Xu
L, Qin Q, Zhan L, Lu J, Cheng H and Sun X: Eukaryotic elongation
factor 2 kinase confers tolerance to stress conditions in cancer
cells. Cell Stress Chaperones. 20:217–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu B, Cheng Y, Liu Q, Bao JK and Yang M:
Autophagic pathways as new targets for cancer drug development.
Acta Pharmacol Sin. 31:1154–1164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tekedereli I, Alpay SN, Tavares CD,
Cobanoglu ZE, Kaoud TS, Sahin I, Sood AK, Lopez-Berestein G, Dalby
KN and Ozpolat B: Targeted silencing of elongation factor 2 kinase
suppresses growth and sensitizes tumors to doxorubicin in an
orthotopic model of breast cancer. PLoS One. 7:e411712012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Zhu X, Zhang X, Liu B and Huang L:
Nanoparticles modified with tumor-targeting scFv deliver siRNA and
miRNA for cancer therapy. Mol Ther. 18:1650–1656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi M, Huang X, Zhou L and Zhang J:
Identification of differentially expressed microRNAs in metastatic
melanoma using next-generation sequencing technology. Int J Mol
Med. 33:1117–1121. 2014.PubMed/NCBI
|
|
27
|
Kruger NJ: The Bradford method for protein
quantitation. Basic Protein and Peptide Protocols. Walker JM:
32:(Totowa, NJ). Humana Press. 9–15. 1994. View Article : Google Scholar
|
|
28
|
Creel PA: Optimizing patient adherence to
targeted therapies in renal cell carcinoma. Clin J Oncol Nurs.
18:694–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen C, Ai H, Ren J, Li W, Li P, Qiao R,
Ouyang J, Yang M, Ma J and Huang L: A global view of porcine
transcriptome in three tissues from a full-sib pair with extreme
phenotypes in growth and fat deposition by paired-end RNA
sequencing. BMC Genomics. 12:4482011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lyons PJ, Lang-Ouellette D and Morin P Jr:
CryomiRs: Towards the identification of a cold-associated family of
microRNAs. Comp Biochem Physiol Part D Genomics Proteomics.
8:358–364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kosaka N, Iguchi H and Ochiya T:
Circulating microRNA in body fluid: A new potential biomarker for
cancer diagnosis and prognosis. Cancer Sci. 101:2087–2092. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang Y, Dai Y, Yang J, Chen T, Yin Y,
Tang M, Hu C and Zhang L: Microarray analysis of microRNA
expression in renal clear cell carcinoma. Eur J Surg Oncol.
35:1119–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang H, Guo Y, Shang C, Song Y and Wu B:
miR-21 downregulated TCF21 to inhibit KISS1 in renal cancer.
Urology. 80:1298–1302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Knebel A, Haydon CE, Morrice N and Cohen
P: Stress-induced regulation of eukaryotic elongation factor 2
kinase by SB 203580-sensitive and -insensitive pathways. Biochem J.
367:525–532. 2002. View Article : Google Scholar : PubMed/NCBI
|