Introduction
Intramuscular hemangioma (IMH) of the head and neck
region is extremely rare, accounting for <1% of all cases of
hemangioma in Caucasian populations (1). In America, IMHs accounted for ~0.8% of
all benign vascular neoplasms in 1940 (1), and in 1975 13.8% of IMHs were observed
in the head and neck region (2). The
masseter muscle is the most common region, followed by the
trapezius and sternocleidomastoid muscle (3). IMHs predominantly occur before the age
of 30 years without gender predominance (4); however, intramasseteric IMH is
male-dominated (3). There are two
major hypotheses for IMH: The first is the hereditary type and the
second type is trauma-related (4).
The presenting symptoms and signs include gradually enlarging
lesions leading to cosmetic problems and certain IMHs are
associated with pain (3). Due to the
location and characteristics of IMHs, it is difficult to make a
correct diagnosis prior to surgery (5). IMH is often misdiagnosed as salivary
neoplasms. Other differential diagnoses include cysts,
lymphangiomas, rhabdomyosarcomas, masseteric hypertrophy and
schwannomas (3). The treatment is
variable, including cryotherapy, radiotherapy and steroid injection
(4,6);
however, surgery is the primary treatment strategy (3,7,8). When intramasseteric IMHs are located
proximal to the facial nerve, an approach of superficial
parotidectomy with facial nerve preservation and masseter muscle
excision is generally adopted (3,4,7,8). In
consideration of local recurrence, total excision of the masseter
muscle may be recommended (3).
However, total extirpation of a suitable intramasseteric IMH with
functional preservation of the facial nerve and masseter muscle may
be achieved via a transcervical approach, which shortens the
duration of the operation and hospital stay, and also significantly
improves cosmesis. The current study presents the case of a
57-year-old male with intramasseteric IMH who underwent surgery via
the less invasive route. In addition, available literature
regarding the clinical radiology, pathology and treatment of
intramasseteric IMH are reviewed. Written informed consent was
obtained from the patient.
Case report
In July 2011, a 57-year-old male with no previous
medical history presented to the outpatient department at Taipei
City Hospital Renai Branch (Taipei City, Taiwan) with a painful
mass on the right side of the face, which had been present for ~6
months. Physical examination identified a 3×3 cm, movable mass in
the right cheek, located just above the mandible. The mass became
firmer upon forceful mastication. Nasopharyngoscopy revealed a
smooth nasopharynx and no evidence of tumor in the upper
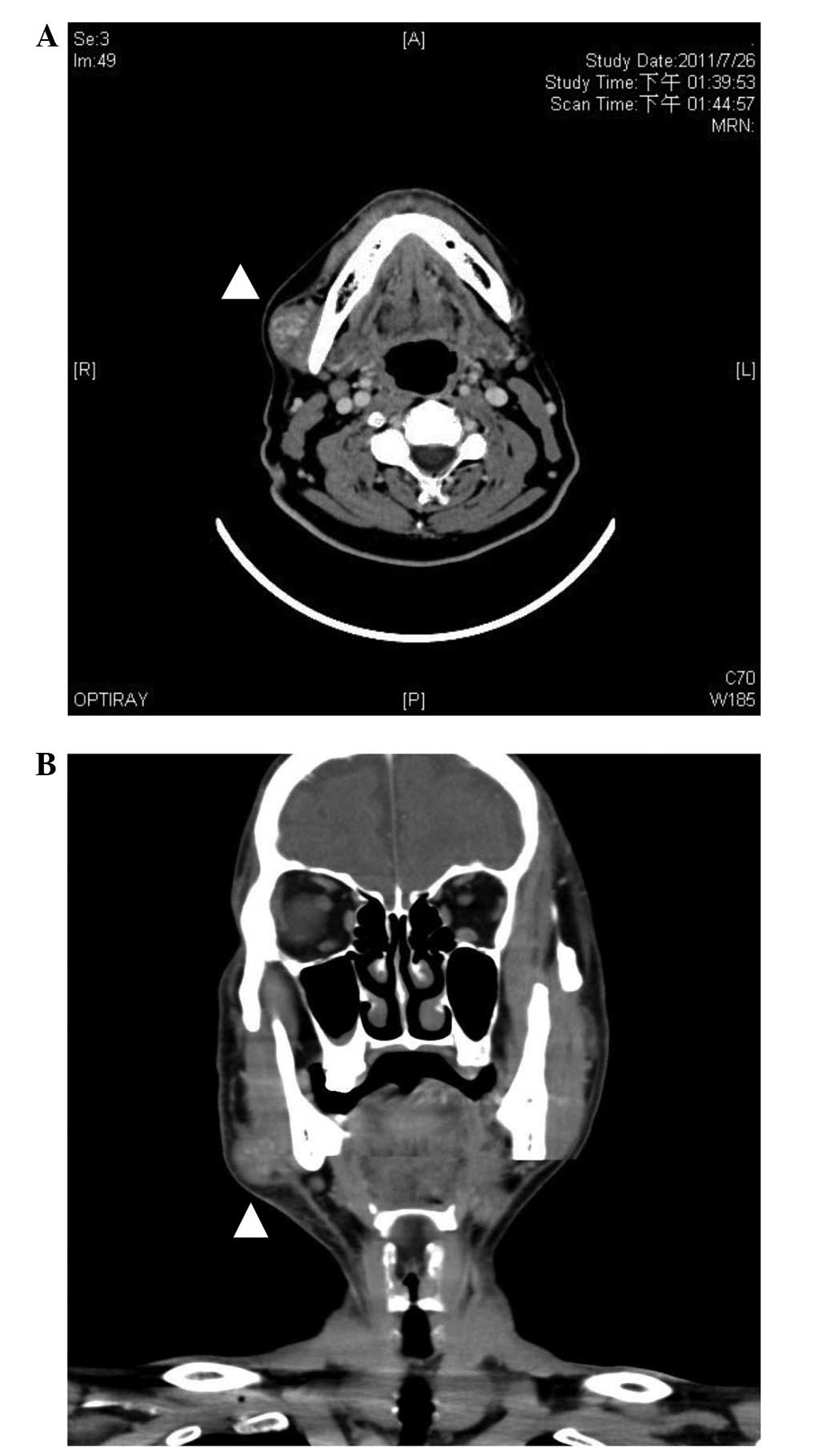
aerodigestive tract. Computed tomography (CT) of the neck showed a
highly-vascularized, heterogeneous mass, 3 cm in diameter, in the
superficial layer of the masseter muscle, with intratumor
whirl-like contrast enhancement (Fig.
1). Fine needle aspiration identified no malignant cells, and
the infiltration of a small number of eosinophils and lymphocytes
was observed.
The patient was subsequently referred for tumor
excision. Under general anesthesia, a collar incision of ~5 cm was
made in the submandibular region, approximately two-finger widths
below the inferior border of the mandible. The platysmal flap was
then elevated cephalad, and the mandibular branch of the facial
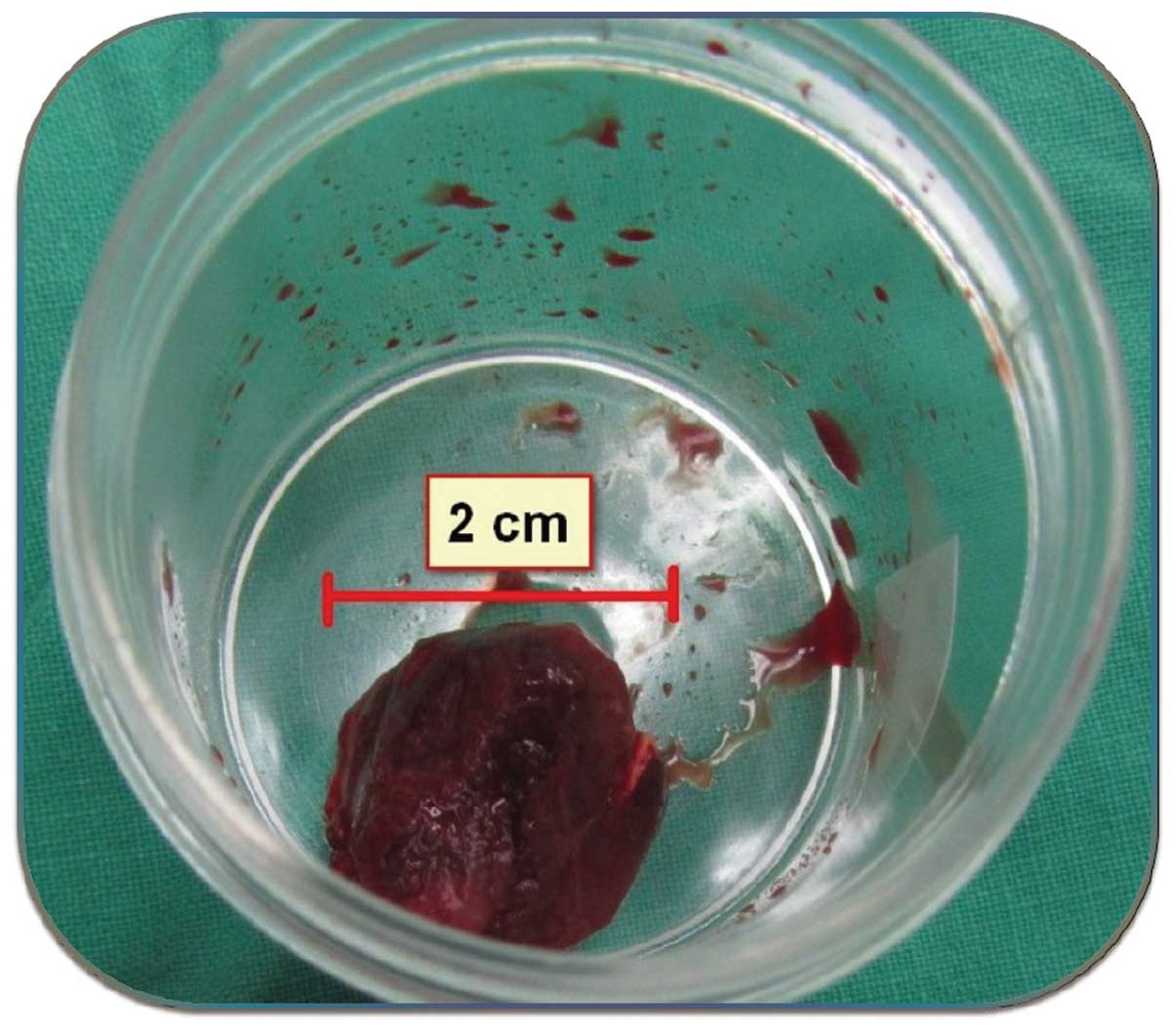
nerve was identified and preserved. The tumor at the superficial
layer of the masseter muscle was removed en bloc (Fig. 2). After hemostasis and placing of the
drainage tube, wound layered closure was performed and the wound
was dressed with dry gauze. The drainage tube was removed two days
after surgery, and the right corner of the patient's mouth regained
normal movement.
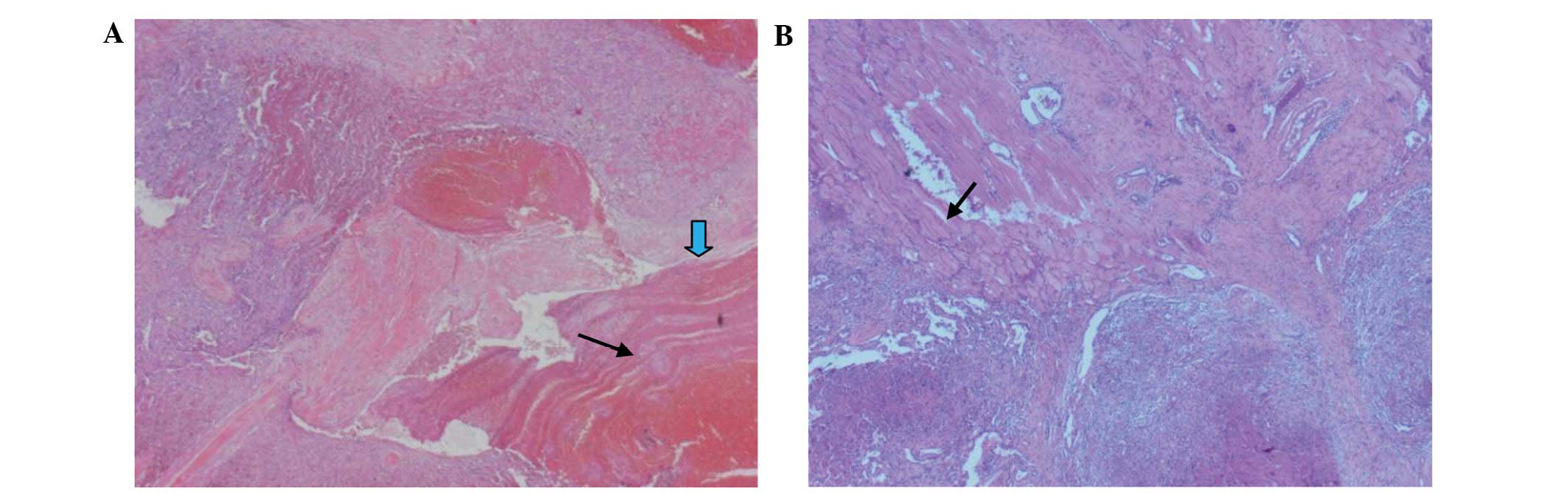
Based on pathological examination of the excised
specimen, a diagnosis of cavernous IMH was determined (Fig. 3). No complications occurred during the
postoperative period and no further treatment was administered. The
functional and cosmetic results were excellent, and no recurrence
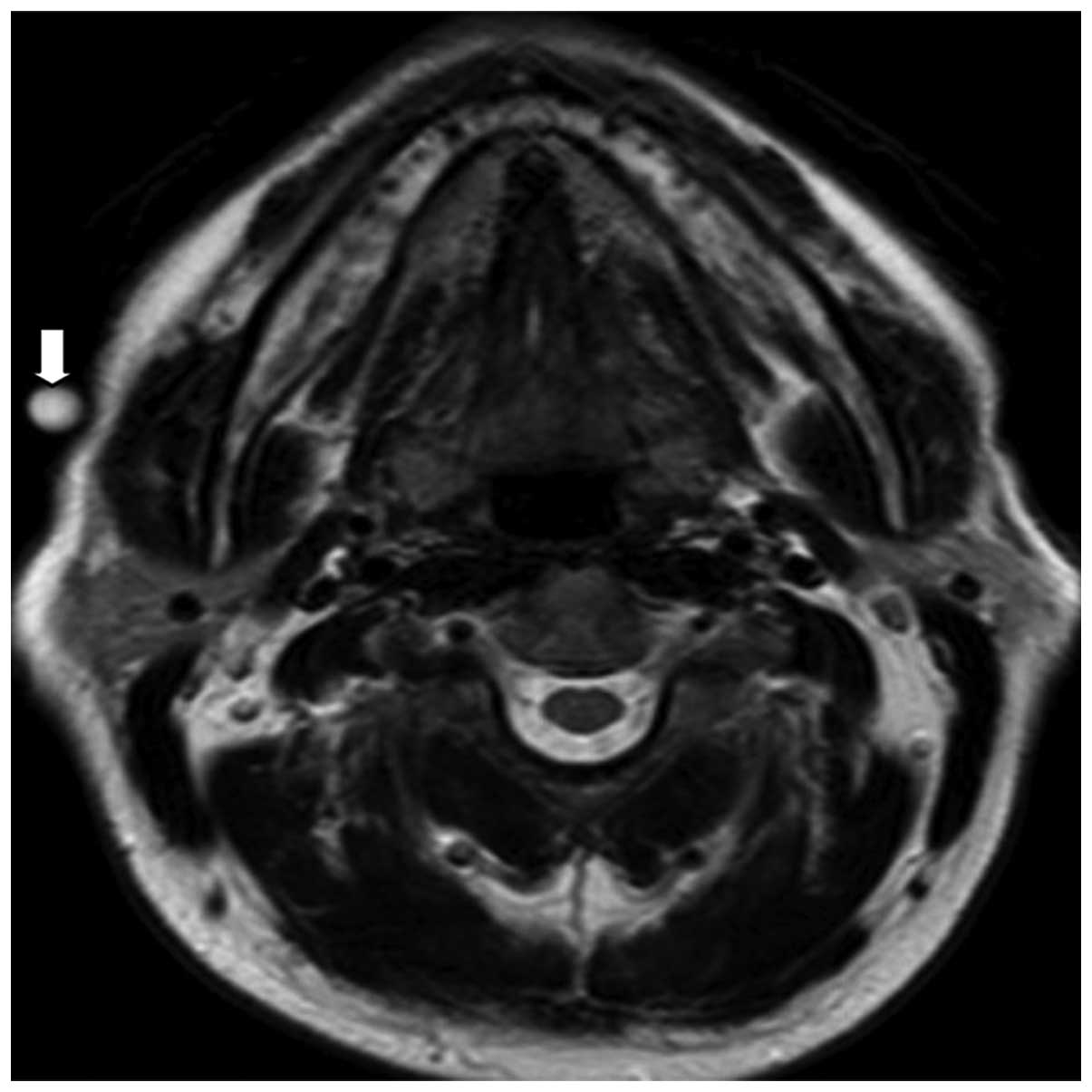
was identified on the 1 year magnetic resonance imaging (MRI)
follow-up scans (Fig. 4). To date,
the 4-year follow-up course has remained uneventful.
Discussion
Hemangioma is the most common type of benign tumor
in infants (6,9), and also one of the most common
manifestations of birth defects (9).
Hemangioma in skeletal muscles accounts for only ~0.8% of all
benign vascular neoplasms (3), and
15% of IMHs occur in the head and neck region (6). The masseter muscle is the most common
location, followed by the trapezius and sternocleidomastoid muscles
(3). In the majority of cases, IMH
occurs in patients <30 years of age, with no gender predilection
(3,5).
Among the classification methods of vascular tumors,
the histological classification is most commonly accepted (4). According to Allen and Enzinger's
definition of hemangiomas, these tumors are classified into a large
vascular type (vessel diameter, >140 mm), small vascular type
(vessel diameter, <140 mm) and mixed vascular type corresponding
to cavernous, capillary and mixed types, respectively (10). This classification is useful and is
consistent with clinical performance and recurrence (10). Capillary-type IMH is the most common,
accounting for 50% of all skeletal muscle hemangiomas (4). It occurs most commonly in the head and
neck region, accounting for ~68% of cases (4,8).
Histologically, capillary-type IMH is hypercellular and thus, it is
difficult to diagnose preoperatively (10). By contrast, the cavernous- and
mixed-types of IMHs occur most commonly in the lower extremities
and trunk, with reported incidence rates of 26 and 5%, respectively
(8,11). These types of tumor tend to have
prolonged symptoms and, thus, such tumors are typically diagnosed
earlier than capillary-type IMH. Notably, mixed-type IMH exhibits
the highest recurrence rate, at ≤28% (8,10,11).
IMHs gradually increase in size, typically over a
period of <1 year (6). The most
common manifestation is a painful lump, with 50–60% of patients
reporting pain. However, pain is not the only symptom in patients
with tumors >3 cm in size (6).
There are generally no overlying skin changes, and thrills, bruits,
compressibility and pulsation are typically absent; however,
situations that increase the venous pressure in the head increase
IMH size (5). The ‘turkey wattle’
sign, a swelling that becomes most apparent when the head is in a
dependent position, may be observed (8,11).
Forceful mastication can cause the lesion to become hard and
difficult to move, as observed upon preoperative physical
examination in the present case.
Correctly diagnosing IMH prior to surgery is
extremely difficult; the tumor occurs within the muscle and
overlaps the parotid gland; thus, <8% of IMH cases are diagnosed
preoperatively (10). Based on the
location and morphology of the tumor, IMH may be differentiated
from other benign and malignant tumors, including
neurofibromatosis, aneurysms, lymphangiomas, rhabdomyosarcomas,
masseteric hypertrophy, schwannomas and other head and neck
malignancies (10). It is difficult
to make a definitive diagnosis clinically by fine needle aspiration
cytology, as pathology often only shows blood cells. In the present
case, fine needle aspiration cytology ruled out malignancy;
however, it did not provide any further information. Hemangioma was
not suspected, therefore, arteriography was not arranged. Although
arteriography and flow dynamics may confirm the existence of a
hemangioma and its vascular type, the low-flow lesion or
capillary-type of IMH makes the diagnosis more difficult.
Furthermore, plain films have not been reported to be useful in the
diagnosis (5,7). With regard to CT and MRI, the majority
of case reports indicate that MRI is of more diagnostic value for
this type of soft tissue tumor than CT. However, in the present
case, CT scans showed a 3.1-cm slightly heterogeneous iso-dense
lesion in the superficial portion of the right masseter muscle.
Following injection of the contrast agent iopamidol (Iopamiro 370;
Bracco, High Wycombe, UK) during the CT exam, heterogeneous
whirl-like enhancement was noted rapidly, revealing a
highly-vascularized lesion (Fig. 1).
Therefore, hemangioma, arteriovenous malformation and vascular-rich
malignancies were all considered. However, as the lesion exhibited
an intact border and no invasion was evident, the possibility of
malignancy was ruled out and IMH was diagnosed. Therefore, the
results of the present case indicate that CT is helpful for the
accurate diagnosis of IMH.
Appropriate treatment must be selected based on the
conditions of the case; factors to be considered include patient
age, tumor size, location and depth of invasion (3,4). Various
treatment modalities have been reported, including cryotherapy,
radiation therapy, steroid injections, vascular thrombosis,
injections of sclerosing material and surgical excision (5,6). However,
surgery remains the most common treatment for IMH. Determining the
tumor entity during surgery is a major difficulty due to the
tumor's ambiguous capsule. The local recurrence rate following
surgical resection is 9–28%; however, removing the masseter muscle
completely has been reported to reduce the recurrence rate
(6). In addition, the facial nerve
may be damaged easily during surgery. Surgery to remove an IMH in
the head and neck region may be approached transorally or via
superficial parotidectomy with a preauricular incision (6,12).
Superficial parotidectomy with a preauricular incision removes the
tumor completely and preserves the facial nerve with minimal
damage, and thus it should be performed when tumors proximal to the
parotid gland require resection. Previous studies have also
reported that parotidectomy is a safer technique for the removal of
rare tumors located in the head and neck region compared with a
transoral approach. Additionally, this type of surgery reduces the
risk of recurrence (6,12,13).
In the present case, a collar incision of the neck
was used instead of a preauricular incision. This approach was
considered appropriate for the patient for the following reasons:
Firstly, a smaller wound results in a shorter operative time,
whilst allowing for complete removal of the tumor, as the tumor was
small in size and located at an appropriate location; and secondly,
the mandibular branch of the facial nerve could also be preserved
without sacrificing the superficial lobe of the parotid gland.
After elevating the platysmal flap, the mandibular branch of the
facial nerve was identified and preserved. The tumor was then
removed safely en bloc. One-year postoperative follow-up MRI showed
no recurrence (Fig. 4).
In conclusion, minimally invasive surgery for
certain IMH patients who exhibit small tumors in an appropriate
location may be performed safely and efficiently. This type of
surgery preserves the cosmetic appearance of the patient, without
compromising their prognosis, typically resulting in no tumor
recurrence or facial nerve dysfunction.
References
|
1
|
Watson WL and McCarthy WD: Blood and lymph
vessel tumors. A report of 1056 cases. Surg Gynecol and Obstet.
71:569–588. 1940.
|
|
2
|
Clemis JD, Briggs DR and Changus GW:
Intramuscular hemangioma in the head and neck. Can J Otolaryngol.
4:339–346. 1975.PubMed/NCBI
|
|
3
|
Narayanan CD, Prakash P and Dhanasekaran
CK: Intramuscular hemangioma of the masseter muscle: A case report.
Cases J. 2:74592009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Odabasi AO, Metin KK, Mutlu C, Başak S and
Erpek G: Intramuscular hemangioma of the masseter muscle. Eur Arch
Otorhinolaryngol. 256:366–369. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zengin AZ, Celenk P and Sumer AP:
Intramuscular hemangioma presenting with multiple phleboliths: A
case report. Oral Surg Oral Med Oral Pathol Oral Radiol.
115:e32–e36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wolf GT, Daniel F, Krause CJ and Kaufman
RS: Intramuscular hemangioma of the head and neck. Laryngoscope.
95:210–213. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith WP, Prince S and Phelan S: The role
of imaging and surgery in the management of vascular tumors of the
masseter muscle. J Oral Maxillofac Surg. 63:1746–1752. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mandel L and Surattanont F: Clinical and
imaging diagnoses of intramuscular hemangiomas: The wattle sign and
case reports. J Oral Maxillofac Surg. 62:754–758. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartzell LD and Buckmiller LM: Current
management of infantile hemangiomas and their common associated
conditions. Otolaryngol Clin N Am. 45:545–556. 2012. View Article : Google Scholar
|
|
10
|
Allen PW and Enzinger FM: Hemangioma of
skeletal muscle. An analysis of 89 cases. Cancer. 29:8–22. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rai P, Setia S, Kalra N and Upreti L:
Intramuscular vascular malformation of the masseter muscle
presenting with turkey wattle sign. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 102:6182006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okabe Y, Ishikawa S and Furukawa M:
Intramuscular hemangioma of the masseter muscle: Its characteristic
appearance on magnetic resonance imaging. ORL J Otorhinolaryngol
Relat Spec. 53:366–369. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rossiter JL, Hendrix RA, Tom LW and Potsic
WP: Intramuscular hemangioma of the head and neck. Otolaryngol Head
Neck Surg. 108:18–26. 1993. View Article : Google Scholar : PubMed/NCBI
|