Introduction
Ductal adenocarcinoma of the prostate (DAP) was
initially identified by Melicow and Patcher in 1967 (1). Initially, DAP was considered to arise
from a Müllerian remnant of the prostatic utricle verumontanum
(2). However, subsequent studies have
clearly confirmed the prostatic origin of DAP, which is supported
by a favorable response to orchiectomy, the expression of
prostatic-specific antigen (PSA) and ultrastructural findings
(3).
DAP is a rare subtype of prostate cancer (PCa) that
has a poorly-understood etiology, and more commonly occurs in
Caucasian males that are >70 years of age (4). A previous study that reviewed the
histological slides of 2,600 prostatic carcinomas reported a
prevalence of 0.4–0.8% for pure ductal adenocarcinoma and 5% for
mixed ductal adenocarcinoma (5).
According to the latest Surveillance, Epidemiology and End Results
cancer registry database, 693/731,262 patients (0.09%) with acinar
PCa have been identified as possessing ductal adenocarcinoma since
the 1970s (6).
DAP is even more rare in the Chinese population, and
few cases of DAP in Chinese men have been reported at present. The
present study reports the case of a Chinese male with DAP, and
reviews the relevant literature in order to summarize the clinical
features, histological characteristics and therapeutic strategies
for DAP.
Case report
In August 2011, a 55-year-old male presented to
Nanjing Drum Tower Hospital with progressive dysuria, with urgency
and frequency of urination, which had lasted for over a year. A
digital rectal examination revealed a slightly swollen prostate
gland, and no nodules or induration was detected. The laboratory
findings included a normal urinalysis, a serum total
prostate-specific antigen level of 0.27 ng/ml, a tumor marker
carbohydrate antigen 19-9 level of 67.47 units/ml and a
carcinoembryonic antigen (CEA) level of 14.40 ng/ml. The prostate
volume (27.6 ml) was calculated via the transabdominal ultrasound.
There was no requirement for a needle biopsy, according to the
National Comprehensive Cancer Network guidelines for PCa (7). The patient was diagnosed with benign
prostate hyperplasia initially and was scheduled for a
transurethral resection of the prostate (TURP). During the
cysto-urethroscopy, multiple gray fragile papillary lesions in the
region of the verumontanum were identified protruding at the
surface of the prostate. All visual lesions were resected.
Tissue samples were fixed in 10% neutral
formaldehyde solution (Sigma-Aldrich Shanghai Trading Co., Ltd.,
Shanghai, China), dehydrated, embedded in paraffin (Leica
Biosystems, Shanghai, China), and conventionally hematoxylin-eosin
stained. A microscopic examination (BX41; Olympus America, Center
Valley, PA, USA) of the specimen revealed that the majority of the
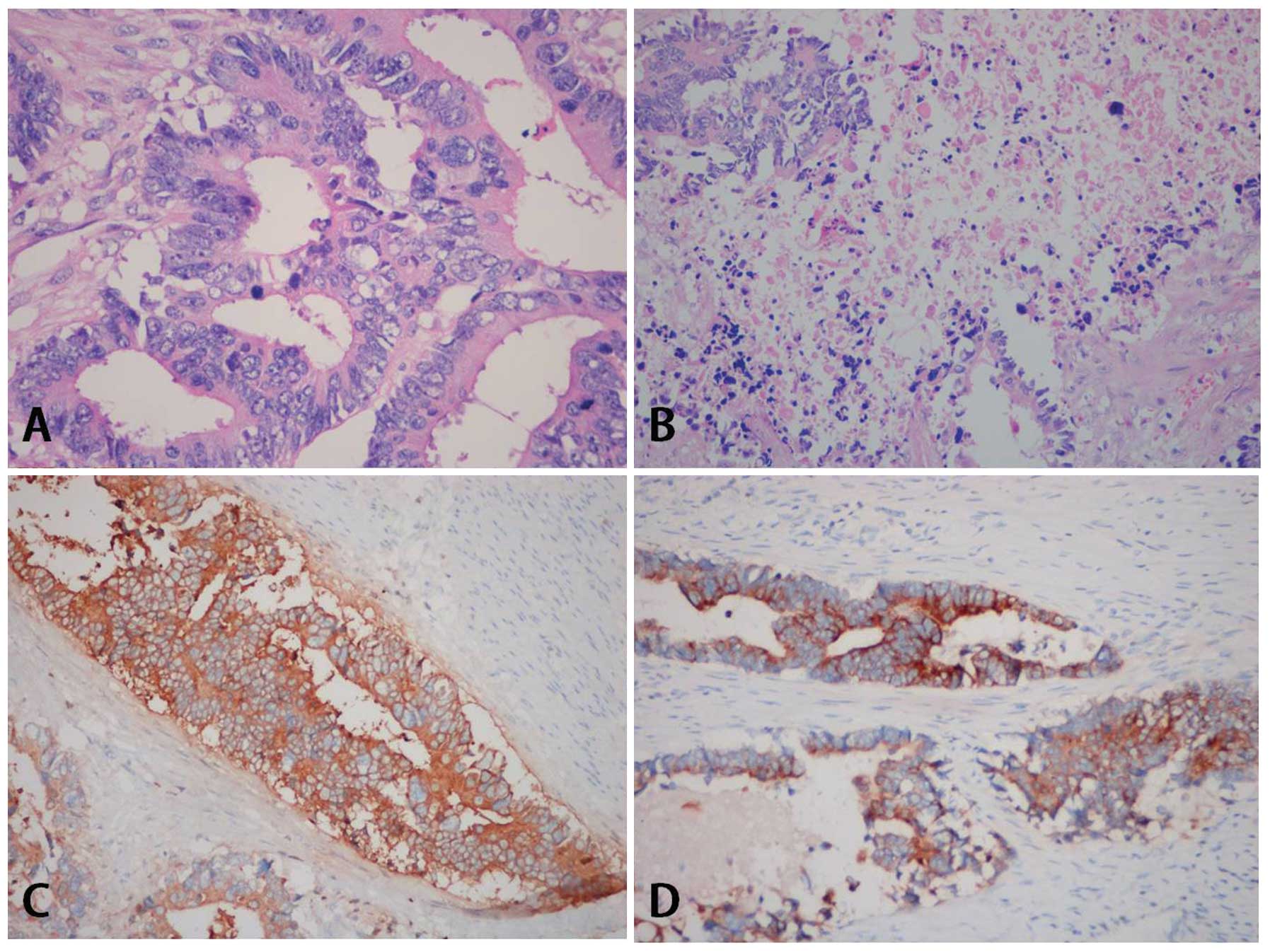
gland was affected. The major components of the tumor were composed
of tall pseudostratified columnar cells, with abundant eosinophilic
cytoplasm, prominent necrosis and stromal fibrosis (Fig. 1). The architectural pattern was
considered to be invasive glandular. The tumor cells were poorly
differentiated and assigned a Gleason score of 5+4. The
morphological appearance of the vascular and perineural invasion
was identified. Immunohistochemical staining was performed using
formalin-fixed paraffin-embedded tumor blocks. The results
indicated that the expression of PSA (clone 35H9; mouse anti-human
monoclonal antibody; dilution, 1:400; cat. no. PA0431; Novocastra;
Leica Microsystems, Ltd., Milton Keynes, UK), α methylacyl CoA
racemase (clone 13H4; rabbit anti-human monoclonal antibody;
dilution, 1:200; cat. no. M3616; Dako, Glostrup, Denmark) and Ki67
(clone UMAB107; mouse anti-human monoclonal antibody; dilution,
1:500; cat. no. UM800033; Origene Technologies, Inc., Rockville,
MD, USA), the focal expression of cytokeratin 7 (clone Ep16; rabbit
anti-human monoclonal antibody; dilution, 1:250; cat. no. AC-0020;
Epitomics; Abcam, Cambridge USA), cytokeratin 20 (clone Ep23;
rabbit anti-human monoclonal antibody; dilution, 1:200; cat. no.
AC-0026; Epitomics; Abcam) and CEA (clone COL-1; mouse anti-human
monoclonal antibody; dilution, 1:150; cat. no. CM058; Biocare
Medical, LLC., Concord, CA, USA), and no expression for p63 (clone
UMAB4; mouse anti-human monoclonal antibody; dilution, 1:300; cat.
no. UM500004; Origene Technologies, Inc.) and 34βE12 (clone 34βE12;
mouse anti-human monoclonal antibody; dilution, 1:300; cat. no.
M0630; Dako).
Subsequently, the patient underwent an ultrasonic
colonoscopy. During the procedure, three polypoid lesions were
identified in the sigmoid colon and descending colon regions.
Additionally, a semi-circle protrusion was detected at a 10 cm
distance from the anus. The mucous membrane of the rectum remained
intact. The lesions were biopsied and removed endoscopically. The
pathological findings indicated ductal adenoma associated with
low-grade intraepithelial neoplasia. A bone scan and chest and
abdomen computed tomography (CT) scans indicated no distant
metastases. Due to the long life expectancy of the patient and the
aggressive biological behavior of DAP, an exenteration of the total
pelvis was performed. The surgical procedure was successful. The
postsurgical specimens included the urinary bladder (6.0×5.0×4.5
cm), bilateral seminal vesicle (left, 3.0×1.5×1.0 cm; right,
2.0×1.0×1.0 cm), the entire prostate gland and the rectum (11 cm).
The final pathological diagnosis of the mass was stage IV DAP
(pT4N0M0). During the 40-month follow-up, the patient remained
under close surveillance and remained progression-free with no
evidence of local recurrence or distant metastasis.
Written informed consent was obtained from the
patient for the publication of the present study and accompanying
images.
Discussion
The diagnosis of DAP is based on cytological and
immunohistochemical findings. The classic morphology is
characterized by pleomorphic nuclei, abundant and usually
amphophilic cytoplasm, frequent mitoses and tall pseudostratified
columnar cells lined in particular architectural patterns,
including papillary, cribriform, solid and invasive glandular
(8). The Gleason score is the most
widely accepted grading system based on architectural patterns. DAP
is assigned a grade of at least 4+4, due to the aggressive
biological behavior and distinctive morphology (9). Regardless of variable serum PSA levels,
samples that exhibit DAP histology normally express PSA and
prostatic-specific acid phosphatase in immunostaining; this
supports the prostatic origin of DAP and provides evidence for the
differentiation of DAP tumors from other lesions. In addition,
focal expression of CEA, cytokeratin 7, cytokeratin 20 and caudal
type homeobox 2 have been reported in certain cases (5).
Although the clinical course of DAP remains
controversial, the majority of studies indicate that the variant of
PCa exhibits more aggressive biological behavior, a more advanced
stage, a worse 5-year survival rate and a shorter time to
progression compared with conventional acinar carcinoma (4,6).
Therapies for DAP include radical prostatectomy,
radiation therapy, hormone deprivation, chemotherapy and TURP. Once
an initial diagnosis is confirmed using TURP or a transrectal
ultrasound-guided needle biopsy, patients are advised to undergo a
clinical staging evaluation, including a computed tomography scan
of the abdomen and pelvis, bone scan and chest X-ray. If the
diagnostic examinations rule out distant metastasis, definitive
local therapies, including radical prostatectomies, pelvic lymph
node dissections and external beam radiotherapy, may be offered to
the patient. Notably, DAP is usually under-staged and appears to be
resectable prior to surgery; however, the majority of the patients
with DAP have already developed extracapsular invasion. Neoadjuvant
approaches prior to surgery are recommended for patients in order
to shrink the tumor volume and downstage the DAP. Patients are
recommended to receive androgen deprivation following definitive
therapies as DAP is prostatic in origin and androgen dependent. In
one previous study, radiation therapy was recommended as a more
adequate treatment option compared with radical surgery (10).
DAP is a rare entity of PCa. DAP may be challenging
to detect during the early stages of disease due to the lack of
distinctive clinical features, and is usually diagnosed at an
advanced stage. DAP is more likely to pursue an aggressive clinical
course and an unfavorable prognosis compared with other PCas. An
aggressive management strategy is recommended, even for patients
with metastatic disease.
References
|
1
|
Melicow MM and Pachter MR: Endometrial
carcinoma of proxtatic utricle (uterus masculinus). Cancer.
20:1715–1722. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carney JA and Kelalis PP: Endometrial
carcinoma of the prostatic utricle. Am J Clin Pathol. 60:565–569.
1973.PubMed/NCBI
|
|
3
|
Lee SS: Endometrioid adenocarcinoma of the
prostate: A clinicopathologic and immunohistochemical study. J Surg
Oncol. 55:235–238. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morgan TM, Welty CJ, Vakar-Lopez F, Lin DW
and Wright JL: Ductal adenocarcinoma of the prostate: Increased
mortality risk and decreased serum prostate specific antigen. J
Urol. 184:2303–2307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Epstein JI and Woodruff JM: Adenocarcinoma
of the prostate with endometrioid features. A light microscopic and
immunohistochemical study of ten cases. Cancer. 57:111–119. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meeks JJ, Zhao LC, Cashy J and Kundu S:
Incidence and outcomes of ductal carcinoma of the prostate in the
USA: Analysis of data from the Surveillance, Epidemiology, and End
Results program. BJU Int. 109:831–834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carroll PR, Parsons JK, Andriole G,
Bahnson RR, Barocas DA, Castle EP, Catalona WJ, Dahl DM, Davis JW,
Epstein JI, et al: Prostate Cancer Early Detection, Version 2.2015.
J Natl Compr Canc Netw. 13:1534–1561. 2015.PubMed/NCBI
|
|
8
|
Brinker DA, Potter SR and Epstein JI:
Ductal adenocarcinoma of the prostate diagnosed on needle biopsy:
Correlation with clinical and radical prostatectomy findings and
progression. Am J Surg Pathol. 23:1471–1479. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Epstein JI: An update of the Gleason
grading system. J Urol. 183:433–440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Orihuela E and Green JM: Ductal prostate
cancer: Contemporary management and outcomes. Urol Oncol.
26:368–371. 2008. View Article : Google Scholar : PubMed/NCBI
|