Introduction
Oral lichen planus (OLP), characterized by a chronic
mucocutaneous inflammatory condition (1), is considered to be a T-cell-mediated
immunological process, although the etiology and pathogenesis of
OLP is not completely understood (2).
The prevalence of OLP has been estimated to be between 0.5 and 3%
in a number of studies (3–5). A previous review reported an overall
age-standardized prevalence of 1.27% (0.96% in males and 1.57% in
females) (6). These data reflect that
OLP is a common mucosal disease of the oral cavity.
One of the most significant issues concerning OLP is
the question of its potential for malignant transformation into
oral squamous cell carcinoma (OSCC). Since the World Health
Organization (WHO) developed diagnostic criteria for OLP that
included clinical and histopathological standards in 1978 (7), certain authors have considered OLP to be
a precancerous lesion based on retrospective and prospective
epidemiological data (8–11). Van der Meij et al proposed a
modification of the WHO diagnostic criteria for OLP to include the
definition of an entity referred to as ‘oral lichenoid lesion’
(OLL) and to differentiate between OLL and OLP clinically and
histopathologically. By applying clinically and histologically
diagnostic modified WHO criteria, these authors reported that
patients with OLL have an increased risk of oral cancer, but this
increased risk was not detected in patients with OLP (12).
A cytokine-based microenvironment arising from the
chronic inflammation of OLP may induce genetic alterations of
epithelial cells to progress to malignancy (13). Although a permanent cure of OLP is not
yet possible, various treatment regimens have been designed to
improve management of the symptoms of OLP (14). Several studies have suggested that the
expression of apoptosis- and cell cycle-regulating proteins,
including p53 protein, B-cell lymphoma-2 (Bcl-2) and Bax, was also
involved in the transformation process (15,16).
However, the mechanisms causing the malignant transformation of OLP
remain unclear.
Therefore, the purpose of the present study was to
detect and compare several proteins including myeloid cell
leukemia-1 (Mcl-1) as possible molecules involved in the malignant
transformation of OLP in normal oral mucosa (NOM), OLP and two
human oral cancer cell lines (MC-3 and HSC-3). We also suggest a
strategy on how to prevent malignant transformation of OLP.
Materials and methods
Patients and tissue samples
A total of 14 outpatients were investigated by two
dentists between 2012 and 2013. Three normal samples of gingiva
without pathological lesions were provided from outpatients (one
male and two females; age range from 49 to 56 years) for implant
surgery at the Department of Prosthodontics, Chonbuk National
University Hospital (Jeonju, Korea). Eleven samples of outpatients
(three males and nine females; age range from 49 to 73 years) with
OLP were obtained from the Department of Oral Medicine, of the same
hospital. The materials and methods in the present study were
approved by the ethics committee of Chonbuk National University
Hospital (CUH2013-04-031-001) and written informed consent was
obtained from all patients.
Cell lines and cell culture
MC-3 cells (human mucoepidermoid carcinoma) were
provided by Professor Junzheng Wu (Fourth Military Medical
University, Xi'an, China) and HSC-3 cells (human oral squamous
carcinoma) were obtained from Professor Shindo (Hokkaido
University, Hokkaido, Japan). The two cell lines were cultured in
Dulbecco's modified Eagle's medium/F12 supplemented with 10% fetal
bovine serum (FBS; Welgene, Daegu, Korea) and antibiotics at 37°C
in a 5% CO2 incubator.
Reagents and antibodies
A DC protein assay kit was obtained from Bio-Rad
Laboratories, Inc. (Madison, WI, USA). Trypan blue solution was
purchased from Gibco Life Technologies (Paisley, UK). Antibodies
against Mcl-1 and Bcl-2 were obtained from Cell Signaling
Technology, Inc. (Charlottesville, VA, USA). Actin was purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Western blot analysis
To determine the levels of protein expression,
whole-cell lysates were prepared with lysis buffer and protein
concentrations were measured. Equal amounts of proteins were loaded
on sodium dodecyl sulphate-polyacrylamide gel, transferred to
polyvinylidene membranes and assessed using an enhanced
chemiluminescence western blotting reagent (Luminol; Santa Cruz
Biotechnology, Inc.).
Trypan blue exclusion assay
The effects of sorafenib (LC Laboratories, Woburn,
MA, USA) and mithramycin A (Sigma-Aldrich, St. Louis, MO, USA) on
cell viability were determined using a trypan blue exclusion assay.
Cells were incubated with sorafenib and mithramycin A for 48 h,
stained with trypan blue (0.4%), and then viable cells were counted
using a hemocytometer (Thermo Fisher Scientific, Waltham, MA,
USA).
Anchorage-independent growth
assay
MC-3 and HSC-3 cells were treated with various
concentrations of sorafenib or mithramycin A in 1 ml 0.3% basal
medium Eagle's agar containing 10% FBS. The culture was incubated
at 37°C in a 5% CO2 incubator for 20 days, and then
colonies were counted.
Statistical analysis
Student's t-test was used to determine the
significance of differences between the control and treatment
groups; P<0.05 was considered to indicate a statistically
significant difference.
Results
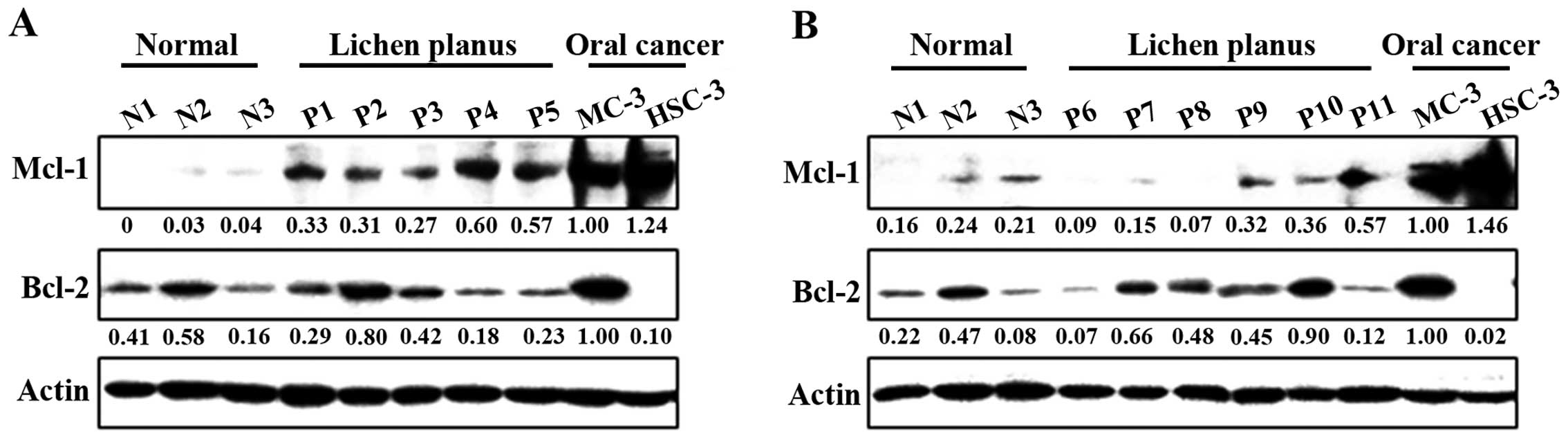
Correlation between Mcl-1 expression
and pathogenesis of OLP
To clarify the potential role of anti-apoptotic
Bcl-2 family proteins on the pathogenesis of OLP, we compared three
samples of NOM and eleven samples from outpatients with proven OLP.
Notably, the expression of Mcl-1 was significantly higher in the
OLP than in the NOM samples, although in both samples it was lower
than in the MC-3 and HSC-3 human oral cancer cell lines (Fig. 1A and B). However, there was no
association between Bcl-2 expression and the pathogenesis of OLP.
These results indicate that Mcl-1 expression may contribute to
malignant transformation in OLP.
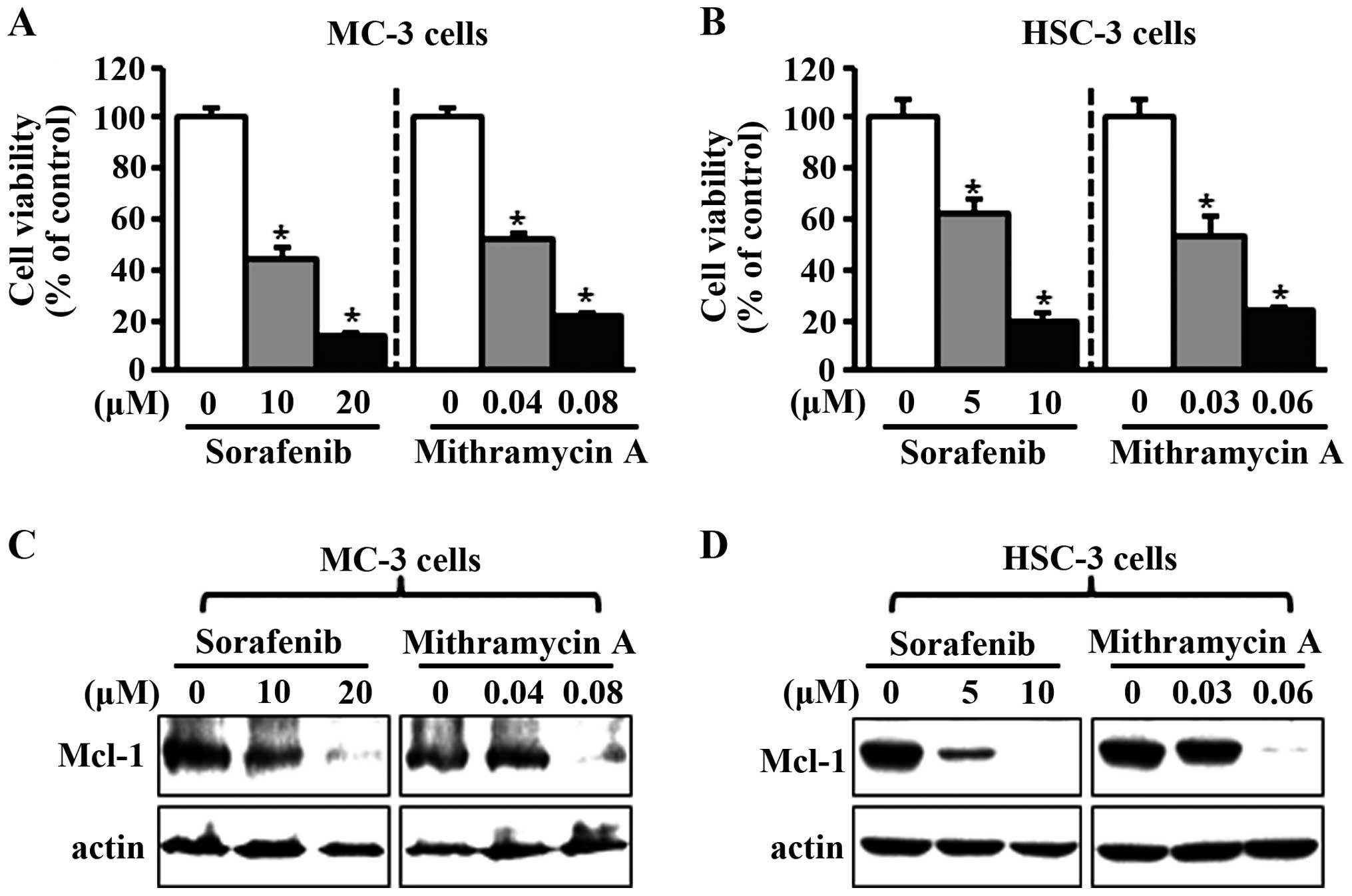
Effects of sorafenib and mithramycin A
on cell growth and Mcl-1 expression in human oral cancer cell
lines
To investigate the potential effects of sorafenib
and mithramycin A on cell viability in human oral cancer cell
lines, we treated the cells with dimethyl sulfoxide or various
concentrations of sorafenib and mithramycin A for 48 h. As shown in
Fig. 2A and B, the two chemicals
notably decreased cell viability in MC-3 and HSC-3 cell lines. To
evaluate whether the effects of sorafenib and mithramycin A on
growth inhibition were associated with the regulation of Mcl-1
expression, we used western blot analysis. As shown in Fig. 2C and D, sorafenib and mithramycin A
reduced Mcl-1 expression in the two cell lines. To further confirm
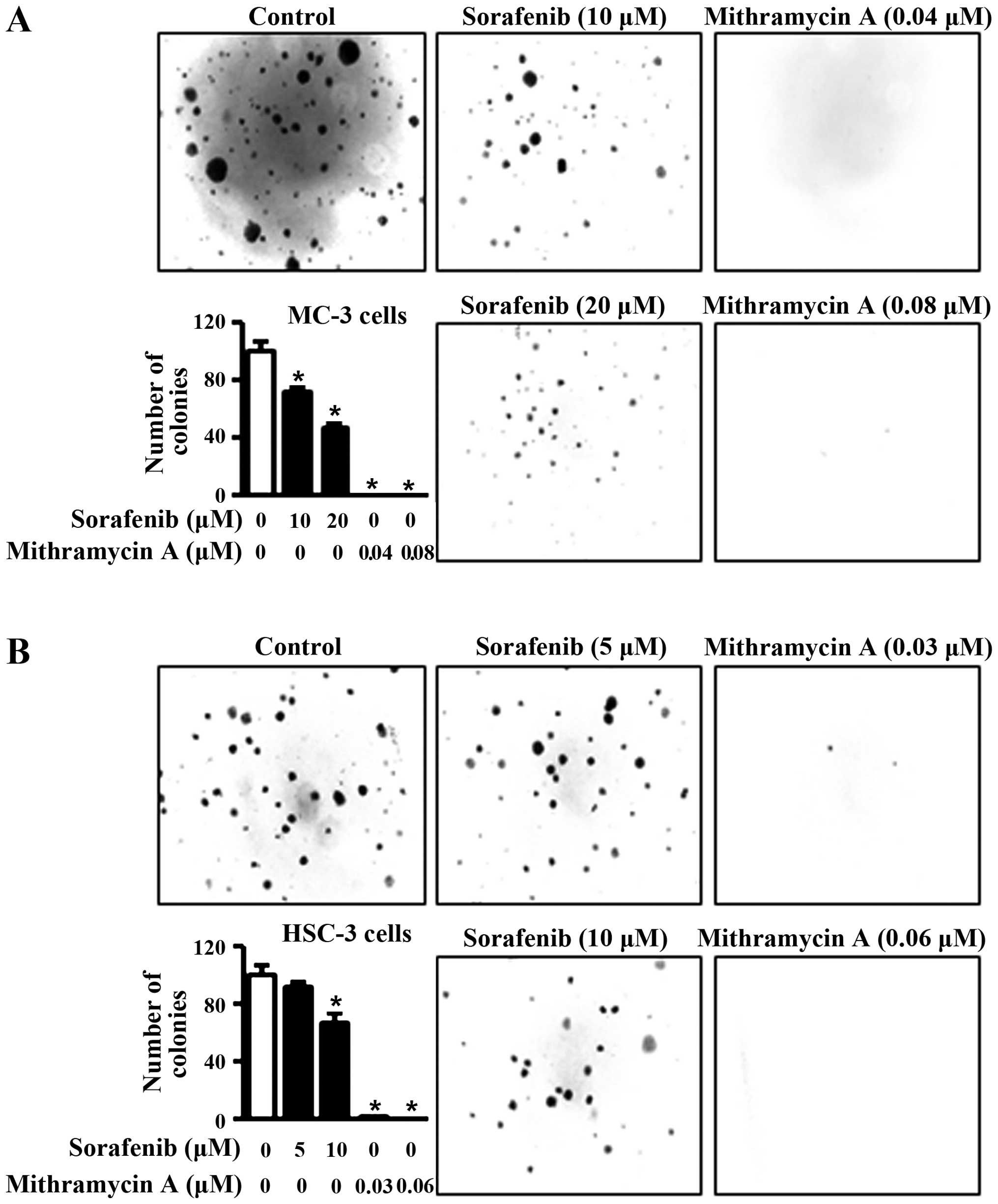
the anti-proliferative effects of sorafenib and mithramycin A, we
carried out anchorage-independent colony formation assay. The
results in Fig. 3A and B reveal that
sorafenib and mithramycin A effectively decreased neoplastic cell
transformation in MC-3 and HSC-3 cells. These results indicate that
sorafenib and mithramycin A may have the ability to prevent
neoplastic transformation from OLP to oral cancer through
downregulation of Mcl-1.
Discussion
Apoptosis, also termed programmed cell death, may be
triggered by various stimuli from outside or inside the cell and
characterized by well-defined biochemical and morphological changes
(17). Bcl-2 family members control
mitochondrial outer membrane permeabilization, and may be either
pro-apoptotic (Bax, Bak, Bid, Bim and Bad, among others) or
anti-apoptotic (Bcl-2, Bcl-xL and Mcl-1, among others) (18). Bcl-2 has the function of inhibiting
apoptosis at various stages and is strongly associated with cancer
development, increasing the genetically altered cell survival rate.
In oral cancer, it is observed from the initial stages of
carcinogenesis up to the appearance of metastasis (19–21). Mcl-1
has been reported to be a critical survival-promoting molecule in
hematopoietic cells, and its overexpression is known to be
associated with a variety of human hematopoietic and lymphoid
cancers (22). The anti-apoptotic
splice variant of Mcl-1 was also augmented in human oral cancer
(23), and our previous study
demonstrated that the overexpression of Mcl-1 may be strongly
correlated with oral cancer development (24).
In the present study, we hypothesized that Bcl-2 or
Mcl-1 may play a significant role in potential malignant
transformation of OLP. To test this hypothesis, our approach was to
evaluate their expression levels in OLP samples compared with NOM
samples and MC-3 and HSC-3 human oral cancer cell lines. Our
results demonstrated that Mcl-1 expression was notably higher in
patient samples of OLP than NOM, with both levels being lower than
in MC-3 and HSC-3 human oral cancer cell lines, while there was no
difference in Bcl-2 expression levels among the samples and cell
lines. We demonstrated the difference in expression levels of Mcl-1
protein among NOM, OLP and human oral cancer cell lines for the
first time. These results indicate that Mcl-1 expression may
contribute to the pathogenesis of OLP and its possible progression
to oral cancer. In a previous comparative study (25), no statistically significant
differences between the expression of Bcl-2 in OLP and OSCC were
observed, which is consistent with our results. This suggests no
association between Bcl-2 expression and pathogenesis of OLP and
progression to oral malignancy.
Several studies have reported a high expression
level of Mcl-1 in tumor cells of human primary squamous cell
carcinoma (SCC) and SCC cell lines (24,26). It
has been demonstrated that the blockage of its function may play a
potentially critical and novel role by the use of either specific
siRNA or inhibitor in the treatment of SCC (26). To date, several therapeutic strategies
have been designed to abrogate the anti-apoptotic function of Mcl-1
in a variety of tumor types (27,28).
Therefore, we hypothesized that downregulation of Mcl-1 may prevent
malignant transformation of OLP to oral cancer. Previously, in
vitro and in vivo studies by our group reported that
sorafenib and mithramycin A regulated Mcl-1 protein to inhibit the
proliferation of oral cancer (24,29). Thus,
we assessed the anti-proliferative effect of sorafenib and
mithramycin A on MC-3 and HSC-3 cells in order to demonstrate the
function of Mcl-1 in the malignant transformation of OLP to oral
cancer. The results revealed that the two chemicals notably
decreased cell viability and neoplastic cell transformation in the
two cell lines. Although we did not directly treat the OLP
specimens with sorafenib and mithramycin A, these results indicate
that the inhibition of Mcl-1 may prevent malignant transformation
of OLP to oral cancer.
In summary, we demonstrated that Mcl-1 protein is
expressed at a higher level in OLP than in NOM, and lower than in
oral cancer cell lines. We also observed that the decreases in
Mcl-1 protein by sorafenib and mithramycin A reduced cell growth
and inhibited neoplastic cell transformation. Our study indicates
that the malignant potential of OLP may be correlated with the
expression of Mcl-1, and a strategy to downregulate Mcl-1 may
prevent its malignant potential. However, further studies are
required in order to support this hypothesis.
Acknowledgements
This study was supported by the Fund of Biomedical
Research Institute, Chonbuk National University Hospital and Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (2014R14A1005309).
References
|
1
|
Barnes L, Eveson JW, Reichart P and
Sidransky D: Tumor of the oral cavity and oropharynx. Pathology and
Genetics of Head and Neck Tumours (World Health Organization
Classification of Tumours) (Lyon). IARC Press. 2005.
|
|
2
|
Roopashree MR, Gondhalekar RV, Shashikanth
MC, George J, Thippeswamy SH and Shukla A: Pathogenesis of oral
lichen planus - a review. J Oral Pathol Med. 39:729–734. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farhi D and Dupin N: Pathophysiology,
etiologic factors and clinical management of oral lichen planus,
part I: facts and controversies. Clin Dermatol. 28:100–108. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ismail SB, Kumar SK and Zain RB: Oral
lichen planus and lichenoid reactions: etiopathogenesis, diagnosis,
management and malignant transformation. J Oral Sci. 49:89–106.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parashar P: Oral lichen planus.
Otolaryngol Clin North Am. 44:89–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McCartan BE and Healy CM: The reported
prevalence of oral lichen planus: a review and critique. J Oral
Pathol Med. 37:447–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kramer IR, Lucas RB, Pindborg JJ and Sobin
LH: Definition of leukoplakia and related lesions: an aid to
studies on oral precancer. Oral Surg Oral Med Oral Pathol.
46:518–539. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Silverman S Jr..Gorsky M and Lozada-Nur F:
A prospective follow-up study of 570 patients with oral lichen
planus: persistence, remission and malignant association. Oral Surg
Oral Med Oral Pathol. 60:30–34. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murti PR, Daftary DK, Bhonsle RB, Gupta
PC, Mehta FS and Pindborg JJ: Malignant potential of oral lichen
planus: observations in 722 patients from India. J Oral Pathol.
15:71–77. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Silverman S Jr, Gorsky M, Lozada-Nur F and
Giannotti K: A prospective study of findings and management in 214
patients with oral lichen planus. Oral Surg Oral Med Oral Pathol.
72:665–670. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sigurgeirsson B and Lindelöf B: Lichen
planus and malignancy. An epidemiologic study of 2071 patients and
a review of the literature. Arch Dermatol. 127:1684–1688. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van der Meij EH, Mast H and van der Waal
I: The possible premalignant character of oral lichen planus and
oral lichenoid lesions: a prospective five-year follow-up study of
192 patients. Oral Oncol. 43:742–748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mignogna MD, Fedele S, Lo Russo L, Lo
Muzio L and Bucci E: Immune activation and chronic inflammation as
the cause of malignancy in oral lichen planus: is there any
evidence? Oral Oncol. 40:120–130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lodi G, Scully C, Carrozzo M, Griffiths M,
Sugerman PB and Thongprasom K: Current controversies in oral lichen
planus: report of an international consensus meeting. Part 2.
Clinical management and malignant transformation. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 100:164–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sousa FA, Paradella TC, Carvalho YR and
Rosa LE: Immunohistochemical expression of PCNA, p53, bax and bcl-2
in oral lichen planus and epithelial dysplasia. J Oral Sci.
51:117–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Sousa FA, Paradella TC, Carvalho YR and
Rosa LE: Comparative analysis of the expression of proliferating
cell nuclear antigen, p53, bax and bcl-2 in oral lichen planus and
oral squamous cell carcinoma. Ann Diagn Pathol. 13:308–312. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuwana T, Bouchier-Hayes L, Chipuk JE,
Bonzon C, Sullivan BA, Green DR and Newmeyer DD: BH3 domains of
BH3-only proteins differentially regulate Bax-mediated
mitochondrial membrane permeabilization both directly and
indirectly. Mol Cell. 17:525–535. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jordan RC, Catzavelos GC, Barrett AW and
Speight PM: Differential expression of bcl-2 and bax in squamous
cell carcinomas of the oral cavity. Eur J Cancer B Oral Oncol.
32B:394–400. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh BB, Chandler FW Jr, Whitaker SB and
Forbes-Nelson AE: Immunohistochemical evaluation of bcl-2
oncoprotein in oral dysplasia and carcinoma. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 85:692–698. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sulkowska M, Famulski W, Chyczewski L and
Sulkowski S: Evaluation of p53 and bcl-2 oncoprotein expression in
precancerous lesions of the oral cavity. Neoplasma. 48:94–98.
2001.PubMed/NCBI
|
|
22
|
Warr MR and Shore GC: Unique biology of
Mcl-1: therapeutic opportunities in cancer. Curr Mol Med.
8:138–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mallick S, Patil R, Gyanchandani R, Pawar
S, Palve V, Kannan S, Pathak KA, Choudhary M and Teni TR: Human
oral cancers have altered expression of Bcl-2 family members and
increased expression of the anti-apoptotic splice variant of Mcl-1.
J Pathol. 217:398–407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shin JA, Jung JY, Ryu MH, Safe S and Cho
SD: Mithramycin A inhibits myeloid cell leukemia-1 to induce
apoptosis in oral squamous cell carcinomas and tumor xenograft
through activation of Bax and oligomerization. Mol Pharmacol.
83:33–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leyva-Huerta ER, Ledesma-Montes C,
Rojo-Botello RE and Vega-Memije E: P53 and bcl-2 immunoexpression
in patients with oral lichen planus and oral squamous cell
carcinoma. Med Oral Patol Oral Cir Bucal. 17:e745–e750. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagata M, Wada K, Nakajima A, Nakajima N,
Kusayama M, Masuda T, Iida S, Okura M, Kogo M and Kamisaki Y: Role
of myeloid cell leukemia-1 in cell growth of squamous cell
carcinoma. J Pharmacol Sci. 110:344–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang S, Okumura K and Sinicrope FA: BH3
mimetic obatoclax enhances TRAIL-mediated apoptosis in human
pancreatic cancer cells. Clin Cancer Res. 15:150–159. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rahmani M, Davis EM, Bauer C, Dent P and
Grant S: Apoptosis induced by the kinase inhibitor BAY 43–9006 in
human leukemia cells involves down-regulation of Mcl-1 through
inhibition of translation. J Biol Chem. 280:35217–35227. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu HJ, Shin JA, Jung JY, Nam JS, Hong IS,
Cho NP and Cho SD: Inhibition of myeloid cell leukemia-1:
Association with sorafenib-induced apoptosis in human
mucoepidermoid carcinoma cells and tumor xenograft. Head Neck.
37:1326–1335. 2015. View Article : Google Scholar : PubMed/NCBI
|