Introduction
Pseudolaric acid B (PAB) is a diterpene acid
isolated from the root and trunk bark of Pseudolarix
kaempferi Gord. (Pinaceae; also known as Tu Jing Pi in
traditional Chinese medicine), and has been used to treat
dermatological fungal infections (1).
PAB exerts potent antifungal (1),
antifertility (2) and cytotoxic
activities in vitro (3).
Breast cancer is the most common invasive cancer in women
worldwide. Surgery, medication and radiation may be used in the
treatment of breast cancer; thus it typically has a favorable
prognosis (4,5). Our previous study demonstrated that
treatment of MCF-7 human breast cancer cells with PAB led to the
induction of mitotic arrest and apoptosis in the majority of cells,
while the surviving cells became senescent (6); however, the mechanism by which these
cells were directed towards either cell death or survival has not
yet been determined.
Programmed cell-death may be achieved through two
distinct processes (7). Apoptosis
(type I) is typically characterized by a series of morphological
events, including cell shrinkage, DNA fragmentation and the
formation of membrane-bound apoptotic bodies that are rapidly
phagocytosed by neighboring cells (8). By contrast, autophagy (type II), which
may contribute to cell death or cell survival, is characterized by
the appearance of autophagosomes that engulf bulk cytoplasm and
cytosolic organelles, such as mitochondria and endoplasmic
reticulum. Lysosomes fuse with the autophagic vesicles, leading to
degradation of their cargo. Autophagy recycles cellular material
for survival; however, its continuation leads to organelle
degradation and ultimately cell death (9–13). The
association between autophagy and apoptosis is complex and varies
between different cell types and cellular stress conditions.
Autophagy and apoptosis may facilitate or inhibit each other to
execute alternative functions in the cell (14–16).
The present study aimed to elucidate the role of
autophagy in PAB-induced cell death in the MCF-7 cell line prior to
the onset of cellular senescence.
Materials and methods
Materials
PAB and dracorhodin perchlorate (DP; a synthetic
analogue of the antimicrobial anthocyanin red pigment dracorhodin,
used as positive control for mitochondrial membrane analysis) were
purchased from the National Institute for the Control of
Pharmaceutical and Biological Products (Beijing, China) and
dissolved in dimethyl sulfoxide (DMSO) to create stock solutions
(10 mM). The DMSO concentration was maintained at <0.1% in all
cell cultures and did not exert any detectable effect on cell
growth. The Senescence Detection Kit was purchased from EMD
Millipore (Billerica, MA, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
monodansylcadaverine (MDC), acridine orange, rhodamine 123, and
3-methyladenine (3-MA) were purchased from Sigma-Aldrich (St.
Louis, MO, USA). Polyclonal rabbit anti-human Beclin-1
(#11306-1-AP) and B-cell lymphoma 2 (Bcl-2; #12789-1-AP) IgG
antibodies, and monoclonal mouse anti-human microtubule-associated
protein 1 light chain 3 (LC3) IgG1 antibody
(#66139-1-Ig) were purchased from ProteinTech Group, Inc. (Chicago,
IL, USA). Monoclonal mouse anti-human β-actin (#sc-8432) and
α-tubulin (#sc-23948) IgG1, and alkaline phosphatase
(AP)-labelled rabbit anti-mouse (#sc-358915) and goat anti-rabbit
(#sc-2057) IgG antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Protein A Sepharose
CL-4B was purchased from GE Healthcare Life Sciences (Tokyo,
Japan).
Cell culture
MCF-7 human breast cancer cells were obtained from
the American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in RPMI-1640 medium (GE Healthcare Life Sciences,
Logan, UT, USA) supplemented with 10% fetal calf serum
(heat-inactivated, 56°C for 30 min), 2 mM glutamine (both Gibco;
Thermo Fisher Scientific, Carlsbad, CA, USA), penicillin (100,000
U/l; Sigma-Aldrich) and streptomycin (100 mg/l; Amresco, Solon, OH,
USA), and maintained at 37°C in a 5% CO2 humidified
atmosphere. Cells were treated with various concentrations of PAB
dissolved in DMSO, with a final DMSO concentration <0.1%, or
with DMSO alone. DMSO-treated cells were used as a control. PAB
doses and incubation times were selected based on a previous study
(6).
Senescence-associated
(SA)-β-galactosidase detection
The MCF-7 cells (1.5×105 cells/well) were
seeded into 24-well culture plates (Nalge Nunc International,
Penfield, NY, USA). Following incubation overnight, 4 µM PAB was
added and the plates were incubated for 3 days. The senescence
detection kit was used according to the manufacturer's
instructions, as previously described (6). Finally, the cells were observed under a
phase contrast microscope (Olympus IX51; Olympus Corporation,
Tokyo, Japan) for the development of a blue color.
MDC staining observed by fluorescence
microscopy and flow cytometry
The fluorescent compound MDC is used as a tracer for
autophagic vacuoles (17), and 3-MA
is the most commonly used inhibitor of autophagic sequestration
(18). MCF-7 cells (6×105
cells/well) were seeded into 6-well culture plates (Nunc, Denmark).
Following overnight incubation, the cells were treated with 4 µM
PAB and/or 3 mM 3-MA for 36 h, and subsequently incubated with 0.05
mM MDC at 37°C for 1 h. Following incubation, the cells were washed
once with phosphate-buffered saline (PBS). Intracellular MDC was
observed by fluorescence microscopy at an excitation wavelength of
380 nm with an emission filter of 525 nm (Olympus CKX31; Olympus
Corporation) and analyzed using a FACScan flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA).
Autophagy observed by acridine orange
staining
MCF-7 cells (6×105 cells/well) were
seeded into 6-well culture plates containing 18×18-mm coverslips,
and treated with 0 or 4 µM PAB for 36 h, then rinsed and stained
with acridine orange (10 mg/l) at 37°C for 30 min. After the
coverslips were mounted, the samples were observed using a
fluorescence microscope (Olympus CKX31; Olympus Corporation) to
assess the color change from green to red.
Cell growth inhibition assay
Inhibition of cell growth was determined using an
MTT assay. MCF-7 cells (1.5×104 cells/well) were seeded
into 96-well culture plates (Nalge Nunc International). Following
incubation overnight, 4 µM PAB and/or 3 mM 3-MA were added to the
plates After an incubation of 36 h, cell growth was measured by
further incubation with MTT solution at 37°C for 3 h, followed by
the addition of DMSO (150 µl) to dissolve the formazan crystals.
Absorbance was measured at 492 nm (A492) with an
enzyme-linked immunosorbent assay plate reader (iMark™ Microplate
Reader; Bio-Rad Laboratories Inc., Hercules, CA, USA). The
percentage of inhibition was calculated as follows: Cell death (%)
= A492 [(control - sample)/control] × 100%.
Observation of morphological changes
by light microscopy
MCF-7 cells were treated with PAB (0, 1, 4 or 8 µM)
and/or 3-MA (3 mM) for 36 h. The morphological changes were
observed by phase contrast microscopy (Olympus IX51; Olympus
Corporation).
Western blot protein expression
analysis
MCF-7 cells were treated with 4 µM PAB for 36 h, and
adherent and floating cells were collected and stored at −80°C.
Western blot analysis was performed for the total proteins, as
previously described (19). Cells
were lysed directly in sodium dodecyl sulfate (SDS) sample buffer
(60 mM Tris-HCl, pH 6.8; 2% SDS; 10% glycerol; 5%
2-mercaptoethanol; 0.01% bromophenol blue), followed by boiling for
10 min. Whole-cell lysates were further subjected to
SDS-polyacrylamide gel electrophoresis. Proteins were transferred
to nitrocellulose membranes (Bio-Rad Laboratories, Inc.) and
detected with corresponding primary antibodies against LC3A/B
(dilution, 1:500); Beclin-1 antibody (1:1,000), Bcl-2 antibody
(1:1,000), β-actin antibody (1:2,000), and α-Tubulin antibody
(1:2,000), incubated overnight at 4°C. Subsequently, the membranes
were incubated with AP-conjugated anti-mouse or anti-rabbit
secondary antibodies (1:1,000) for 2 h at room temperature. The
membranes were then reacted with
5-bromo-4-chloro-39-indolylphosphate and nitro-blue tetrazolium
substrate (Sigma-Aldrich).
Mitochondrial membrane potential
analysis by rhodamine 123 staining
MCF-7 cells were harvested and rinsed with PBS. The
cells were then stained with 5 µg/ml of rhodamine 123 at 37°C for
30 min. Following incubation, the cells were washed once with PBS.
The intensity of rhodamine 123 staining was observed by
fluorescence microscopy at an excitation wavelength of 505 nm with
an emission filter of 534 nm (Olympus CKX31; Olympus Corporation)
and analyzed using a FACScan flow cytometer.
Immunoprecipitation analysis of Bcl-2
protein complexes
MCF-7 cells (1×106 cells/bottle) were
cultured in a 25-ml culture bottle overnight. Subsequently, the
cells were treated with 4 µM PAB for 36 h. Immunoprecipitation
analysis was conducted as described in a previous study (20). Briefly, radioimmunoprecipitation assay
buffer [50 mM Tris-HCl; 1% NP-40; 0.25% sodium deoxycholate; 150 mM
NaCl; 1 mM EDTA; 1 mM phenylmethylsulfonylfluoride; 1 mM sodium
orthovanadate (Na3VO4); 1 mM NaF; 1 µg/ml
aprotinin; 1 µg/ml leupeptin; 1 µg/ml pepstanin] was used for cell
lysis. Subsequently, Bcl-2 antibody (dilution, 1:100) was added to
precipitate Bcl-2 and the Bcl-2-binding protein Beclin-1 with
Protein A Sepharose beads. Finally western blotting was used to
analyze the expression of Bcl-2 and Beclin-1 (dilution,
1:1,000).
Statistical analysis
All data represent at least three independent
experiments and are expressed as the mean ± standard deviation.
Statistical comparisons were performed using the Student's t-test
through Microsoft Excel 2007 (Microsoft, Franklin, TN, USA.
P<0.05 was considered to represent a statistically significant
difference.
Results
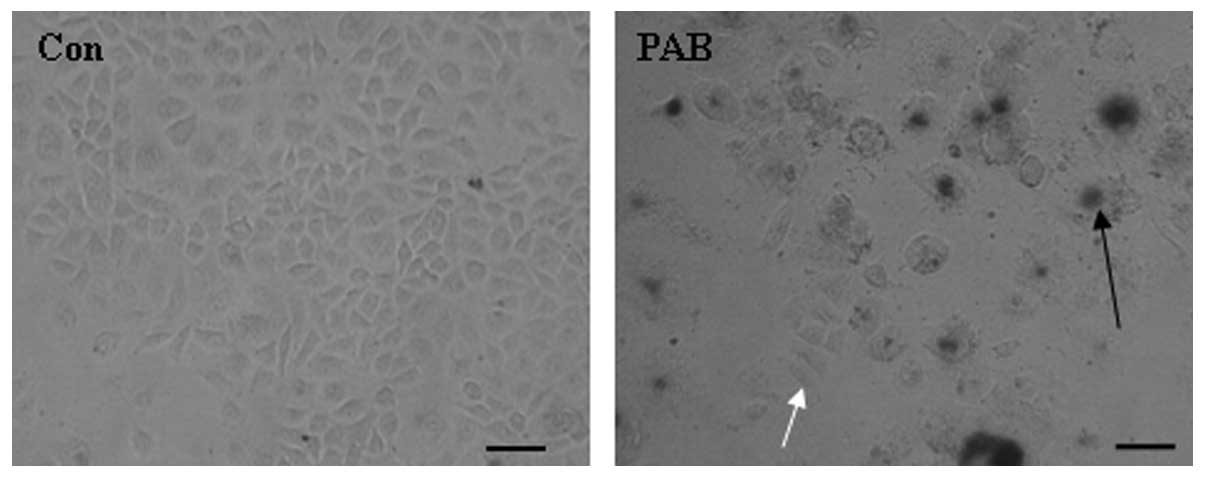
PAB does not induce complete tumor
cell death over a period of 3 days
MCF-7 cells were treated with 4 µM PAB for 3 days
and senescence was monitored using a senescence detection kit. The
results of this assay demonstrated that larger cells were positive
(Fig. 1, black arrow) for the
senescence marker SA-β-galactosidase, indicating that larger cells
became senescent following PAB treatment. In addition, a number of
cells retained a similar morphology to control cells and stained
negative for SA-β-galactosidase (Fig.
1). Therefore, a number of MCF-7 cells were able to resist
PAB-induced cell death and senescence at this time point.
PAB induces autophagy
Previous studies have demonstrated that PAB
treatment induces apoptosis in breast cancer cells (3,6,21). However, the present study demonstrated
that certain cells survived treatment with PAB, therefore, the
mechanism by which cell survival is promoted following PAB
treatment was investigated. Western blot analysis of MCF-7 cells
following 36 h of PAB treatment revealed increased protein
expression of Beclin-1 and enhanced conversion of LC3-I to LC3-II
(Fig. 2A). Fluorescence microscopy of
PAB-treated MCF-7 cells demonstrated the appearance of bright green
dots from MDC staining and a color change from green to red
following acridine orange staining, compared with the untreated
control group at 36 h (Fig. 2B and
C). Therefore, PAB induced autophagy in MCF-7 cells.
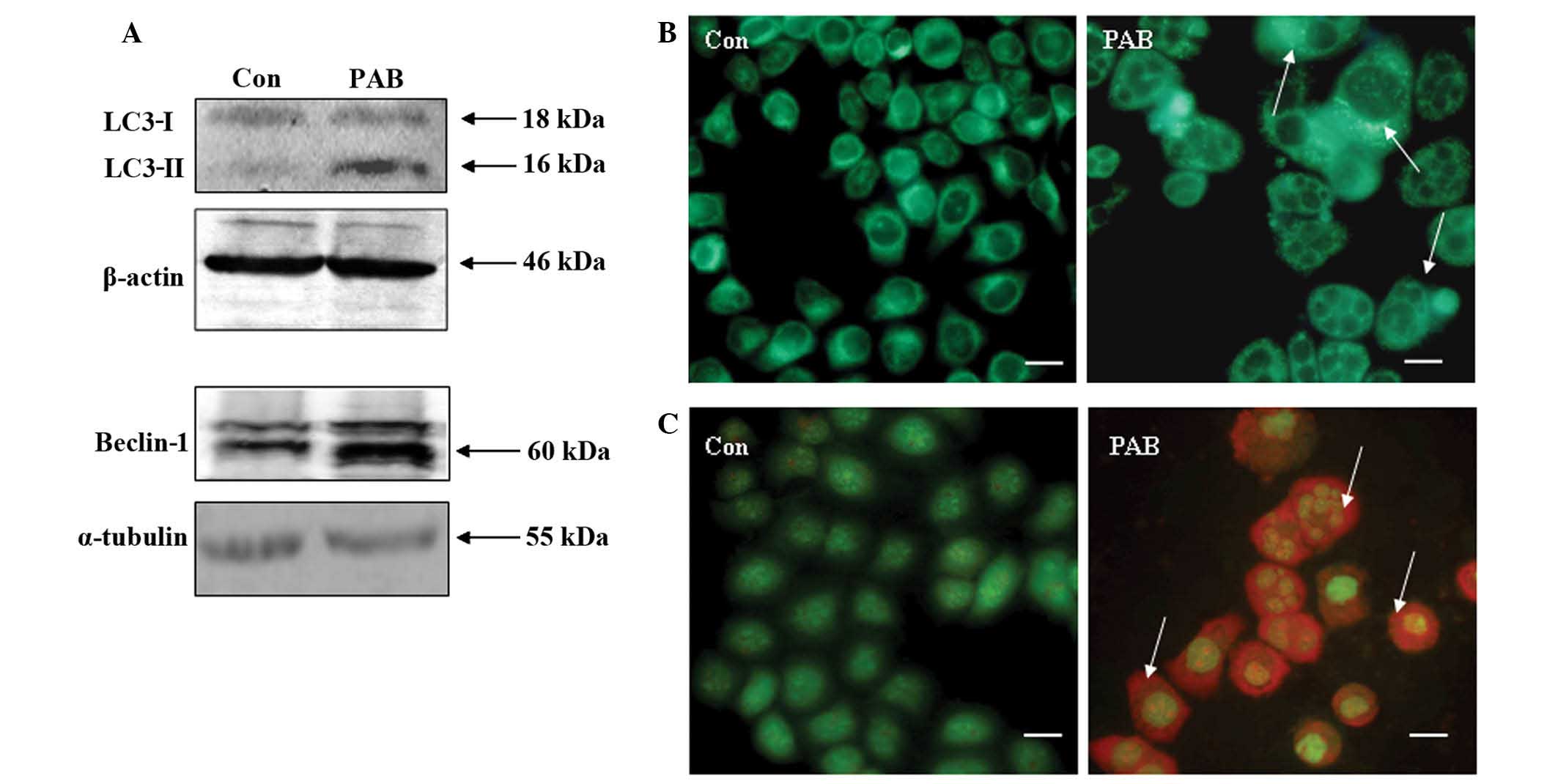 | Figure 2.Autophagy was induced in MCF-7 cells
at 36 h following PAB (4 µM) treatment. (A) Expression of LC3-I,
LC3-II and Beclin-1 was detected by western blot analysis, using
β-actin/α-tubulin as the loading control. PAB was found to promote
the conversion from LC3-I to LC3-II, and to increase the expression
of Beclin-1 compared with the Con group. (B) Monodansylcadaverine
staining of autophagic vacuoles, revealing that PAB increased the
number of autophagic vacuoles compared with the Con group (arrows
indicate positive staining; scale bar, 15 µm). (C) Acridine orange
staining, revealing that PAB increased the number of autophagic
vacuoles compared with the Con group (arrows indicate red positive
staining; scale bar, 15 µm). All experiments were performed in
triplicate. PAB, pseudolaric acid B, LC3, light chain 3; Con,
control. |
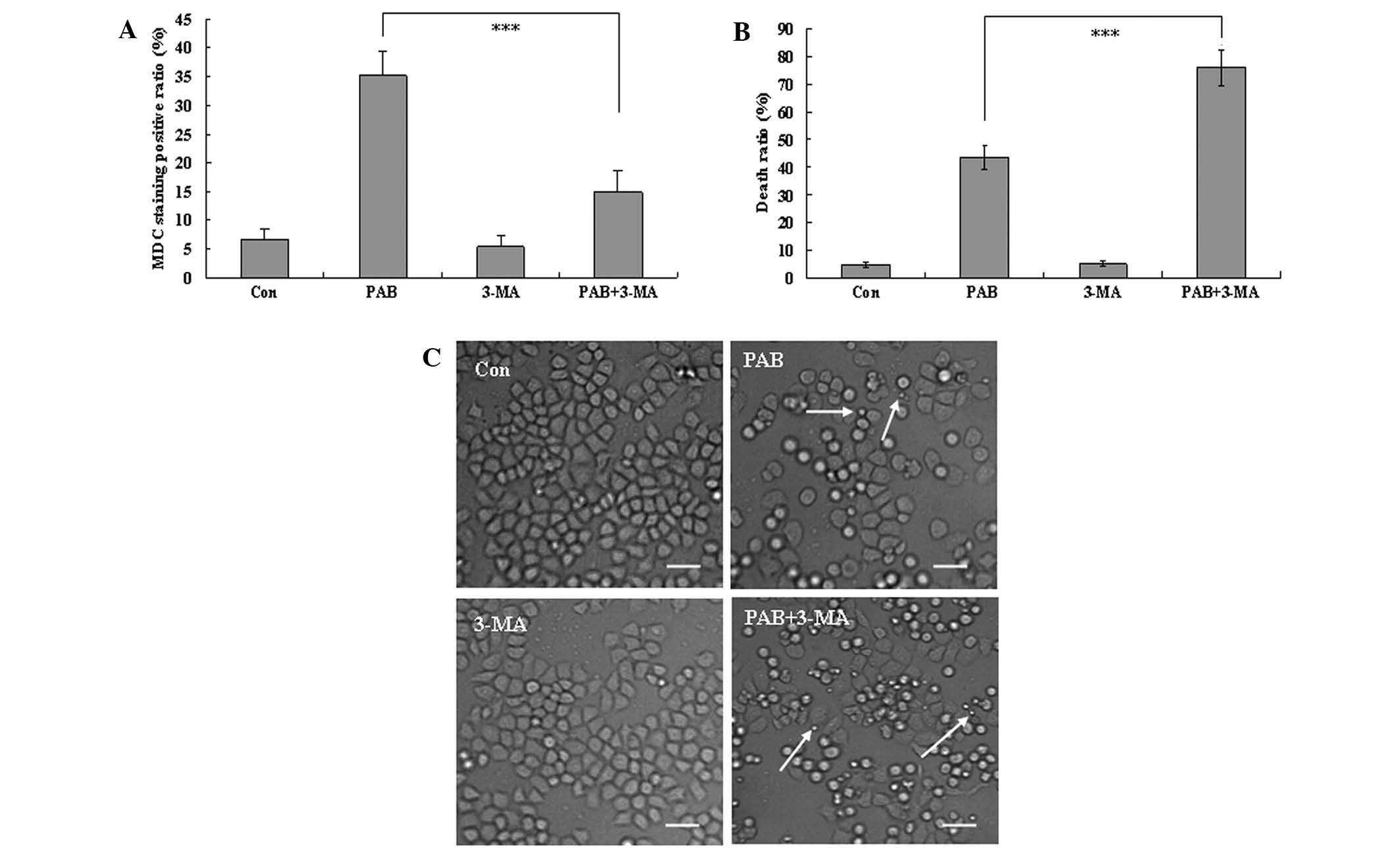
Autophagy promotes cell survival
At 36 h, the autophagic ratio of PAB-treated cells
was 35.30±4.12%; however, in combination with 3-MA treatment, the
autophagic ratio of PAB-treated cells was 14.87±3.73% (P<0.001;
Fig. 3A). The MTT assay demonstrated
that the apoptotic ratio of PAB-treated cells was 43.56±4.36%;
while the apoptotic ratio of PAB and 3-MA-treated cells was
76.12±6.46% (P<0.001; Fig. 3B).
Therefore, 3-MA significantly increased the inhibitory effect of
PAB. To confirm the results, morphological changes were observed by
phase contrast microscopy. A decrease in the total cell number, an
increase in floating cells, and the appearance of apoptotic bodies
were observed at 36 h after 4 µM PAB treatment. PAB and 3-MA
combination treatment resulted in a further decrease in the total
cell number, and a further increase in the number of floating cells
and apoptotic bodies. Therefore, 3-MA and PAB promoted the
induction of cell death compared with PAB treatment alone (Fig. 3C), and autophagy appears to promote
cell survival.
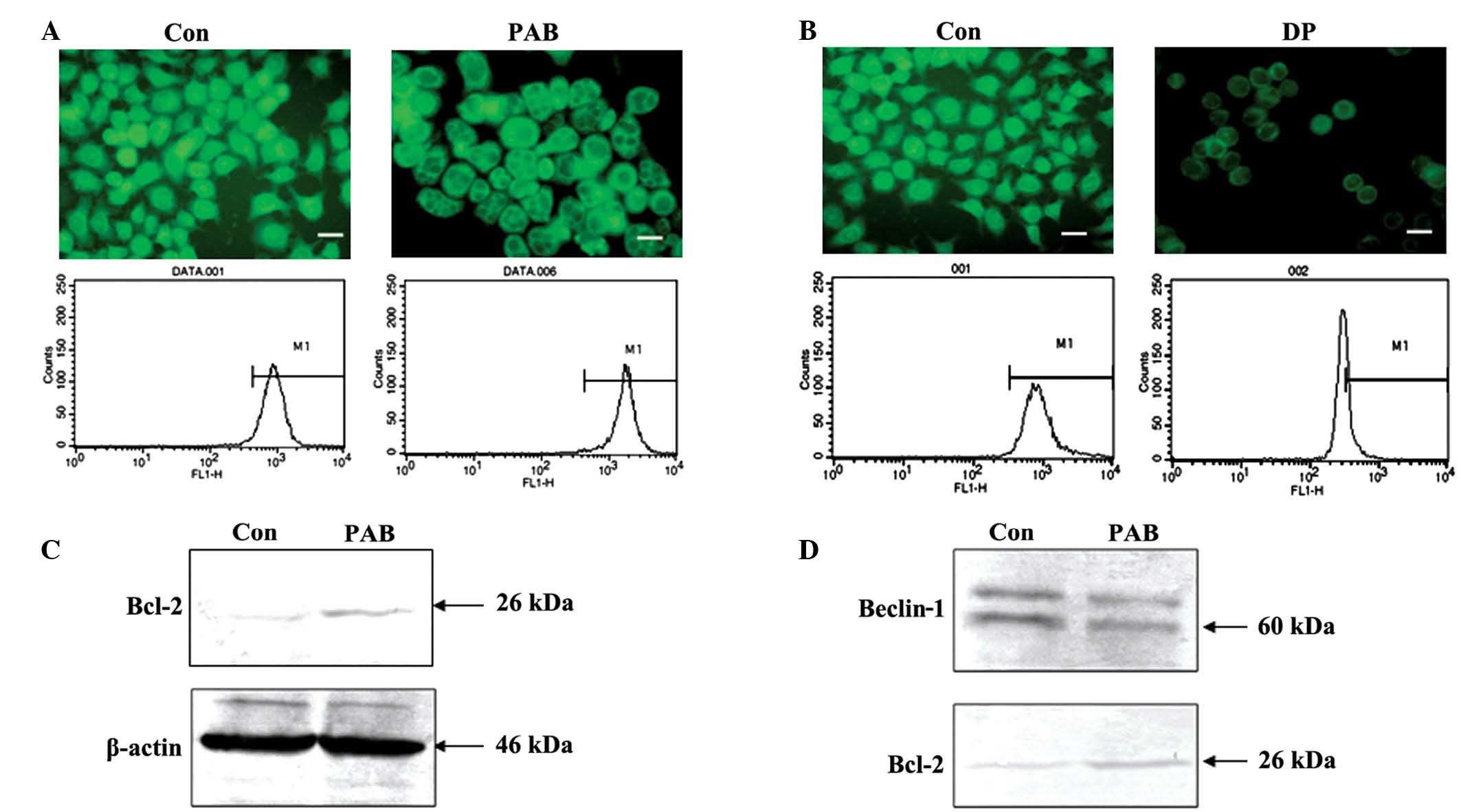
PAB does not affect the mitochondrial
membrane potential, and inhibits the binding of Bcl-2 and
Beclin-1
Decreased mitochondrial membrane potential leads to
cell death (22,23). In the present study, fluorescence
microscopic results demonstrated that the density of green
fluorescence from rhodamine 123 staining following PAB treatment
was same as control group, and flow cytometric analyses revealed
that fluorescence density following rhodamine 123 staining did not
differ between the PAB treatment group and the control group
(P>0.05); thus PAB did not affect the mitochondrial membrane
potential following 36 h of treatment (4 µM; Fig. 4A). However, following 12 h of
treatment with 60 µM DP, the mitochondrial membrane potential
decreased, indicating that the current method for analyzing the
mitochondrial membrane was viable (Fig.
4B). Expression of Bcl-2 was investigated with regard to the
mitochondrial membrane potential. PAB treatment was found to
increase Bcl-2 expression (Fig. 4C).
Therefore, it is possible that increased Bcl-2 sustained the
mitochondrial membrane potential. It has been previously reported
that the binding of Bcl-2 and Beclin-1 is important for promoting
autophagy (20). The current
immunoprecipitation analysis indicated that, following PAB
treatment, the level of Beclin-1 bound with Bcl-2 was reduced;
however, PAB increased Bcl-2 expression (Fig. 4D). Therefore, the lack of change in
mitochondrial membrane potential following PAB treatment, may be
associated with autophagy.
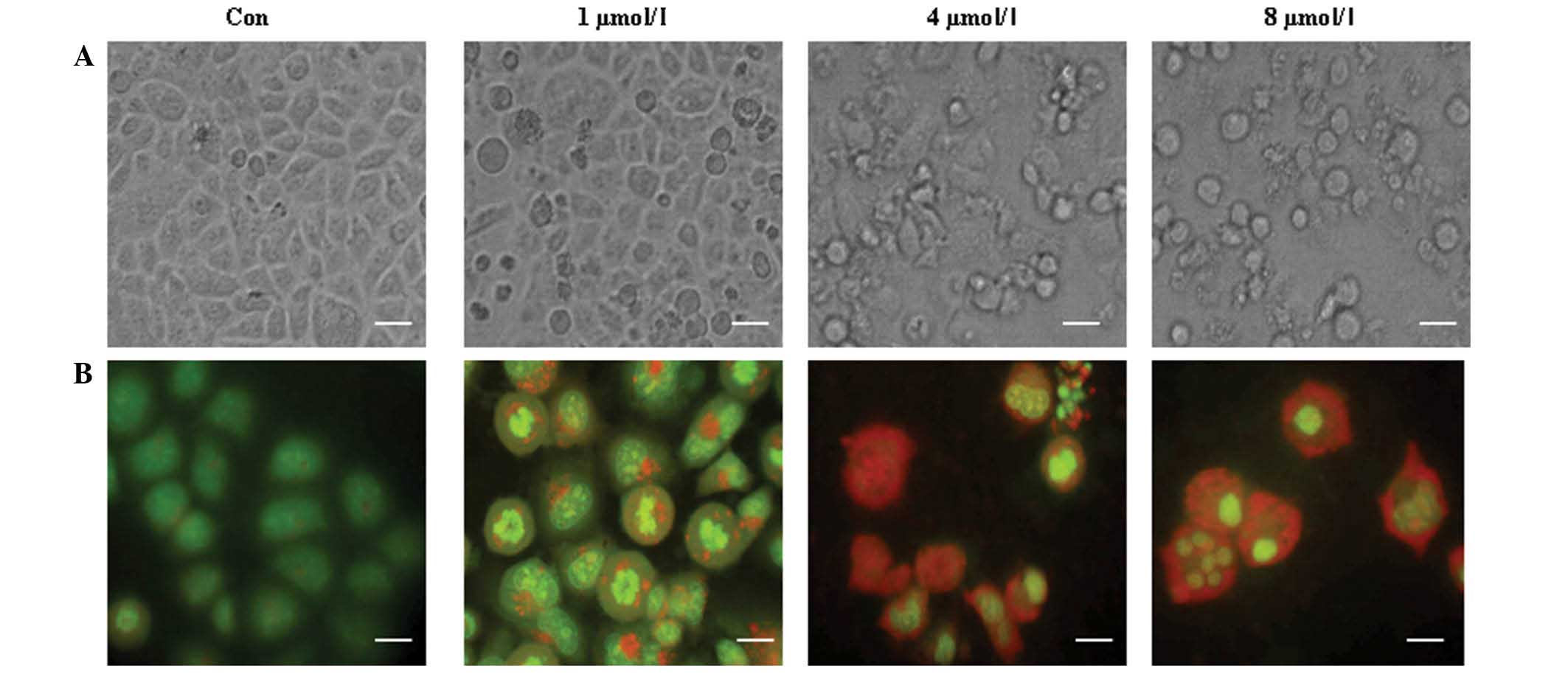
PAB increased autophagy and apoptosis
in a dose-dependent manner
To confirm whether autophagy is associated with cell
death in MCF-7 cells, different doses of PAB (0, 1, 4 or 8 µM) were
used in an attempt to further stimulate autophagy. The appearance
of increasing cell morphological changes, including floating cells
and apoptotic bodies (Fig. 5A),
indicated that cell apoptosis was increased with an increase of PAB
dose. Meanwhile, a color change from green to red was observed with
increasing PAB doses (Fig. 5B),
indicating that autophagy was also increased. Therefore the results
demonstrated a concurrent increase in apoptosis and autophagy when
the dose of PAB was increased.
Discussion
A previous study demonstrated that PAB-induced
apoptosis kills tumor cells; however, not all tumor cells were
killed within a short time period (48 h), whereas over a longer
period (≥72 h) a proportion of cells became senescent and certain
cells exhibited normal morphology following PAB treatment (7). Thus, the present study investigated why
certain cells undergo apoptotic cell death and why other cells
survive following PAB treatment.
The current study initially investigated whether
autophagy occurred in PAB-treated cells to sustain cell survival by
recycling cell material. The results indicated that PAB increased
the expression of Beclin-1 and promoted the conversion of LC3-I to
LC3-II. Furthermore, there was an increase in positive MDC and
acridine orange staining (a marker of autophagy), whereas the
autophagy inhibitor 3-MA abolished PAB-induced autophagy.
To confirm whether autophagy promotes cell survival,
autophagy was inhibited with 3-MA, demonstrating that the ratio of
cell death of PAB increases following treatment of 3-MA. This
finding indicated that, in PAB-treated MCF-7 cells, inhibition of
autophagy promotes cell death, thus providing an explanation for
how cells could survive following PAB treatment. In previous study,
the phenomena of inhibition of autophagy promoting cell death was
also found in the murine fibrosarcoma L929 cell line (20). This is consistent with the current
study; thus, inhibition of autophagy promoting cell death following
PAB treatment appears to be a common characteristic, regardless of
cell type and cell origin.
To further investigate the association between
autophagy and cell death, the dose of PAB was increased, with the
aim of increasing autophagy. The results demonstrated that
autophagy increases with a concurrent increase in the dose of PAB.
However, cell death was also increased. Therefore, we hypothesize
that: i) Autophagy and cell death are activated by the same factor
that is upregulated by PAB treatment, and ii) cell death is
accompanied by autophagy, which decreases the effects of PAB.
Therefore, combined application of PAB with an inhibitor of
autophagy may increase the effectiveness of PAB treatment, which is
important for the clinical application of PAB in breast cancer.
The mitochondrial membrane potential typically
decreases during cell death (22,23);
however, the present study demonstrated that PAB did not affect
mitochondrial membrane potential. This may explain why certain
cells may survive following PAB treatment. Bcl-2 is associated with
mitochondrial membrane potential and autophagy (20), and the present study demonstrated that
expression of Bcl-2 increases following PAB treatment, possibly
stabilizing the mitochondrial membrane potential. Furthermore, it
has been reported that Bcl-2 inhibits autophagy through direct
binding with Beclin-1 (24). By
contrast, the PAB-induced Bcl-2 expression observed in the present
study was also accompanied by enhanced autophagy. Therefore, the
binding of Bcl-2 with Beclin-1 was analyzed, and the results
demonstrated that the binding of Bcl-2 with Beclin-1 was inhibited
following PAB treatment. Thus, we hypothesize that Bcl-2 may
stabilize the mitochondrial membrane potential through binding with
Bax or other proteins that decrease mitochondrial membrane
potential and, consequently, is unable to bind with Beclin-1 to
participate in autophagy.
In conclusion, the results of the current study
demonstrate that autophagy protected breast cancer cells from
death, thus, decreasing the anti-tumor effects of PAB. Considering
our previous study using the L929 cell line (20), it is concluded that the development of
PAB as anti-tumor medicine must include combination with an
autophagy inhibitor. Although it remains unclear which other
anti-tumor medicines are best able to inhibit autophagy to increase
the effect of PAB, the current study provides a direction for
research into medicine combination.
Acknowledgements
This study was supported in part by funded by grants
from Jilin Provincial Science and Technology Department (no.
20140204004YY), National Natural Science Foundation of China (no.
81301416), the Chinese Ministry of Science and Technology (nos.
2012CB911100 and 2013ZX10001005), State Grade III Laboratory of
Traditional Chinese Medicine, Immunology and Molecular Biology
Laboratory and the Chinese Ministry of Education (no. IRT1016), and
the Key Laboratory of Molecular Virology of Jilin Province (no.
20102209).
References
|
1
|
Li E, Clark AM and Hufford CD: Antifungal
evaluation of pseudolaric acid B, a major constituent of
Pseudolarix kaempferi. J Nat Prod. 58:57–67. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang WC, Lu RF, Zhao SX and Gu ZP:
Comparison of early pregnancy-terminating effect and toxicity
between pseudolaric acids A and B. Zhongguo Yao Li Xue Bao.
9:445–448. 1988.(In Chinese). PubMed/NCBI
|
|
3
|
Pan DJ, Li ZL, Hu CQ, Chen K, Chang JJ and
Lee KH: The cytotoxic principles of Pseudolarix kaempferi:
Pseudolaric acid-A and -B and related derivatives. Planta Med.
56:383–385. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lv M, Lv M, Chen L, Qin T, Zhang X, Liu P
and Yang J: Angiomotin promotes breast cancer cell proliferation
and invasion. Oncol Rep. 33:1938–1946. 2015.PubMed/NCBI
|
|
5
|
Tarasewicz E and Jeruss JS:
Phospho-specific Smad3 signaling Impact on breast oncogenesis. Cell
Cycle. 11:2443–2451. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu JH, Cui Q, Jiang YY, Yang W, Tashiro S,
Onodera S and Ikejima T: Pseudolaric acid B induces apoptosis,
senescence, and mitotic arrest in human breast cancer MCF-7. Acta
Pharmacol Sin. 28:1975–1983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bursch W, Grasl-Kraupp B, Ellinger A,
Török L, Kienzl H, Müllauer L and Schulte-Hermann R: Active cell
death: Role in hepatocarcinogenesis and subtypes. Biochem Cell
Biol. 72:669–675. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bursch W: The autophagosomal-lysosomal
compartment in programmed cell death. Cell Death Differ. 8:569–581.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Duve C and Wattiaux R: Functions of
lysosomes. Annu Rev Physiol. 28:435–492. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edinger AL and Thompson CB: Death by
design: Apoptosis, necrosis and autophagy. Curr Opin Cell Biol.
16:663–669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klionsky DJ: The molecular machinery of
autophagy: Unanswered questions. J Cell Sci. 118:7–18. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leist M and Jäättelä M: Four deaths and a
funeral: From caspases to alternative mechanisms. Nat Rev Mol Cell
Biol. 2:589–598. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giansanti V, Torriglia A and Scovassi AI:
Conversation between apoptosis and autophagy: ‘Is it your turn or
mine?’. Apoptosis. 16:321–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lindqvist LM and Vaux DL: BCL2 and related
prosurvival proteins require BAK1 and BAX to affect autophagy.
Autophagy. 10:1474–1475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lockshin RA and Zakeri Z: Apoptosis,
autophagy, and more. Int J Biochem Cell Biol. 36:2405–2419. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Biederbick A, Kern HF and Elsässer HP:
Monodansylcadaverine (MDC) is a specific in vivo marker for
autophagic vacuoles. Eur J Cell Biol. 66:3–14. 1995.PubMed/NCBI
|
|
18
|
Seglen PO and Gordon PB: 3-Methyladenine:
Specific inhibitor of autophagic/lysosomal protein degradation in
isolated rat hepatocytes. Proc Natl Acad Sci USA. 79:1889–1892.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Wu Y, Cheng Y, Zhao Z, Tashiro S,
Onodera S and Ikejima T: Fas-mediated autophagy requires JNK
activation in HeLa cells. Biochem Biophys Res Commun.
377:1205–1210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu J, Li X, Tashiro S, Onodera S and
Ikejima T: Bcl-2 family proteins were involved in pseudolaric acid
B-induced autophagy in murine fibrosarcoma L929 cells. J Pharmacol
Sci. 107:295–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu JH, Wang HJ, Li XR, Tashiro S, Onodera
S and Ikejima T: Protein tyrosine kinase, JNK, and ERK involvement
in pseudolaric acid B-induced apoptosis of human breast cancer
MCF-7 cells. Acta Pharmacol Sin. 29:1069–1076. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cregan SP, Dawson VL and Slack RS: Role of
AIF in caspase-dependent and caspase-independent cell death.
Oncogene. 23:2785–2796. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui Q, Yu JH, Wu JN, Tashiro S, Onodera S,
Minami M and Ikejima T: P53-mediated cell cycle arrest and
apoptosis through a caspase-3-independent, but caspase-9-dependent
pathway in oridonin-treated MCF-7 human breast cancer cells. Acta
Pharmacol Sin. 28:1057–1066. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng P, Ni Z, Dai X, Wang B, Ding W,
Smith Rae A, Xu L, Wu D, He F and Lian J: The novel BH-3 mimetic
apogossypolone induces Beclin-1- and ROS-mediated autophagy in
human hepatocellular carcinoma cells. Cell Death Dis. 4:e4892013.
View Article : Google Scholar : PubMed/NCBI
|