Introduction
Cervical cancer, which is associated with sexual,
bowel and bladder function, is caused by the infection of the human
papillomavirus in 99.8% of cases (1).
It is the leading malignancy among females and a common cause of
mortality among middle-aged females (2,3).
Statistics have revealed that in 2008, the incidence and mortality
rates were 9.0% and 3.2% in more developed areas, and 17.8% and
9.8% in less developed areas, respectively (4). The estimated numbers of new cases and
mortalities, which have been increasing in recent years, reached
12,360 and 4,020, respectively, in 2014 in the United States
(5–7).
In view of these figures, it is essential to the health of females
to improve the efficacy of treatment for cervical cancer.
At present, radiotherapy continues to be the
cornerstone in the treatment of cervical cancer (8,9). However,
the radiosensitivity of cervical cancer cells, which is associated
with genetic factors, restricts the efficacy of radiotherapy
(10). Thus, it is essential to
investigate the mechanism of radiosensitivity of cervical cancer
cells in order to improve the efficacy of radiotherapy. It was
reported that radiotherapy-induced expression of encoding
immediate-early response 5 gene (IER5) affected the
radiosensitivity of HeLa cells by disturbing radiation-induced cell
cycle checkpoints (11,12). In addition, it was reported that the
radiation-induced expression of IER5 in human lymphoblastoid
cells was dose-dependent (13,14). Thus,
we speculated that the expression of IER5 might be also
induced by radiotherapy, and then correlated the efficacy of
radiotherapy through its influence on radiosensitivity in cervical
cancer cells.
However, no studies exist concerning the correlation
between radiotherapy-induced expression of IER5 and the
efficacy of radiotherapy in cervical cancer. Thus, we investigated
the expression of IER5 in cervical cancer patients treated
with various radiation doses to explore the association between the
expression of IER5 and radiotherapy. In addition, the
correlation between the expression of IER5 and clinical
outcomes of radiotherapy was also analyzed. These investigations
are likely to provide a new direction for assessing the improvement
and predicting the clinical outcomes of radiotherapy in treating
cervical cancer.
Materials and methods
Patients and treatment
A total of 53 cervical cancer patients aged between
47 and 68 years old (average, 58.3±3.2) and treated in the
Department of Gynecology, Liaoning Cancer Hospital and Institute,
China, between October 2011 and July 2013 were included in this
study. The inclusion criteria were: i) patients were first
diagnosed and treated; ii) patients were diagnosed with cervical
squamous cell carcinoma by biopsy; iii) patients were in clinical
stage II–III of cervical cancer based on the International
Federation of Gynecology and Obstetrics (FIGO) cancer staging
system (15); iv) the results of
complete blood count (CBC), urinalysis, electrocardiogram, and
liver and kidney function tests were normal; v) there were no
contraindications to radiotherapy in the patients. The exclusion
criteria were: i) patients had bone marrow suppression with white
blood cells less than 3×109/l−1 and platelets
less than 7×109/l−1 in the CBC examination;
ii) patients had complications due to other critical diseases,
including serious cardiovascular and cerebrovascular diseases,
acute hepatitis and uremia; iii) acute or subacute pelvic
inflammatory disease was not under control.
All the included patients were treated with pelvic
external irradiation by a 10-MV X-ray at a dose of 180–200
centigrays (cGy) once a day and five times a week. The treatment
was continued until all tumors had fully regressed. For all
patients, the maximum cumulative dose of radiation was 50 Gy.
The study was approved by the Liaoning Provincial
Tumor Hospital Ethics Committee and all included patients provided
their informed consent.
Specimen collection
The 3–5 mm3 cervical cancer tissues of
patients were obtained by surgery or biopsy before and after
radiotherapy. Each fresh tissue was immediately placed into an
Eppendorf tube (RNase-free; Eppendorf, Hamburg, Germany) and
preserved in liquid nitrogen. As a result, a total of 53 specimens
from tissues after radiotherapy and 16 specimens from tissues
before radiotherapy were obtained. According to the cumulative dose
of radiation, the specimens were randomly divided into three
groups: the 0 Gy group (16 specimens which were obtained from
tissues before radiotherapy), the <20 Gy group (20 specimens)
and the ≥20 Gy group (33 specimens).
Immunohistochemistry
The immunohistochemical staining was performed using
a SuperPolymer rabbit and mouse horseradish peroxidase (HRP) kit
(CoWin Bioscience Co., Ltd., Beijing, China). Firstly, 4-µm
paraffin-embedded sections were deparaffinized with xylene and
dehydrated in alcohol. The sections were then subjected to
microwave antigen retrieval in 10 mM sodium citrate buffer at pH 6
(CoWin Bioscience Co., Ltd.) for 10 min. After cooling for 20 min
and washing in phosphate-buffered saline (PBS), these sections were
immersed in methanol with 0.3% hydrogen peroxide for 10 min to
inactivate endogenous peroxidase activity, followed by normal horse
and goat serum for 30 min to block non-specific reactions.
Secondly, these sections were incubated in primary antibody
solution (1:20) for 60 min. After washing with PBS, they were
incubated with secondary antibody solution for 10 min at room
temperature. Then sections were rewashed with PBS and incubated
with streptavidin-HRP solution for 10 min at room temperature.
Finally, sections were stained with 3,3-diaminobenzidine solution
and counterstained with hematoxylin (CoWin Bioscience Co., Ltd.).
In addition, the PBS instead of the primary antibody solution was
considered as the negative control. Color images of
immunohistochemically stained sections were captured with a
microscopic imaging system (Leica Q500MC; Leica, Cambridge, UK).
The shade of positive tissue staining was observed, with brown
color representing positive expression of IER5 protein. The optical
density (OD) value of positive tissue staining was measured and
analyzed using an Alpha Imager 2000 (Alpha Innotech Corp., CA,
USA).
RNA extraction and reverse
transcription
Frozen samples were thawed, and then total RNA was
extracted using TRIzol (Invitrogen Life Technologies, Carlsbad, CA,
USA). The quantity and quality of RNA were analyzed by NanoDrop
(NanoDrop Technologies, Wilmington, DE, USA) using 260/280 nm and
gel analysis. Moreover, pretreatment of the RNA samples with
RNAse-free DNAse was conducted to avoid genetic DNA causing false
positive amplifications (16).
Complementary DNA (cDNA) was synthesized from RNA using a reverse
transcription kit (Toyobo Biotech Co., Ltd., Shanghai, China)
according to the manufacturer's instructions. The reaction
conditions were 37°C for 15 min, followed by 50°C for 5 min and
98°C for 5 min.
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed using a real-time PCR instrument
(Bio-Rad Laboratories, Hercules, CA, USA) and data were analyzed by
MJ Opticon Monitor software (Bio-Rad Laboratories). IER5 and
β-actin were used as shown in Table
I. The reactions were carried out in a volume of 20 µl
containing 9 µl 2.5X Real Master mix/20X SYBR solution, 4 µl each
primer, 0.33 µl cDNA template and 6.77 µl nuclease-free water. The
amplification of cDNA was started with an initial denaturation step
at 95°C for 1 min, then 40 consecutive cycles of the following
series of steps were performed: denaturation at 95°C for 30 sec,
annealing at 63°C for 45 sec and extension at 68°C for 45 sec.
Results were collected and analyzed with MJ Opticon Monitor
analysis software. The comparative Ct method (ΔΔCt) was used for
quantification of gene expression. The relative expression was
calculated as 2−ΔΔCt according to the Perkin Elmer
Instruction Manual (17), where ΔΔCt
= [Ct (IER5) - Ct (β-actin)] - [Ct (IER5, calibrator) - Ct
(β-actin, calibrator)].
 | Table I.Primers of IER5 and
β-actin. |
Table I.
Primers of IER5 and
β-actin.
| Gene name | Forward primer (5′→
3′) | Reverse primer
(5′→3′) |
|---|
| IER5 |
GGACGACACCGACGAGGAG |
GCTTTTCCGTAGGAGTCCCG |
| β-actin |
GCGCGGCTACAGCTTCA |
CTTAATGTCACGCACTTTCC |
Western blotting
Frozen tissue samples were pulverized under liquid
nitrogen using a mortar and pestle immersed in liquid nitrogen.
Then tissue cells were lysed on ice by using protein lysates (CoWin
Bioscience Co., Ltd.) and phenylmethylsulfonyl fluoride (CoWin
Bioscience Co., Ltd.). Cell disruption was performed in an ice bath
using an ultrasonic processor (Q700 Sonicator; Qsonica, LLC,
Newtown, CT, USA) for 5 min. The proteins were released after cell
disruption. Protein content was quantitated using a bicinchoninic
acid protein assay kit (Tiangen Biotech Co., Ltd., Beijing, China).
Western blotting was performed as follows. Firstly, proteins were
heated for 2 min in a boiling water bath prior to loading on a
sodium dodecyl sulfate (SDS) polyacrylamide gel (12%), and then
electrophoretically transferred to a nitrocellulose membrane. After
blocking, membranes were incubated overnight at 4°C with goat
polyclonal anti-IER5 (1:500; Abcam Inc., Cambridge, MA, USA) or
mouse anti-β-actin (1:1000; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) monoclonal antibody. Afterwards, membranes were
washed three times with Tris-buffered saline containing 0.05%
Tween-20 (8 min each time) before and after incubating with
anti-goat IgG and anti-mouse IgG for 1 h at room temperature
(1:1000, Santa Cruz Biotechnology, Inc.). Finally, the membrane was
assayed using an enhanced chemiluminescent kit (ECL; Thermo
Scientific, Rockford, IL, USA) and scanned with a ChemiDoc™Doc XRS+
system (Bio-Rad Laboratories). The relative protein content was
represented through the gray value ratio of IER5 protein
bands/β-actin protein bands, and the results were analyzed with
Quantity One software (Version 4.3.0, Bio-Rad Laboratories).
Follow-up
The outcomes of treatment were obtained by telephone
follow-up or medical record review with the deadline of March 2014.
The survival and recurrence rate during the follow-up were
calculated.
Data analysis
Data are presented as the means ± standard
deviation. The data were analyzed by using the SPSS statistical
package 19.0 (IBM SPSS, Armonk, NY, USA). One-way analysis of
variance was used to compare differences among groups. The least
significant difference method was used to test the difference
between groups. Spearman's rank correlation method was used to
assess the association. A likelihood ratio Chi-square test was used
to compare the difference between two values. For all above
statistical analyses, P<0.05 was considered to indicate a
statistically significant difference.
Results
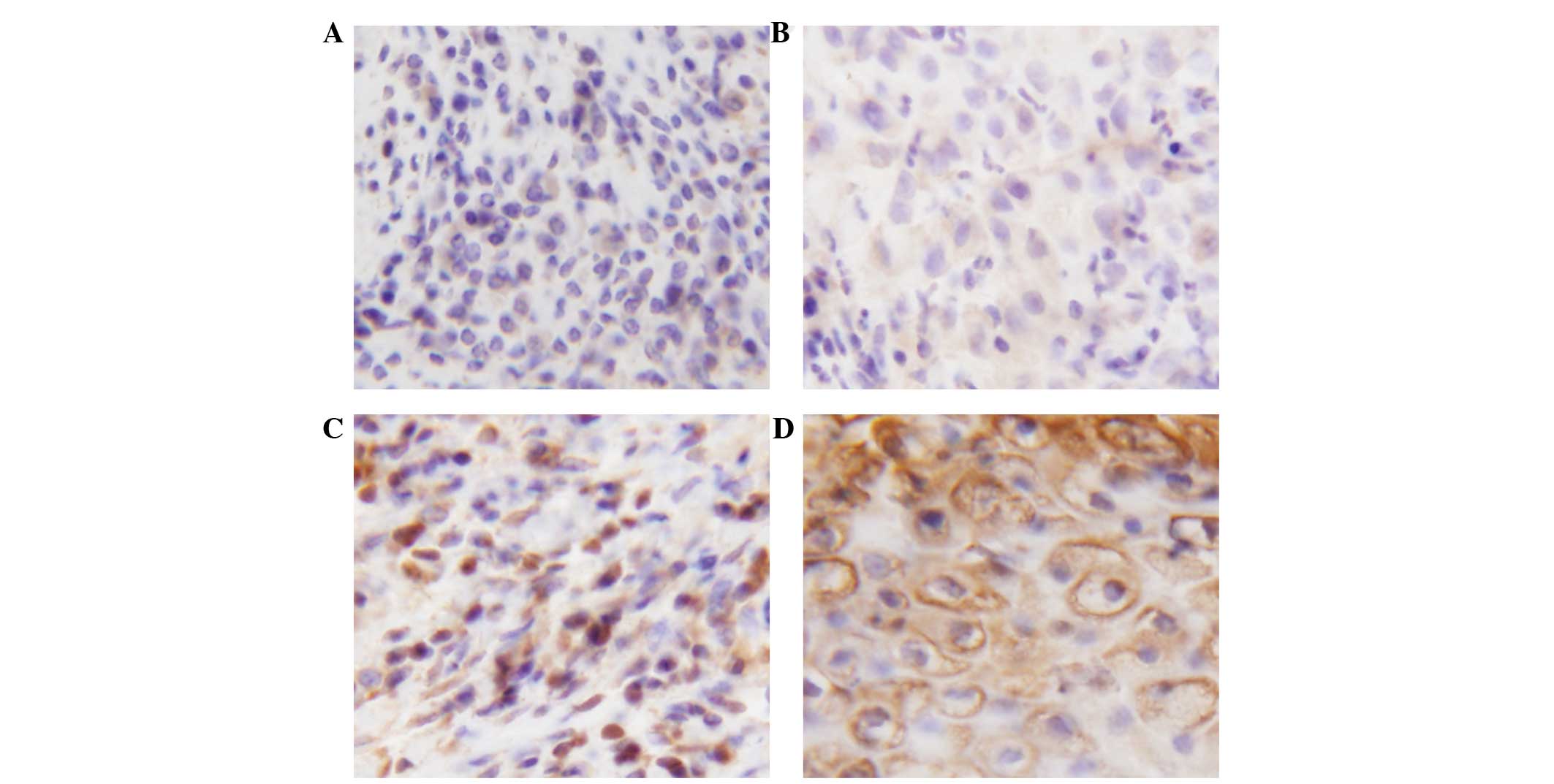
Immunohistochemistry analysis
A total of 18 tissue specimens (0 Gy group, 6
specimens; <20 Gy group, 4 specimens; ≥20 Gy group, 8 specimens)
were randomly selected for the immunohistochemistry analysis. We
observed that the brown staining was increasingly deep with the
increase of radiation dose in immunohistochemically stained
sections (Fig. 1), indicating that
the protein expression level of IER5 was raised with the increase
in radiation dose. For the OD values, there was no significant
difference between the 0 Gy and <20 Gy groups (0 Gy group,
0.241±0.030; <20 Gy group, 0.239±0.014; P=0.915; Table II). However, the protein expression
level of IER5 in the ≥20 Gy group was significantly higher than
that in the other two groups (≥20 Gy group, 0.272±0.019; compared
with <20 Gy group, P=0.030; compared with 0 Gy group, P=0.021;
Table II). Moreover, the results of
the correlation analysis indicated that there was a significant
positive correlation between the protein expression of IER5 and the
dose of radiation (r=0.548, P=0.019).
 | Table II.mRNA and protein expression of
IER5 in each group. |
Table II.
mRNA and protein expression of
IER5 in each group.
| Groups | Immunohistochemistry
expression levels of IER5 protein | qPCR mRNA expression
of IER5 | Western gray scale
ratio of IER5/β-actin |
|---|
| 0 Gy group | 0.241±0.030 | 0.813±0.145 | 0.653±0.154 |
| <20 Gy group | 0.239±0.014 | 0.785±0.238 | 0.847±0.359 |
| ≥20 Gy group |
0.272±0.019a,b |
1.227±0.216a,b |
1.300±0.376a,b |
mRNA level of IER5
A total of 18 tissue specimens (0 Gy group, 6
specimens; <20 Gy group, 4 specimens; ≥20 Gy group, 8 specimens)
were randomly selected for qPCR. The results revealed that there
was no significant difference between the 0 Gy and <20 Gy groups
(0 Gy group, 0.813±0.145; <20 Gy group, 0.785±0.238; P=0.830;
Table II). However, the mRNA level
of IER5 in the ≥20 Gy group was significantly higher than that in
the other two groups (≥20 Gy group, 1.227±0.216; compared with
<20 Gy group, P=0.003; compared with 0 Gy group, P=0.002;
Table II). In addition, the results
of the correlation analysis indicated that there was a significant
positive correlation between the mRNA expression of IER5 and the
dose of radiation (r=0.671, P=0.002).
Western blotting
A total of 33 tissue specimens (0 Gy group, 4
specimens; <20 Gy group, 12 specimens; ≥20 Gy group, 17
specimens) were randomly selected for western blot analysis. The
results of SDS-polyacrylamide gel electrophoresis are shown in
Fig. 2. Based on the gray scale ratio
of IER5/β-actin, the protein expression level of IER5 in ≥20 Gy
group was significantly higher than that in the other two groups
(≥20 Gy group, 1.300±0.376; compared with <20 Gy group, P=0.002;
compared with 0 Gy group, P=0.003; Table
II). However, no significant difference was observed between
the 0 Gy and <20 Gy groups (0 Gy group, 0.653±0.154; <20 Gy
group, 0.847±0.359; P=0.349; Table
II). In addition, the results of the correlation analysis
indicated that there was a significant positive correlation between
the protein expression of IER5 and the dose of radiation (r=0.573,
P<0.0001).
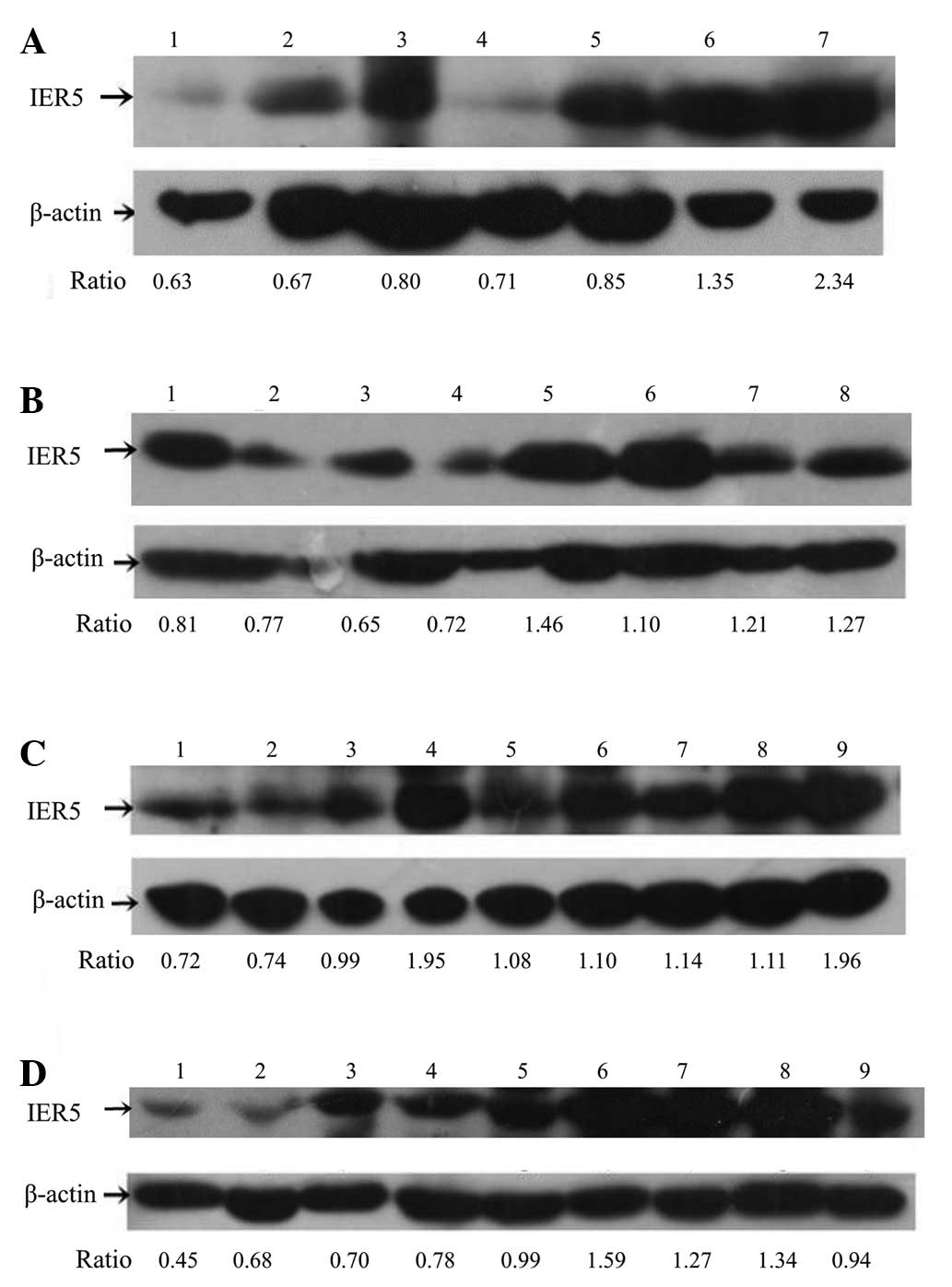 | Figure 2.Results of sodium dodecyl sulfate
polyacrylamide gel electrophoresis of immediate-early response 5
(IER5) and β-actin. (A) 1, 0 Gy; 2, 3 Gy; 3, 10 Gy+1
TC; 4, 10 Gy; 5, 20 Gy; 6, 30 Gy; 7, 30 Gy. (B) 1, 0 Gy+1 TC; 2, 2
Gy; 3, 3 Gy; 4, 10 Gy; 5, 20 Gy; 6, 30 Gy; 7, 40 Gy; 8, 40 Gy. (C)
1, 0 Gy; 2, 3 Gy; 3, 6 Gy; 4, 10 Gy; 5, 20 Gy; 6, 30 Gy; 7, 40 Gy;
8, 50 Gy; 9, 50 Gy. (D) 1, 0 Gy; 2, 3 Gy; 3, 6 Gy; 4, 10 Gy; 5, 20
Gy; 6, 20 Gy+1 TC; 7, 30 Gy; 8, 40 Gy; 9, 50 Gy. 1 TC, one course
of paclitaxel-cisplatin chemotherapy. Ratio, gray scale ratio of
IER5/β-actin. |
Correlation between expression of IER5
and clinical outcomes of radiotherapy
Based on the mRNA and protein expression of IER5,
the patients were divided into two groups: a low IER5 expression
group (including the 0 Gy group and <20 Gy group) and a high
IER5 expression group (including the ≥20 Gy group). There were 36
patients in the low expression group and 33 patients in the high
expression group. The results of the Chi-square test indicated that
there was no significant difference between the two groups with
regard to the survival rate (low expression group, 93.3%; high
expression group, 96.8%; Chi-square test; P=0.490) and recurrence
rate (low expression group, 6.67%; high expression group, 6.25%;
Chi-square test; P=0.914).
Discussion
IER5 is possibly an intronless gene, which
encodes a transcript of 2110 nucleotides in length and shares a
number of nucleic acid and protein homologies with other members of
the growth factor-inducible genes (18,19).
Previous studies have reported that the expression of IER5
may be induced by radiation in cells including HeLa cells, human
lymphoblastoid cells, HepG2 and A549 (20–23). In
this study, we investigated the radiation-induced mRNA and protein
expression levels of IER5 in cervical cancer. The results
indicated that the protein and mRNA expression of IER5 in
the ≥20 Gy group was significantly higher than that in the other
two groups, which suggested that the expression of IER5 in
cervical cancer tissue was significantly increased after receiving
radiotherapy at a dose of ≥20 Gy. Moreover, the mRNA and protein
expression of IER5 was significantly positively correlated
with the radiation dose. The results suggested that radiotherapy
could induce the upregulation of mRNA and protein expression of
IER5 in cervical cancer and that this induction was
dose-dependent. In addition, there was no significant difference
between the low IER5 expression group and high IER5
expression group in terms of the survival and recurrence rate. This
suggested that the radiation-induced upregulation of IER5
expression was not associated with the clinical outcomes of
radiotherapy in cervical cancer.
The results of this study were consistent with those
of Kis et al, who observed that the mRNA expression of
IER5 was dependent on radiation dose and time (24). Moreover, the dose- and time-dependent
patterns of radiation-induced expression of IER5 varied with
cell types (18). This indicated that
there was a complex transcriptional responsiveness of IER5
to ionizing radiation. In addition, in an study of
estrogen-dependent gene expression in the rat uterus, investigators
identified that the expression of IER5 would decrease
following ovariectomy but increase following an injection of
estrogen (25). In addition, Tavakoli
et al observed that the radiation-induced transcription
alterations of IER5 were associated with gender (13). Given that cervical cancer is a female
malignancy, we inferred that the radiation-induced expression of
IER5 might be associated with the secretion of estrogen in
the cervical cancer tissues.
Furthermore, the radiation-induced expression of
IER5 may be associated with the radiosensitivity of cervical
cancer. Ding et al observed that suppression of IER5
potentiated radiation-induced arrest at the G2/M transition and led
to an increase in the fraction of S-phase cells (11). It was also reported that low-dose
hyper-radiosensitivity is linked to the early G2/M checkpoint
through the damage response of G2-phase cells (26–28). Thus,
we inferred that the radiation-induced expression of IER5
was associated with the radiosensitivity of cervical cancer via the
damage response of G2-phase cells. Further studies are required to
prove this speculation.
Certain limitations must be noted in this study.
Firstly, due to the lack of detailed information on follow-up,
survival curves could not be obtained. More studies should be
carried out to investigate the correlation between the expression
of IER5 and the efficacy of radiotherapy. Secondly, three of
the patients were treated with a combination of radiotherapy and
chemotherapy, which may affect the results of this study. Thus,
further studies are required to verify the results of this
study.
In conclusion, we observed that radiotherapy could
induce the upregulated expression of IER5 in cervical cancer
and that the induction was dependent on the dose of radiation.
IER5 may play crucial roles in the radiosensitivity of
cervical cancer cells. This study provides data for the
investigation of the radiosensitivity mechanism of cervical cancer,
which is the main constraint in the efficacy of radiotherapy.
However, no association between the radiation-induced expression of
IER5 and the clinical outcomes of radiotherapy in cervical
cancer was identified in this study. The results suggested that
although IER5 may be a key gene in the radiosensitivity
mechanism of cervical cancer, there may be no direct association
between the expression of IER5 and clinical efficacy of
radiotherapy. The possible roles of IER5 in the
radiosensitivity mechanism of cervical cancer still require further
investigation.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 30770573 and 31170806),
Beijing Municipal Education Commission (Science and Technology for
Development Program Km7092013) and the Key Project of Science and
Technology of Shenyang, Liaoning, China (F12193948).
References
|
1
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arbyn M, Castellsagué X, De Sanjose S,
Bruni L, Saraiya M, Bray F and Ferlay P: Worldwide burden of
cervical cancer in 2008. Ann Oncol. 22:2675–2686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forouzanfar MH, Foreman KJ, Delossantos
AM, Lozano R, Lopez AD, Murray CJ and Naghavi M: Breast and
cervical cancer in 187 countries between 1980 and 2010: A
systematic analysis. Lancet. 378:1461–1484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanderup K, Georg D, Pötter R, Kirisits C,
Grau C and Lindegaard JC: Adaptive management of cervical cancer
radiotherapy. Semin Radiat Oncol. 20:121–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barillot I, Horiot JC, Maingon P, Truc G,
Chaplain G, Comte J and Brenier JP: Impact on treatment outcome and
late effects of customized treatment planning in cervix carcinomas:
baseline results to compare new strategies. Int J Radiat Oncol Biol
Phys. 48:189–200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitahara O, Katagiri T, Tsunoda T, Harima
Y and Nakamura Y: Classification of sensitivity or resistance of
cervical cancers to ionizing radiation according to expression
profiles of 62 genes selected by cDNA microarray analysis.
Neoplasia. 4:295–303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding KK, Shang ZF, Hao C, Xu QZ, Shen JJ,
Yang CJ, Xie YH, Qiao C, Wang Y, Xu LL and Zhou PK: Induced
expression of the IER5 gene by gamma-ray irradiation and its
involvement in cell cycle checkpoint control and survival. Radiat
Environ Biophys. 48:205–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yili Z, Xiaoyan H, Hongwen D, Yun Z, Xin
C, Peng W and Youmin G: The value of diffusion-weighted imaging in
assessing the ADC changes of tissues adjacent to breast carcinoma.
BMC Cancer. 9:182009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tavakoli H, Manoochehri M, Mosalla
Modarres SM, Ghafori M and Karimi P: Dose-dependent and
gender-related radiation-induced transcription alterations of
Gadd45a and Ier5 in human lymphocytes exposed to gamma ray emitted
by (60)Co. Radiat Prot Dosimetry. 154:37–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Long XH, Zhao ZQ, He XP, et al:
Dose-dependent expression changes of early response genes to
ionizing radiation in human lymphoblastoid cells. Int J Mol Med.
19:607–615. 2007.PubMed/NCBI
|
|
15
|
Lanciano RM, Won M and Hanks GE: A
reappraisal of the international federation of gynecology and
obstetrics staging system for cervical cancer. A study of patterns
of care. Cancer. 69:482–487. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martel F, Gründemann D and Schömig E: A
simple method for elimination of false positive results in RT-PCR.
J Biochem Mol Biol. 35:248–250. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aune TM, Penix LA, Rincón MR and Flavell
RA: Differential transcription directed by discrete gamma
interferon promoter elements in naive and memory (effector) CD4 T
cells and CD8 T cells. Mol Cell Biol. 17:199–208. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Williams M, Lyu MS, Yang YL, Lin EP,
Dunbrack R, Birren B, Cunningham J and Hunter K: Ier5, a novel
member of the slow-kinetics immediate-early genes. Genomics.
55:327–334. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gregory S, Barlow K, Mclay K, Kaul R,
Swarbreck D, Dunham A, Scott CE, Howe KL, Woodfine K, Spencer CC,
et al: The DNA sequence and biological annotation of human
chromosome 1. Nature. 441:315–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li XN, Li L, Yang CJ, et al: The influence
of IER5 gene on the radiosensitivity of HeLa cells. Prog Biochem
Biophys. 36:847–853. 2009.
|
|
21
|
Lu WY, Liu ZX, Li L, Ma HP, Zheng KY and
Ding KK: Quantitation analysis to radiation effect on tumor cell by
MATLAB. Chinese Journal of Medical Physics. 5:0242010.
|
|
22
|
Guo WF, Lin RX, Huang J, Zhou Z, Yang J,
Guo GZ and Wang SQ: Identification of differentially expressed
genes contributing to radioresistance in lung cancer cells using
microarray analysis. Radiat Res. 164:27–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H, Long X, Sun ZZ, Rigaud O, Xu QZ,
Huang YC, Sui JL, Bai B and Zhou PK: Identification of
differentially transcribed genes in human lymphoblastoid cells
irradiated with 0.5 Gy of gamma-ray and the involvement of low dose
radiation inducible CHD6 gene in cell proliferation and
radiosensitivity. Int J Radiat Biol. 82:181–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kis E, Szatmári T, Keszei M, Farkas R,
Esik O, Lumniczky K, Falus A and Sáfrány G: Microarray analysis of
radiation response genes in primary human fibroblasts. Int J Radiat
Oncol Biol Phys. 66:1506–1514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu X, Pang ST, Sahlin L, Blanck A,
Norstedt G and Flores-Morales A: Gene expression profiling of the
effects of castration and estrogen treatment in the rat uterus.
Biol Reprod. 69:1308–1317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fernet M, Mégnin-Chanet F, Hall J and
Favaudon V: Control of the G2/M checkpoints after exposure to low
doses of ionising radiation: Implications for
hyper-radiosensitivity. DNA Repair (Amst). 9:48–57. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marples B, Wouters B, Collis S, Chalmers A
and Joiner MC: Low-dose hyper-radiosensitivity: a consequence of
ineffective cell cycle arrest of radiation-damaged G2-phase cells.
Radiat Res. 161:247–255. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Krueger SA, Wilson GD, Piasentin E, Joiner
MC and Marples B: The effects of G2-phase enrichment and checkpoint
abrogation on low-dose hyper-radiosensitivity. Int J Radiat Oncol
Biol Phys. 77:1509–1517. 2010. View Article : Google Scholar : PubMed/NCBI
|