Introduction
Osteosarcoma (OS) is the most common primary
malignant bone tumor, occurring frequently in adolescents and
possessing a high malignant severity (1–4). OS is
commonly identified on the distal femur and proximal tibia,
possessing high rates of recurrence and metastasis, and a poor
prognosis. Previously, surgical resection therapy resulted in a
poor prognosis for OS patients (2).
At present, the molecular pathogenesis and etiology of OS remain
unclear. Therefore, the identification of the effector molecules or
signaling pathways responsible for regulating tumor growth and
metastasis is critical for improving OS treatment.
MicroRNAs (miRNAs/miRs) are a type of highly
conserved, small non-coding RNA that are vital to the
post-transcriptional regulation of gene expression via base pairing
with target mRNA 3′-untranslated regions (3′-UTRs) (5,6). Previous
studies have indicated that the abnormal expression of miRNAs is
closely associated with cell proliferation, apoptosis, metastasis
and invasion in human cancers, including OS (7,8). miRNAs
function as either tumor suppressors or oncogenes, depending on the
role of the target genes. Previous studies have indicated that the
inhibition of miR-26a may induce increased apoptosis in primary
cultured chronic lymphocytic leukemia cells through suppression of
phosphatase and tensin homolog (9).
In addition, miR-26a inhibits hepatitis B virus transcription and
replication by targeting the host factor cysteine and
histidine-rich domain-containing, zinc-binding protein 1 (10).
In the present study, the miRNA expression profiles
of human OS samples and cell lines were compared with those of
adjacent normal skeletal muscle and normal cell lines. miR-26a was
indicated to be upregulated in human OS and cell lines, and the
expression of miR-26a affected the proliferation, migration and
invasion of Saos-2 cells subsequent to transfection with a miR-26a
inhibitor or mimic. Additionally, miR-26a regulated glycogen
synthase kinase-3β (GSK-3β) expression through binding to the
3′-UTR of GSK-3β mRNA. miR-26a expression was negatively correlated
with GSK-3β expression in OS tissues.
Materials and methods
Cell culture and tissue samples
In total, 20 paired OS and matched normal non-tumor
tissues were obtained during biopsy from patients at the Department
of Orthopedics, Chinese People's Liberation Army (PLA) General
Hospital (Beijing, China). All tissues were immediately stored in
liquid nitrogen until use. The present study was approved by the
Ethics Committee of the Chinese PLA General Hospital.
Human OS U2OS, Saos-2, HOS and MG-63 cell lines were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and cultured in Dulbecco's modified Eagle's medium (DMEM;
Hyclone, Beijing, China) and Roswell Park Memorial Institute
(RPMI)-1640 medium (Hyclone), supplemented with 10% fetal bovine
serum (FBS; Hyclone), 100 mg/ml streptomycin (Hyclone) and 100
IU/ml penicillin (Hyclone) at 37°C, in 5% CO2. The human
osteoblast hFOB 1.19 cell line (American Type Culture Collection)
was maintained in DMEM/F12 medium (Hyclone) supplemented with 10%
FBS, 100 mg/ml streptomycin and 100 IU/ml penicillin at 37°C, in 5%
CO2.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA and miR were isolated using the RNeasy
Mini and miRNeasy Mini kits (Qiagen, Inc., Valencia, CA, USA),
according to the manufacturer's protocol. For the miRNA expression
assay, reverse transcription and RT-qPCR were performed using
Applied Biosystems TaqMan miRNA assay kits (Thermo Fisher
Scientific, Waltham, MA, USA) and Applied Biosystems hsa-miR-26a
(cat. no. 4395166; Thermo Fisher Scientific). PCR reactions were
performed using a Bio-Rad iCycler iQ RealTime PCR Detection System
(Bio-Rad Laboratories, Hercules, CA, USA), ~20 ng cDNA and the
following primers (Sangon Biotech Co., Ltd., Shanghai, China):
Forward, 5′-TTGGATCCGTCAGAAATTCTCTCCCGAGG-3′ and reverse,
5′-GGTCTAGATGTGAACTCTGGTGTTGGTGC-3′ for miR-26a; forward,
5′-GGAGAACTGGTCGCCATCAAG-3′ and reverse,
5′-ACATTGGGTTCTCCTCGGACC-3′ for GSK-3β; and forward,
5′-GCGCGTCGTGAAGCGTTC-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′ for
U6. PCR was performed under the following conditions: Denaturation,
95°C for 10 min, followed by 35 cycles of 95°C for 15 sec and 60°C
for 1 min, and melting curve analysis at 95°C for 15 sec, 60°C for
1 min, 95°C for 15 sec and 60°C for 15 sec. The RT-qPCR data were
normalized using the 2−ΔΔCq method (11), relative to glyceraldehyde-3-phosphate
dehydrogenase or U6 small nuclear RNA.
miRNA transfection
Transfections of miRNA were performed using
Invitrogen Lipofectamine 3000 (Thermo Fisher Scientific), according
to the manufacturer's protocols. miR-26a mimic and control mimics
(Ribobio Co., Ltd., Guangzhou, China) were used at a final
concentration of 100 nM. For the miR-26a inhibitor, an inhibitor
control (Ribobio Co., Ltd.) was added to the transfection complexes
at a final concentration of 20 nM. The medium was changed following
4–6 h incubation in a CO2 incubator at 37°C.
Luciferase activity assay
A luciferase activity assay was performed, as
previously described (12). Briefly,
Saos-2 cells were cultured in a 12-well plate (1×105
cells/well), and co-transfected with wild-type (WT) or mutated
(Mut) 3′-UTRs of GSK-3β, luciferase reporter constructs and either
miR-26a or a control using Invitrogen Lipofectamine 3000. The cells
were harvested and the luciferase activity was examined 24 h later,
using the Dual-Luciferase Reporter Assay kit (Promega Corporation,
Madison, WI, USA).
Cell viability assay
The cell counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) assay was used as a
qualitative index of cell viability, which was based on the
conversion of a water-soluble tetrazolium salt,
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
monosodium salt (WST-8), to a water soluble formazan dye upon
reduction by dehydrogenases in the presence of an electron carrier
(13). The cells were plated in
96-well microplates, then the cell count was obtained using the
CCK-8 assay, according to the manufacturer's protocols. Briefly, 10
µl of CCK-8 solution was added to each well, and the samples were
incubated for 1 h, prior to measuring the absorbance at 450 nm.
Colony formation assay
In total, 500 transfected Saos-2 cells were seeded
into a 6-well plate and maintained in DMEM containing 10% FBS for
14 days. Next, the cells were fixed and stained with methanol for
20 min, followed by 0.5% crystal violet for 15 min. Visible
colonies were quantified using an inverted microscope (IX83;
Olympus Corporation, Tokyo, Japan).
In vitro migration and invasion
assay
Migration and invasion assays were performed using
Transwell chambers (BD Biosciences, Franklin Lakes, NJ, USA). For
the migration assay, 5×104 cells were seeded into the
upper chamber of the Transwell. For the invasion assay,
1×105 cells were added into the upper chamber of the
Transwell, which was precoated with Matrigel (BD Biosciences). In
each assay, the cells were maintained in DMEM without serum in the
upper chamber, and DMEM containing 10% FBS was added to the lower
chamber to act as a chemoattractant. Following 24 h of incubation,
the cells that did not migrate or invade through the membrane were
removed. Next, the membranes were fixed and stained with 0.5%
crystal violet. Four random fields were counted per chamber using
an inverted microscope (IX53; Olympus Corporation), and each
experiment was repeated 3 times.
Western blot analysis
The cells or tissues were harvested and lysed using
ice-cold lysis buffer [50 mM Tris-HCl (pH 6.8), 32 mM
2-mercaptoethanol, 2% w/v sodium dodecyl sulfate (SDS) and 10%
glycerol]. Following centrifugation at 20,000 × g for 10 min at
4°C, the proteins in the supernatants were quantified and separated
using 10% SDS polyacrylamide gel electrophoresis and transferred to
a nitrocellulose membrane (GE Healthcare Life Sciences, Chalfont,
UK). Subsequent to blocking with 10% skimmed milk in
phosphate-buffered saline, the membranes were immunoblotted with
antibodies as indicated, followed by horseradish peroxidase-linked
goat anti-rabbit IgG secondary antibodies (cat. no. ab6721;
1:10,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The
signals were detected using the SuperSignal West Pico
Chemiluminescent Substrate kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA), according to the manufacturer's protocols.
Monoclonal rabbit anti-human GSK-3β (cat. no. ab32391; 1:1000),
β-catenin (cat. no. ab32572; 1:3,000), c-Myc (cat. no. ab32072;
1:1,000), Cyclin D1 (cat. no. ab134175; 1:1,000) and β-actin (cat.
no. ab6276; 1:10,000) antibodies were purchased from Abcam
(Cambridge, MA, USA). Protein levels of β-actin were employed as
loading controls. The relative expression level of proteins was
analyzed by Quantity One software (Bio-Rad Laboratories).
Statistical analysis
Data are presented as the mean ± standard deviation,
and were analyzed using the analysis of variance or two-tail
Student's t-test to examine the statistical significance. P<0.05
was considered to indicate a statistically significant difference.
The correlation between GSK-3β and miR-26a expression was assessed
using Spearman's rank correlation coefficient.
Results
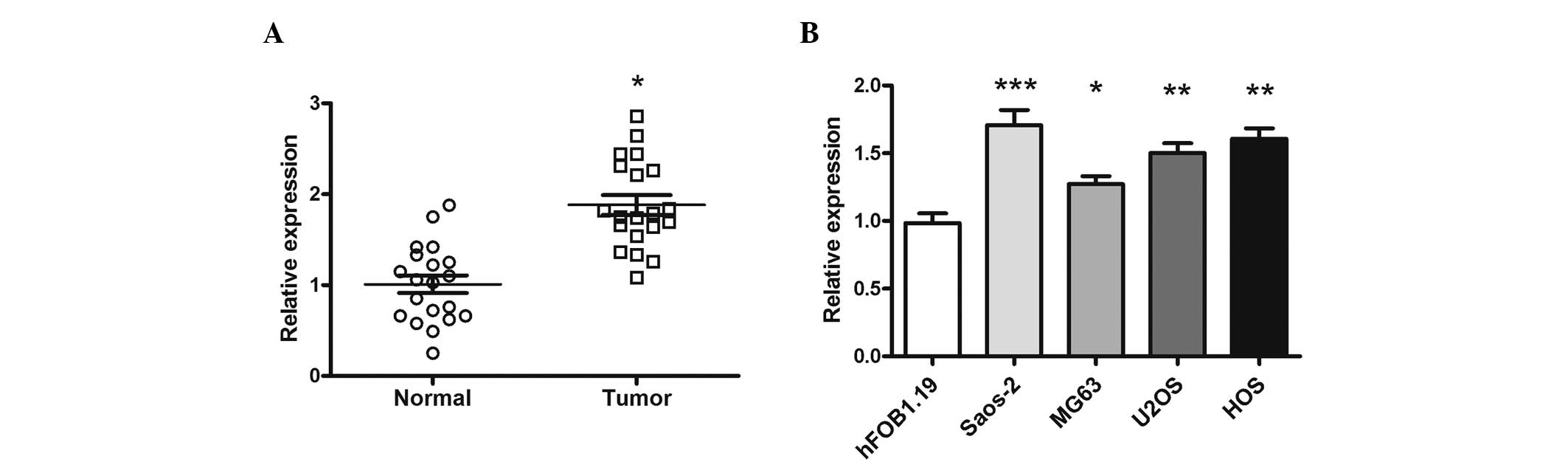
miR-26a is upregulated in human OS
tissues and cell lines
The expression of miR-26a in 20 OS tissues and
matched normal tissues was determined by RT-qPCR. The expression of
miR-26a was significantly increased in the OS tissues compared with
the matched normal tissues (P=0.027; Fig.
1A). In addition, the expression of miR-26a in the OS U2OS
(P=0.0093), Saos-2, HOS (P=0.0088) and MG-63 cell lines was
markedly increased compared with the human osteoblast hFOB 1.19
cell line (Fig. 1B).
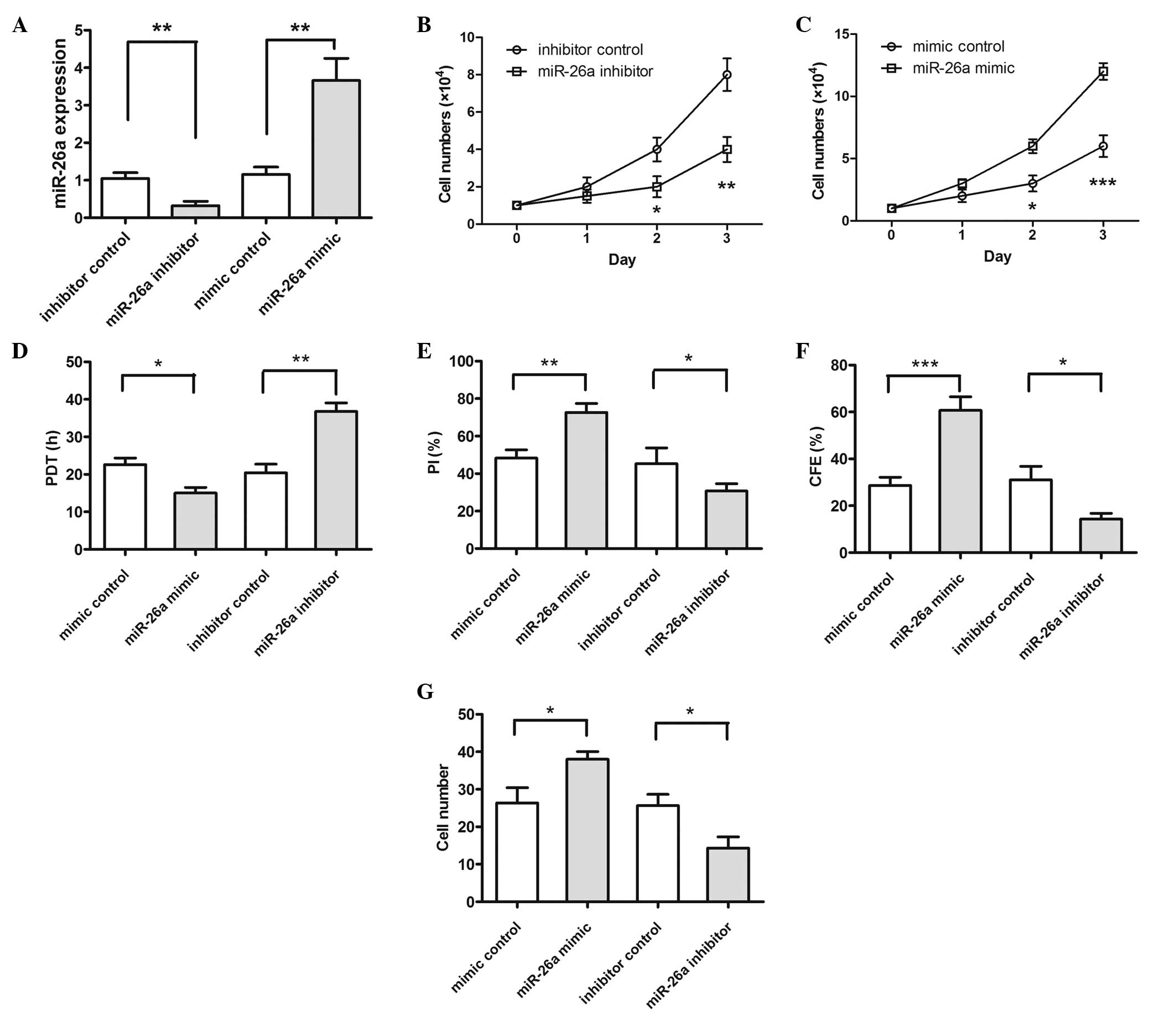
Expression of miR-26a affects the
proliferation, migration and invasion of Saos-2 cells
To examine the role of miR-26a in OS cell growth,
Saos-2 cells were transfected with miR-26a inhibitor/inhibitor
control or miR-26a mimic/mimic control, and the results were
detected by RT-qPCR (Fig. 2A). The
RT-qPCR results indicated that transfection with the miR-26a
inhibitor significantly reduced miR-26a expression (P=0.0075) and
transfection with the miR-26a mimic significantly increased miR-26a
expression (P=0.0084). The results of CCK8 assay indicated that the
proliferation rate of the cells transfected with the miR-26a
inhibitor was significantly decreased compared with the inhibitor
control cells (Fig. 2B): P=0.048 on
day 2 and P=0.0089 on day 3. The transfected miR-26a mimic results
indicated that the proliferation rate of the cells transfected with
miR-26a mimic was significantly increased compared with the mimic
control cells (Fig. 2C): P=0.038 on
day 2 and P=0.0008 on day 3. In addition, the cells transfected
with the miR-26a inhibitor possessed a significantly longer
population doubling time, decreased proliferative index and colony
forming efficiency compared with control cells; furthermore, the
cells transfected with the miR-26a mimic had a shorter population
doubling time (P=0.0041), increased proliferative index (P=0.047)
and colony forming efficiency (P=0.048) compared with the control
group (Fig. 2D–F). Additionally, the
number of cells migrating across the membrane, with or without
Matrigel, that demonstrated miR-26a-knockdown (miR-26a inhibitor)
was significantly decreased (P=0.038) compared with that in the
control cells (Fig. 2G). These data
provide strong evidence that the knockdown of miR-26a may inhibit
proliferation, migration and invasion in Saos-2 cells, and that
transfection with miR-26a may increase cell proliferation,
migration and invasion.
miR-26a downregulates GSK-3β through
binding to the 3′-UTR of GSK-3β mRNA
To screen the function target of miR-32 in OS cells,
bioinformatics software (miRWalk; http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/)
was used. The gene encoding GSK-3β was indicated to harbor a
potential miR-26a binding site (Fig.
3A). Luciferase reporter assays using the 3′-UTR of the GSK-3β
gene demonstrated that the miR-26a mimic significantly decreased
the activity of the GSK-3β 3′-UTR (P=0.0096), but not the binding
motif mutant 3′-UTR (Fig. 3B). In
addition, the overexpression of miR-26a significantly suppressed
the GSK-3β protein level. The intracellular β-catenin protein level
increased, as less β-catenin was degraded through GSK-3β, which
resulted in the activation of Wnt/β-catenin signaling pathways.
Downstream target genes of β-catenin, including Cyclin D1 and
c-Myc, were activated, and the proteins stimulated the growth and
metastasis of the tumor cells (Fig. 3C
and D). The opposite result was indicated in the cells that
were transfected with miR-26a inhibitor.
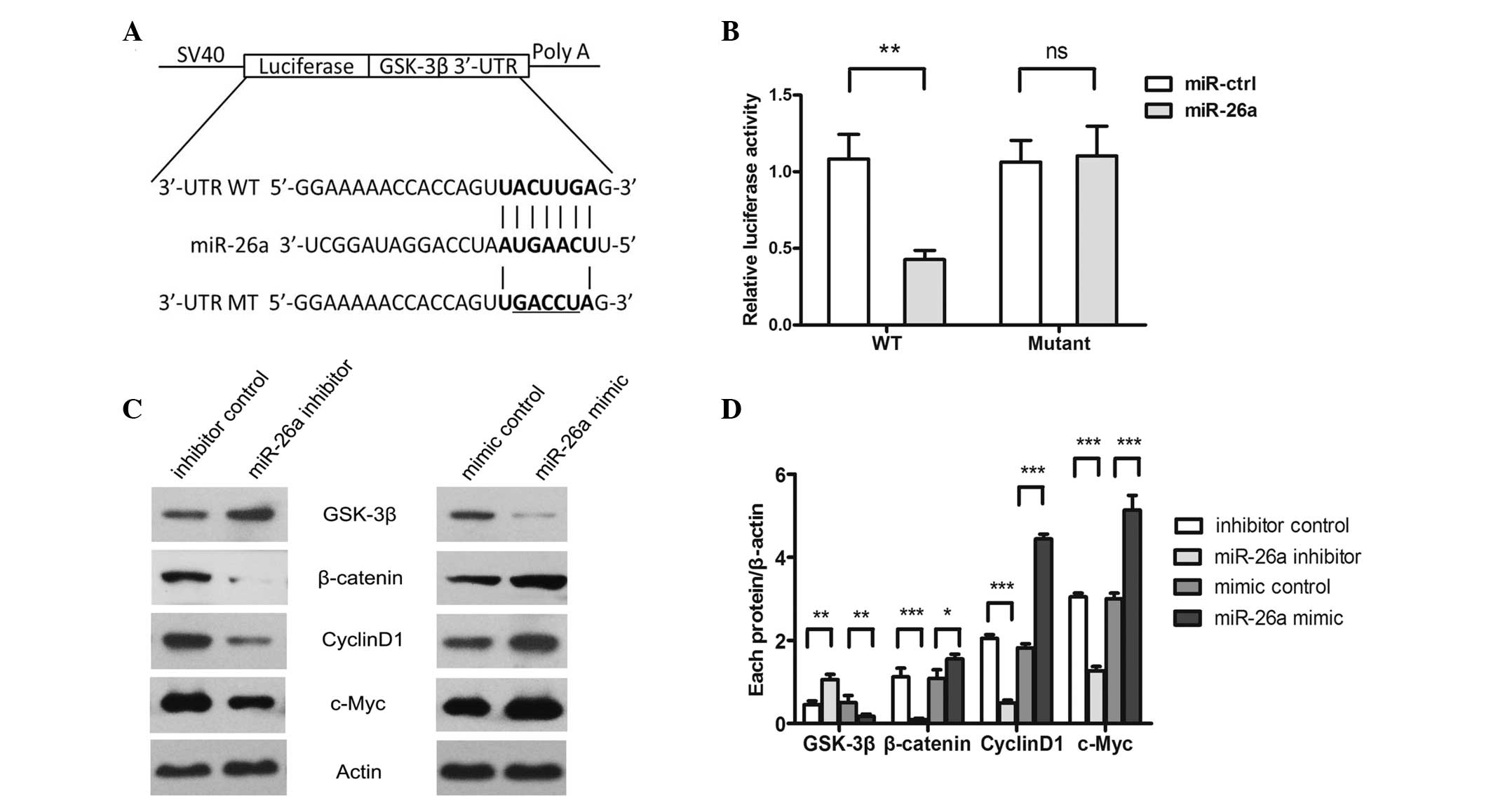 | Figure 3.GSK-3β is a direct target of miR-26a.
(A) Computational analysis showed that miR-26a potentially targeted
GSK-3β. (B) Saos-2 cells were co-transfected with miR-26a and WT or
Mut 3′-UTR luciferase reporter constructs. (C) Protein levels of
GSK-3β, β-catenin, cyclin D1, c-Myc and β-actin were detected using
western blot analysis in Saos-2 cells that were transfected with
miR-26a inhibitor/inhibitor control or miR-26a mimic/mimic control.
(D) Quantitative analysis was performed on each protein level using
Quality One software. *P<0.05, **P<0.01 and ***P<0.001
compared with the control group. GSK-3β, glycogen synthase
kinase-3β; WT, wild-type, Mut, mutated; UTR, untranslated region;
miR, microRNA; ctrl, control. |
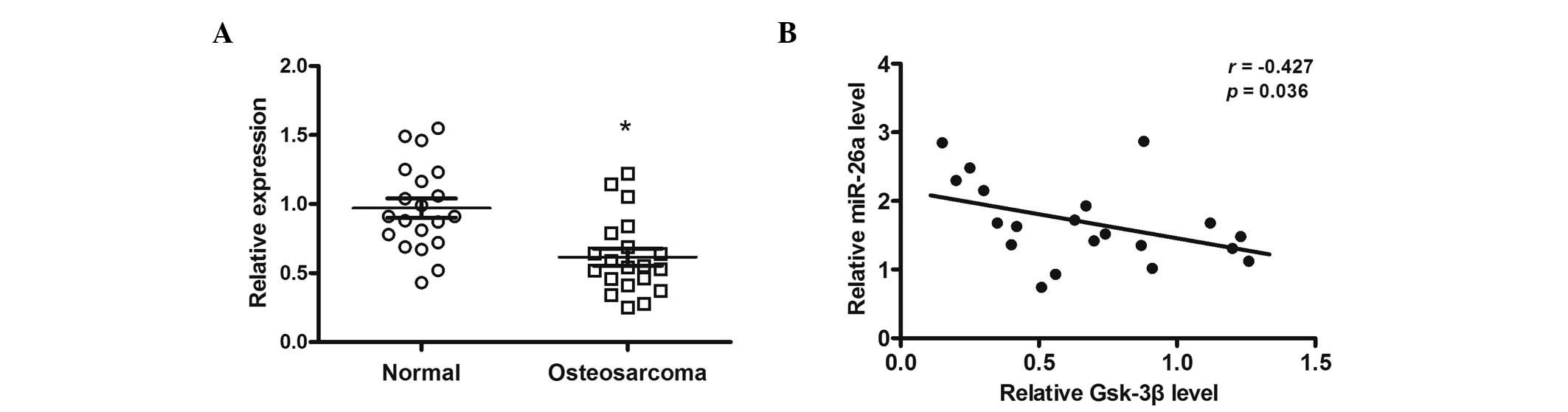
miR-26a expression is negatively
correlated with GSK-3β expression in OS tissues
The expression of GSK-3β mRNA in 20 OS samples and
the corresponding normal tissues was measured. Results indicated
that the expression of GSK-3β mRNA was significantly decreased in
OS tissues compared with the corresponding normal tissues (P=0.044;
Fig. 4A). In addition, GSK-3β
expression was inversely correlated with miR-26a levels in OS
tissues (P=0.036; Fig. 4B).
Discussion
The elucidation of functional targets is one of the
best ways to understand the function of miRNA; this typically
involves an analysis of the changes in target proteins, following a
gain or loss of function of the specific miRNA. The present results
indicated that miR-26a expression was upregulated in the OS tissues
and cell lines. Forced overexpression of miR-26a promoted cell
proliferation, migration and invasion in Saos-2 cells, while
miR-26a inhibition suppressed cell proliferation and metastasis.
Additionally, GSK-3β was indicated to be a target of miR-26a, and
miR-26a expression was negatively correlated with GSK-3β expression
in the OS tissues. However, additional studies are required in
order to investigate the role of miR-26a in vivo.
Abundant miR-26a expression is present in the heart
tissues of rats and humans (14,15), with
decreased levels in ischemic preconditioning. Studies into the role
of miR-26a have also been performed in a number of cancer cell
types. In breast cancer cells, miR-26a has been demonstrated to
initiate cell apoptosis through the extrinsic and intrinsic
pathways by caspase-8 and caspase-9 activation, respectively
(16). In the nasopharyngeal
carcinoma C666-1 cell line, apoptosis induced by ionizing radiation
was dependent on reactive oxygen species, and exogenous miR-26a
expression resulted in significant toxicity in the cells (17). However, the role of miR-26a in OS
remains unclear.
Overall, the present study demonstrated an
association between miR-26a and GSK-3β, and provided a mechanism
for OS cell growth, migration and invasion. These results suggest
that miR-26a may act as an oncogene in OS and represent a potential
molecular target for OS therapy.
References
|
1
|
Bao YP, Yi Y, Peng LL, et al: Roles of
microRNA-206 in osteosarcoma pathogenesis and progression. Asian
Pac J Cancer Prev. 14:3751–3755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He ML, Wu Y, Zhao JM, Wang Z and Chen YB:
PIK3CA and AKT gene polymorphisms in susceptibility to osteosarcoma
in a Chinese population. Asian Pac J Cancer Prev. 14:5117–5122.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia J, Tian Q, Liu Y, Shao ZW and Yang SH:
Interactive effect of bisphenol A (BPA) exposure with −22G/C
polymorphism in LOX gene on the risk of osteosarcoma. Asian Pac J
Cancer Prev. 14:3805–3808. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang W, Huang Y, Wang JP, Yu XY and Zhang
LY: The synergistic anticancer effect of artesunate combined with
allicin in osteosarcoma cell line in vitro and in
vivo. Asian Pac J Cancer Prev. 14:4615–4619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun K and Lai EC: Adult-specific functions
of animal microRNAs. Nat Rev Genet. 14:535–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miao J, Wu S, Peng Z, Tania M and Zhang C:
MicroRNAs in osteosarcoma: Diagnostic and therapeutic aspects.
Tumour Biol. 34:2093–2098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nugent M: MicroRNA function and
dysregulation in bone tumors: The evidence to date. Cancer Manag
Res. 6:15–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zou ZJ, Fan L, Wang L, et al: miR-26a and
miR-214 down-regulate expression of the PTEN gene in chronic
lymphocytic leukemia, but not PTEN mutation or promoter
methylation. Oncotarget. 6:1276–1285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao F, Xu G, Zhou Y, et al: MicroRNA-26b
inhibits hepatitis B virus transcription and replication by
targeting the host factor CHORDC1 protein. J Biol Chem.
289:35029–35041. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H and Yang BB: Stress response of
glioblastoma cells mediated by miR-17-5p targeting PTEN and the
passenger strand miR-17-3p targeting MDM2. Oncotarget. 3:1653–1668.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han SB, Shin YJ, Hyon JY and Wee WR:
Cytotoxicity of voriconazole on cultured human corneal endothelial
cells. Antimicrob Agents Chemother. 55:4519–4523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong S, Cheng Y, Yang J, et al: MicroRNA
expression signature and the role of microRNA-21 in the early phase
of acute myocardial infarction. J Biol Chem. 284:29514–29525. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q, Song XW, Zou J, et al: Attenuation
of microRNA-1 derepresses the cytoskeleton regulatory protein
twinfilin-1 to provoke cardiac hypertrophy. J Cell Sci.
123:2444–2452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang B, Liu XX, He JR, et al:
Pathologically decreased miR-26a antagonizes apoptosis and
facilitates carcinogenesis by targeting MTDH and EZH2 in breast
cancer. Carcinogenesis. 32:2–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alajez NM, Shi W, Hui AB, et al: Enhancer
of Zeste homolog 2 (EZH2) is overexpressed in recurrent
nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and
miR-98. Cell Death Dis. 1:e852010. View Article : Google Scholar : PubMed/NCBI
|