Introduction
Breast cancer is one of the most common cancer types
among women, acting as a distinct cause of mortality, and has a
high incidence of recurrence (1).
Consequently, numerous studies have focused on the development of
breast cancer therapies, including surgery, chemotherapy and
radiotherapy (2–4). The cancer stem cell (CSC) hypothesis
proposes that the preferential targets of oncogenic transformation
are tissue stem cells or progenitor cells that are able to
self-renew (5–8). Therefore, numerous studies have
developed CSC phenotypic assays in order to identify CSCs (9–11). Breast
CSCs have been characterized as cluster of differentiation
(CD)44+/CD24−/low cells that initiate
carcinogenesis in NOD/SCID mice (12).
Hypoxia is involved in various tumors and often
occurs when tumor growth surpasses blood supply (13). In particular, breast cancers are
sensitive to hypoxia, as they outgrow nutrients or oxygen vascular
supplies (14). In addition, hypoxia
often causes chemo- and radiotherapy resistance, and contributes to
tumor metastasis (15). Although the
effect of hypoxia in patients with breast cancer has been widely
reported, the regulatory mechanism of hypoxia in breast CSC and
therapeutic resistance remain unknown (16–18).
17β-estradiol (E2) is the most effective female
estrogen hormone and is pivotal in male and female physiology
(19). Breast cancer is susceptible
to estrogen hormones. Estrogen hormones promote the development and
progression of breast cancer, and induce the invasion and
metastasis of breast cancer cells that express estrogen receptors
(ERs) to distant organs or lymph nodes (20). E2 also enhances the movement and
invasion of breast cancer cells (21). However, due to the complexity of
ER-triggered estrogen signaling, the effects of estrogen hormones
on cancer are occasionally divergent.
The anti-inflammatory cytokine transforming growth
factor (TGF)-β1 is associated with embryonic development and
homeostasis in adult organisms (22,23).
TGF-β1 is critical for angiogenesis, immunoregulation and cancer
progression (24). In addition,
TGF-β1 acts as a tumor suppressor in the early stages of breast
carcinoma, and is involved in the progression of tumors by
resisting inhibited cell growth during the later stages of disease
(25). TGF-β1 also enhances breast
cancer metastasis by inducing Smad family member 2 (Smad2)
(26). However, the regulatory
mechanisms of breast CSCs following treatment with E2 or TGF-β1
have not been investigated.
In the present study, treatment with E2, TGF-β1 and
hypoxia led to breast CSC (CD44+/CD24−/low)
expansion. CSC markers and epithelial-mesenchymal transition
(EMT)-associated factors were expressed in order to investigate the
underlying mechanisms. Additionally, the effects of E2, TGF-β1 and
hypoxia on cell migration and drug and radiation resistance were
determined. The results indicate that E2, TGF-β1 and hypoxia are
important for the regulation of breast CSCs, and that the
modulation of the tumor microenvironment may improve the efficiency
of breast cancer therapy.
Materials and methods
Cell culture
The human breast cancer MCF-7 cell line was obtained
from the American Type Culture Collection (Rockville, MD, USA) and
cultured at 37°C in 20% O2 and 5% CO2 in
Dulbecco's modified Eagle's medium (DMEM; Welgene, Daegu, South
Korea) that contained 10% HyClone fetal bovine serum (FBS; GE
Healthcare Life Sciences, Logan, UT, USA) and 1% Gibco
antibiotic-antimycotic (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The cells were incubated in a chamber containing a 5%
CO2 and 1% O2 atmosphere for 48, 72 and 96 h
and 1 week to create hypoxic conditions. The cells were treated
with 10 nM E2 or 1 ng/ml TGF-β1 (R&D Systems Inc., Minneapolis,
MN, USA).
Immunoblot analysis
The MCF-7 cells (2×106) were collected
using 5 ml cold phosphate buffer solution (PBS; Welgene) and lysed
in 100 µl lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM
NaCl, 1 mM Na2 ethylenediaminetetraacetic acid, 1 mM
ethylene glycol tetraacetic acid, 1% Triton, 2.5 mM sodium
pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4, 1 µg/ml leupeptin and 1 mM
phenylmethanesulfonylfluoride (all Sigma-Aldrich, St. Louis, MO,
USA). Following 1 hour of incubation on ice, samples were
centrifuged at 13,000 × g for 20 min at 4°C. Subsequently, the
supernatant was removed and quantified using the Bio-Rad Protein
Assay kit II (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
according to the manufacturers's protocol. A total of 50 µg of
total protein was loaded and electrophoresed on sodium dodecyl
sulfate-polyacrylamide gels (Bio-Rad Laboratories, Inc.) and
transferred to polyvinylidene difluoride membranes (GE Healthcare
Life Sciences, Chalfont, UK). The transferred membranes were
blocked using 5% milk (BD Biosciences, San Jose, CA, USA) dissolved
in Tris-buffered saline (20 mM Tris; 137 mM NaCl; pH 7.6;
Sigma-Aldrich) containing 0.02% Tween 20 (Sigma-Aldrich), and
incubated overnight at 4°C with specific primary antibodies. The
membranes were subsequently incubated with specific horseradish
peroxidase-conjugated secondary antibodies (detailed below). The
blots were developed with the Super Signal Chemiluminescence
reagent (Pierce Biotechnology, Inc., Rockford, IL, USA) by enhanced
chemiluminescence (Thermo Fisher Scientific, Inc.).
Immunoblot antibodies
The following mouse monoclonal anti-human primary
antibodies (1 µg; dilution, 1:1,000) were utilized for immunoblot
analysis: Anti-CD44 (catalog no., ab78960), anti-CD24 (catalog no.,
ab76514), anti-ATP-binding cassette sub-family G member 2 (ABCG2;
catalog no., ab130244), anti-JMJD1A (catalog no., ab107234) and
anti-Kruppel-like factor 4 (KLF4; catalog no., ab75486) (all
purchased from Abcam, Cambridge, MA, USA); epithelial cell adhesion
molecule (EpCAM; catalog no., 2929) antibody was from Cell
Signaling Technology, Inc. (Danvers, MA, USA); the cytokeratin 5/8
[mouse anti-human immunoglobulin G (IgG)]; catalog no., 550505),
SOX2 (mouse anti-human IgG; catalog no., 561469) and β-catenin
(mouse anti-human IgG; catalog no., 610154) antibodies were from BD
Biosciences; the c-Myc (catalog no., sc-40) and E- and N-cadherin
(catalog nos., sc-71008 and sc-271386, respectively) antibodies
were from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA); the
hypoxia inducing factor (HIF)-1α (catalog no., NB100-105) antibody
was from Novus Biologicals, LLC (Littleton, CO, USA); and the
β-actin (catalog no., A-5441) antibody was from Sigma-Aldrich. The
3 µg of goat anti-mouse polyclonal secondary antibody (catalog no.
62-6520) was from Thermo Fisher Scientific, Inc. and was used at a
dilution of 1:4,000.
Flow cytometry
MCF-7 cells (2×106) were washed twice
using PBS and incubated with 2 µg/ml monoclonal mouse anti-human
CD24-fluorescein isothiocyanate (FITC; catalog no., 555427) and
monoclonal mouse anti-human CD44-allophycocyanin (APC; catalog no.,
559250) antibodies (BD Biosciences; dilution, 1:100) in the dark on
ice for 1 h, and washed twice using cold PBS. The labeled cells
were analyzed using the fluorescence-activated cell sorting
FACSAria cell sorter (BD Biosciences).
Migration assay
An in vitro wound-healing assay was used to
assess two-dimensional cell motility. The MCF-7 cells
(2×106) were treated for 96 h with E2 or TGF-β1 and
hypoxia in 6-well plates. Then, a scratch was made on the cell
layer with a micropipette tip, and the cultures were washed twice
with serum-free medium to remove the floating cells. The cells were
incubated in a chamber containing an atmosphere of 20%
O2 or in hypoxic conditions (5% CO2 and 1%
O2) in DMEM medium at 37°C. Wound healing was visualized
by comparing photographs 48 h later using the Qimaging QI Click
Camera system mounted on a phase-contrast Nikon microscope (TS100;
Nikon, Inc., Tokyo, Japan). For the Transwell assay, MCF-7 cells
(2×104) were seeded onto 8-µm Transwell-inserts (Costar
brand; Corning Life Sciences, Tewksbury, MA, USA). The lower
chambers were filled with DMEM containing 10% FBS. Migrated cells
were stained with crystal violet and counted using the Image-Pro
Plus 7.0 software (Media Cybernetics, Rockville, MD, USA) 24 h
later.
Apoptosis assay
Cell apoptosis was assessed using the Annexin
V/phycoerythrin (PE) Apoptosis Detection kit (BD Biosciences;
catalog no. 559763). Briefly, the MCF-7 cells (1×106)
were seeded in 100-mm dishes and incubated overnight. Then, the
cells were treated with 5-fluorouracil (100 µg/ml) or radiation (10
Gy) for 48 or 72 h, respectively. Subsequently, the cells were
washed twice with 5 ml PBS and stained with 2.5 µg/ml Annexin
V/PE-conjugate and 5 µl 7-aminoactinomycin for 15 min on ice in the
dark. Subsequent to staining, the cells were analyzed using the
FACSAria cell sorter.
Statistical analysis
Statistical analyses were performed using Excel
(Microsoft Corporation, Redmond, WA, USA). A Student's
t-test was used to make statistical comparisons and
P<0.05 was considered to indicate a significant difference.
Results
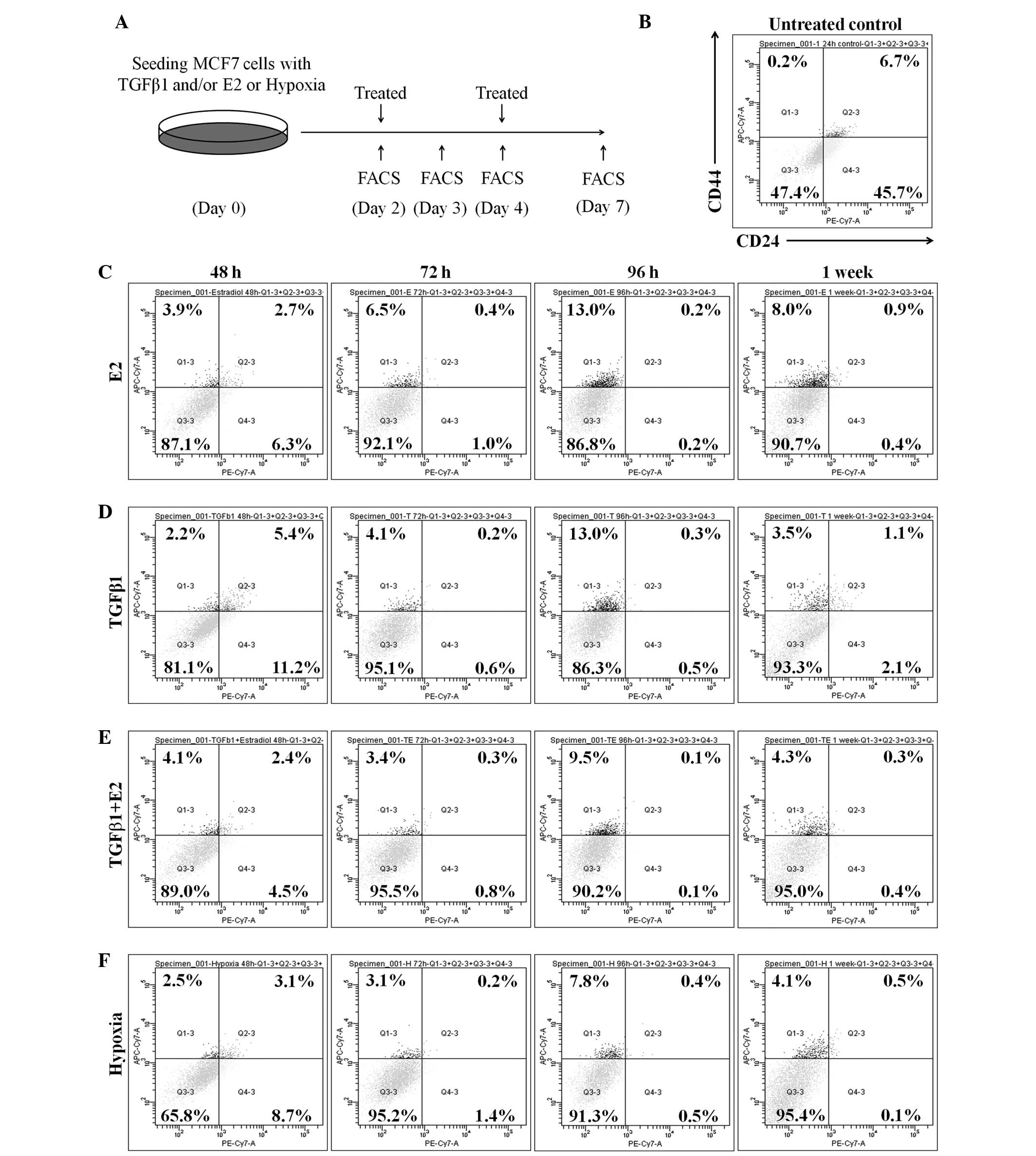
Effect of E2, TGF-β1 and hypoxia on
breast CSC expansion
The percentage of
CD44+/CD24−/low cells is considered to be the
breast CSC subpopulation. This percentage was assessed in MCF-7
cells, in the presence or absence of E2 and TGF-β1, in order to
investigate whether E2 or TGF-β1 treatment affects the size of the
CSC population. ER+ MCF-7 cells were treated with 10 nM
E2, 1 ng/ml TGF-β1 or a combination of the two for 48, 72 and 96 h,
and 1 week. Following treatment, the proportion of stem-like cells
was evaluated using flow cytometry (Fig.
1A). The results indicated that the proportion of
CD44+/CD24−/low cells was significantly
expanded in E2-treated MCF-7 cells (48 h, 2.2%; 72 h, 4.1%; 96 h,
13%; 1 week, 1.1%) compared with untreated control cells (0.2%;
Fig. 1B and C). TGF-β1-treated MCF-7
cells also demonstrated a notable increase in the percentage of
CD44+/CD24−/low cells at 48 (3.9%), 72 (6.5%)
and 96 h (13.0%), and 1 week (8.0%) subsequent to treatment with
TGF-β1 (Fig. 1D). However, no
synergistic induction of the CD44+/CD24−/low
population by the TGF-β1/E2 combined treatment was observed
(Fig. 1E). The effect of hypoxia on
breast CSC expansion was also examined. The results show that the
proportion of CD44+/CD24−/low cells increased
in hypoxia-treated MCF-7 cells (48 h, 2.5%; 72 h, 3.1%; 96 h, 7.8%;
1 week, 4.1%) compared with the proportion in normoxic conditions
(Fig. 1B and F). These results
indicate that treatment with E2, TGF-β1 and hypoxia in isolation
expanded the CSC population of MCF-7 cells, but that the combined
treatment had no synergistic effect.
Effect of E2, TGF-β1 and hypoxia on
the expression of breast CSC markers and EMT-associated
factors
CSC marker expression in E2-, TGF-β1- or
hypoxia-treated MCF-7 cells was examined, as the E2, TGF-β1 and
hypoxia treatment increased the CSC population in MCF-7 cells.
MCF-7 cells were treated and incubated under the same conditions
exhibited in Fig. 1A, and the CD44,
CD24, SOX2, ABCG2 and KLF4 protein levels were measured using
western blot analysis (Fig. 2A).
Consistent with the flow cytometry results, CD44 expression
increased in E2-, TGF-β1- or hypoxia-treated MCF-7 cells. However,
CD24 expression decreased following E2, TGF-β1 and hypoxia
treatment. Notably, the expression of the pluripotency-associated
proteins SOX2 and KLF4 and the putative CSC marker ABCG2 increased
when the cells were treated with E2, TGF-β1 and hypoxia. These
results indicate that SOX2, KLF4 and ABCG2, which are associated
with breast cancer stemness, were actively regulated by the E2,
TGF-β1, and hypoxia treatments.
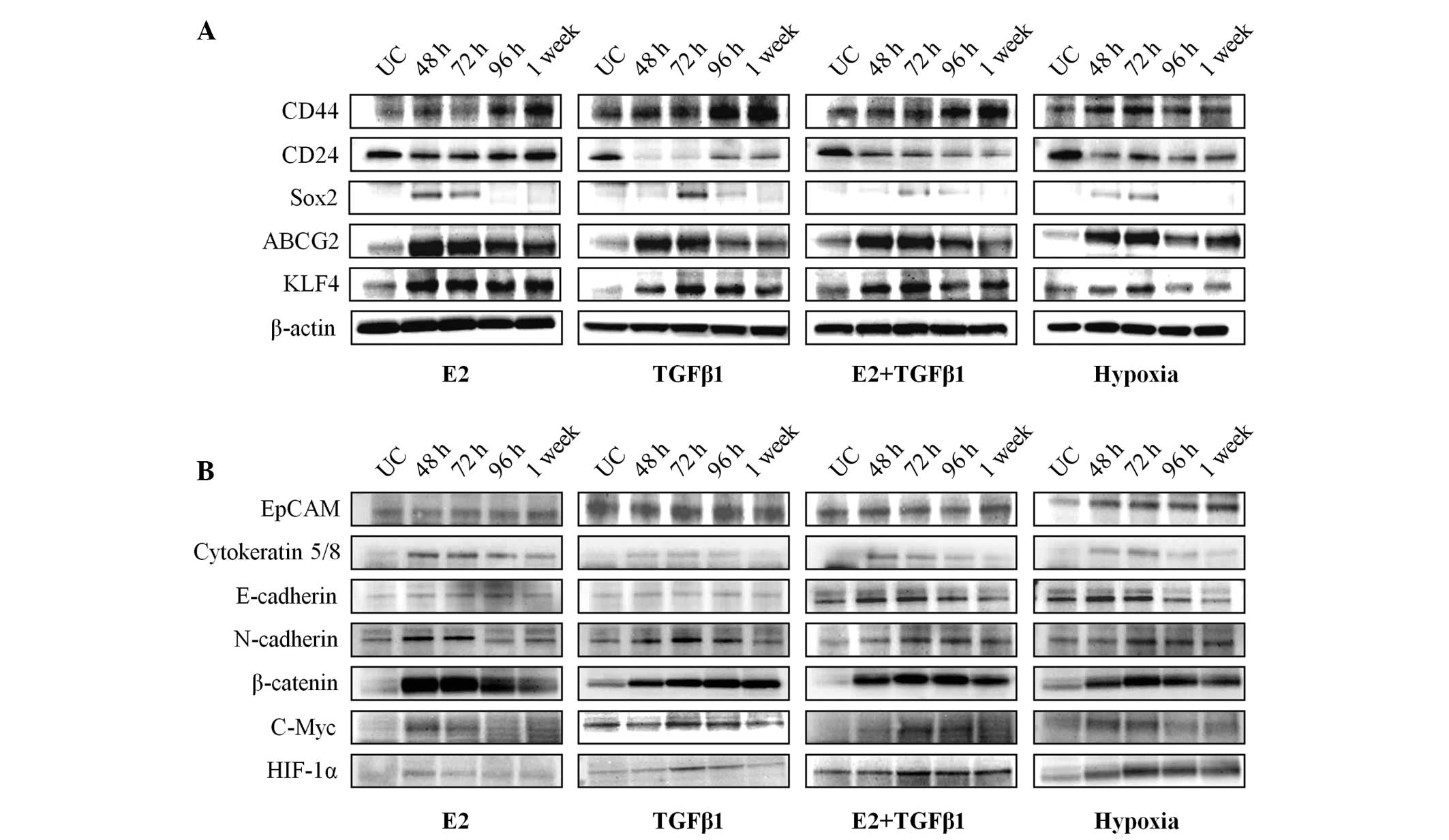 | Figure 2.Patterns of cancer stem cell and
epithelial-mesenchymal transition-associated marker expression in
E2, TGF-β1 and hypoxia-treated MCF-7 cells. (A) The expression of
CD44, CD24, Sox2, ABCG2 and KLF4 was determined in E2, TGF-β1,
combined E2/TGF-β1 and hypoxia-treated MCF-7 cells by western
blotting. β-actin was used as a loading control. Western blotting
was performed at each time point (48, 72 and 96 h and 1 week). (B)
EpCAM, cytokeratin 5/8, E-cadherin, N-cadherin, β-catenin, c-Myc
and HIF-1α expression was determined by western blotting using same
conditions that are indicated in Fig.
2A. E2, estradiol; TGF-β1, transforming growth factor-β1; CD,
cluster of differentiation; Sox2, sex determining region Y-box 2;
ABCG2, ATP-binding cassette sub-family G member 2; KLF4,
Kruppel-like factor 4; EpCAM, epithelial cell adhesion molecule;
HIF, hypoxia-inducible factor. |
Several studies have demonstrated that CSCs and
EMT-phenotypic cells have a tumor aggressiveness phenotype
(27–29). Therefore, the present study
investigated whether treatment with E2, TGF-β1 and hypoxia affects
the expression of EMT-associated factors. The results demonstrate
that EpCAM and E-cadherin expression was not affected by E2- or
TGF-β1 stimulation (Fig. 2B).
However, expression of the EMT markers cytokeratin 5/8 and
N-cadherin, which increase cell motility, clearly increased
following treatment with E2 or TGF-β1. In addition, the levels of
β-catenin and the target gene c-Myc were upregulated in E2 and
TGF-β1-treated MCF-7 cells compared with untreated control cells.
EpCAM expression increased, whereas E-cadherin expression
decreased, in hypoxia-treated MCF-7 cells (Fig. 2B). Cytokeratin 5/8, N-cadherin,
β-catenin and c-Myc levels increased under hypoxic conditions.
These results indicate that the CSC expansion mediated by E2,
TGF-β1 and hypoxia may promote EMT by regulating the expression of
EMT-associated factors.
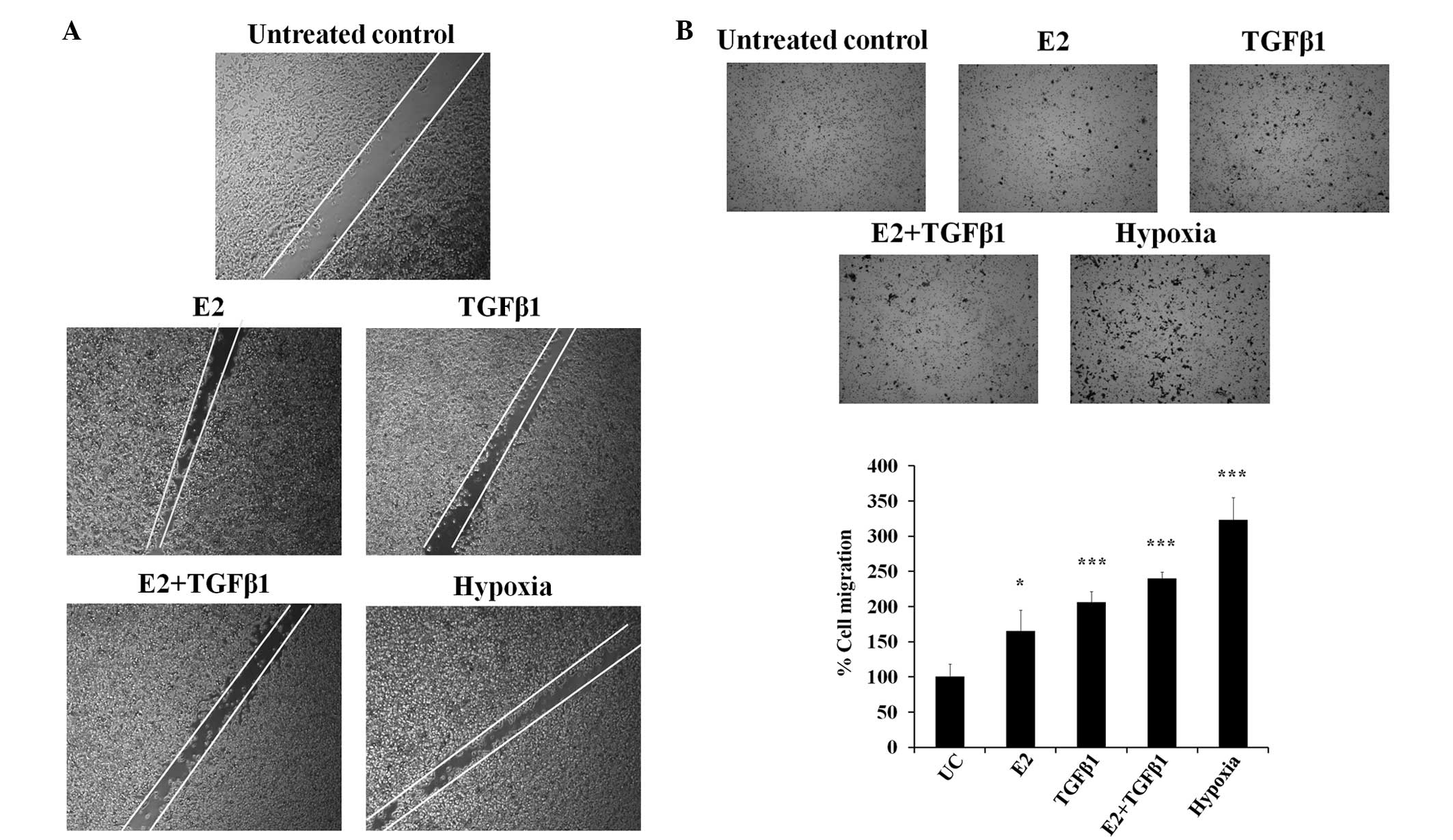
Enhancement of cell motility by E2,
TGF-β1 and hypoxia
The increased number of CSCs and progression of the
EMT are associated with cell motility and invasion. A wound-healing
assay with the E2, TGF-β1 and hypoxia treatments was performed to
visualize the effect on MCF-7 cell motility (Fig. 3A). E2 and TGF-β1-treated cells
demonstrated increased cell migration compared with control cells.
The hypoxia treatment also significantly enhanced cellular
migration ability. Similarly, the Transwell assay results indicated
that E2-, TGF-β1- and hypoxia-treated MCF-7 cells migrated more
compared with control MCF-7 cells (Fig.
3B). The data indicate that treatment with TGF-β1, E2 and
hypoxia induces migration ability in MCF-7 cells.
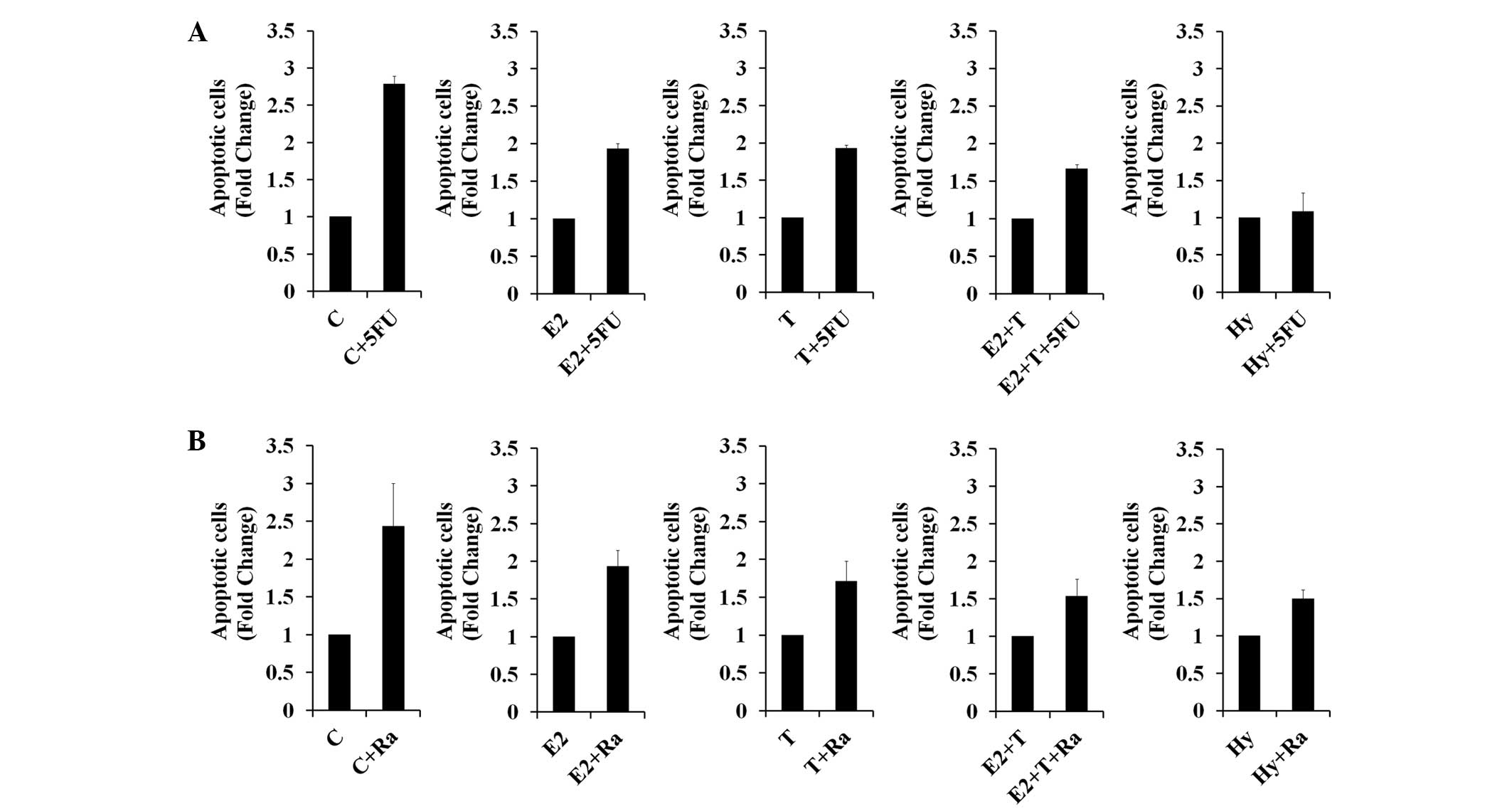
Effect of E2, TGF-β1 and hypoxia on
drug and radioresistance
Fluorouracil (5-FU) is a chemotherapeutic agent used
to treat various types of cancer, including breast cancer (30). In the present study, ABCG2 expression
was induced by E2, TGF-β1 and hypoxia treatments (Fig. 2A). The effect of E2, TGF-β1 and
hypoxia on 5-FU-induced-apoptosis was investigated as ABCG2
mediates multidrug resistance. The results showed that E2-, TGF-β-
and hypoxia-treated MCF-7 cells were more resistant to 5-FU
compared with untreated control MCF-7 cells. In particular,
5-FU-induced apoptosis was entirely abolished in hypoxia-treated
cells (Fig. 4A). E2-, TGF-β1- and
hypoxia-treated cells demonstrated inhibited radiation-induced
apoptosis (Fig. 4B). These results
indicate that the TGF-β1, E2 and hypoxia treatments enhance drug
and radioresistance, which may be due to the increased expression
of CSC-associated proteins, including ABCG2 and β-catenin.
Discussion
CSCs possess the stem cell properties of
self-renewal and differentiation; therefore, tumors may be
initiated, grow, invade and metastasize (31). Anticancer therapies at present
emphasize killing CSCs or inhibiting the induction of the CSC
population (32,33). However, CSC-targeted cancer therapies
are problematic due to the characteristics of CSCs, including
radio- and chemoresistance (34,35). The
aim of the present study was to identify the regulatory mechanisms
for breast CSC expansion. Treatment with E2 induces the
proliferation of human ER+ breast cancer cells (36,37). In
addition, a previous study indicated that breast CSCs are
stimulated by estrogens, through paracrine fibroblast growth
factor/Tbx3 signaling (38). Numerous
studies have demonstrated that estrogen hormones act as a negative
regulator of TGF-β1-stimulated cellular responses (39,40).
Therefore, the present study examined the effects of E2 and TGF-β1
treatment on the regulation of breast CSCs. The results of the
present study indicated that treatment with E2 or TGF-β1 in
isolation expanded the CD44+/CD24−/low cell
subpopulation, but that a combination of E2 and TGF-β1 treatment
did not exhibit a synergistic effect (Fig. 1). These results demonstrate that E2
and TGF-β1 do not affect each other during regulation of the breast
CSC population. Oxygen is a critical regulator of cellular
metabolism and proliferation, and hypoxia regulates a variety of
pro-angiogenic pathways and carcinogenesis (41,42).
Although numerous studies have reported that hypoxia improves
therapeutic efficacy by eliminating the CSC population (43–45), other
studies indicate that hypoxia induces CSC characteristics by
upregulating stemness-associated factors and a more aggressive
phenotype (45,46). Therefore, in the present study, the
CD44+/CD24−/low cell population was monitored
at each time point (48, 72 and 96 h and 1 week) following hypoxia
treatment. As expected, the CD44+/CD24−/low
cell population expanded following hypoxia treatment (Fig. 1F). Although the peak percentage value
varied for each treatment, the induced ratio of
CD44+/CD24−/low cells by E2, TGF-β1 and
hypoxia decreased over time (Fig. 1).
Therefore, breast CSCs with the
CD44+/CD24−/low phenotype appear to maintain
a consistent CSC ratio from external stimuli.
EMT is an essential process in tumor metastasis and
recurrence (47). Previous studies
have demonstrated that the EMT is tightly linked to CSC biology
(27–29). In addition, breast cancer is a
distinct cause of mortality and has a high incidence of recurrence
(48). Therefore, identifying the
mechanisms or molecules used in the EMT is important for breast
cancer therapy. In the present study, the E2, TGF-β1 and hypoxia
treatments induced the expression of EMT-associated factors
(Fig. 2B). Similarly, the migration
ability of MCF-7 cells increased following treatment (Fig. 3). These results indicate that E2,
TGF-β1 and hypoxia may be critical in EMT.
CSCs are hypothesized to demonstrate resistance to
chemo- and radiotherapy (31), and
ABCG2 is a key regulator of chemoresistance (49). The results of the present study
indicated that ABCG2 expression increased significantly while
screening target proteins that were induced by treatment with
TGF-β1, E2 and hypoxia (Fig. 2A). The
Annexin V analysis demonstrated that the E2, TGF-β1 and hypoxia
treatments attenuated 5-FU-induced apoptosis (Fig. 4A). In addition, treatment with E2,
TGF-β1 or hypoxia effectively blocked ionizing radiation-induced
apoptosis (Fig. 4B). These results
indicate that CSCs, which have been expanded by TGF-β1, E2 and
hypoxia, may enhance chemo- and radioresistance. Components of the
cell cycle machinery regulate asymmetric cell division, cell shape,
and protein translation in stem cells (50). In addition, numerous studies have
reported that the regulation of cell cycle-associated factors may
contribute to inhibit the chemotherapeutic resistance of CSCs
(50,51). The present study demonstrated that the
expression of cell cycle-associated factors, including β-catenin
and c-Myc, increased following treatment with E2, TGF-β1 and
hypoxia (Fig. 2A). The present data
suggest that these factors are involved in the regulation of the
breast CSC population.
In conclusion, the results of the present study
indicate that E2, TGF-β1 and hypoxia are important in breast CSC
expansion and in the regulation of CSC-associated protein
expression. In addition, the regulation of hormones, growth factors
and the tumor microenvironment, which are potential breast cancer
therapeutic targets, may improve the efficacy of breast cancer
therapy by inhibiting chemo- and radioresistance.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea (DIRAMS) grant, funded by the Korea
government (grant no. 50590–2015), and the Basic Science Research
Program of the National Research Foundation of Korea (grant no.
NRF-2013 M2A2A 7043665), funded by the Ministry of Science, ICT
& Future Planning.
References
|
1
|
Marmot MG, Altman DG, Cameron DA, Dewar
JA, Thompson SG and Wilcox M: The benefits and harms of breast
cancer screening: An independent review. Br J Cancer.
108:2205–2240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaufmann M, von Minckwitz G, Mamounas EP,
Cameron D, Carey LA, Cristofanilli M, Denkert C, Eiermann W, Gnant
M, Harris JR, et al: Recommendations from an international
consensus conference on the current status and future of
neoadjuvant systemic therapy in primary breast cancer. Ann Surg
Oncol. 19:1508–1516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bear HD, Anderson S, Smith RE, Geyer CE
Jr, Mamounas EP, Fisher B, Brown AM, Robidoux A, Margolese R,
Kahlenberg MS, et al: Sequential preoperative or postoperative
docetaxel added to preoperative doxorubicin plus cyclophosphamide
for operable breast cancer: National Surgical Adjuvant Breast and
Bowel Project Protocol B-27. J Clin Oncol. 24:2019–2027. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
EBCTCG (Early Breast Cancer Trialists'
Collaborative Group). McGale P, Taylor C, Correa C, Cutter D, Duane
F, Ewertz M, Gray R, Mannu G, Peto R, et al: Effect of radiotherapy
after mastectomy and axillary surgery on 10-year recurrence and
20-year breast cancer mortality: Meta-analysis of individual
patient data for 8135 women in 22 randomised trials. Lancet.
383:2127–2135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Charafe-Jauffret E, Ginestier C and
Birnbaum D: Breast cancer stem cells: Tools and models to rely on.
BMC Cancer. 9:2022009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glinsky GV: Stem cell origin of
death-from-cancer phenotypes of human prostate and breast cancers.
Stem Cell Rev. 3:79–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krivtsov AV, Twomey D, Feng Z, Stubbs MC,
Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al:
Transformation from committed progenitor to leukaemia stem cell
initiated by MLL-AF9. Nature. 442:818–822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao RC, Zhu YS and Shi Y: New hope for
cancer treatment: Exploring the distinction between normal adult
stem cells and cancer stem cells. Pharmacol Ther. 119:74–82. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stingl J, Eirew P, Ricketson I, Shackleton
M, Vaillant F, Choi D, Li HI and Eaves CJ: Purification and unique
properties of mammary epithelial stem cells. Nature. 439:993–997.
2006.PubMed/NCBI
|
|
11
|
O'Brien CA, Kreso A and Jamieson CH:
Cancer stem cells and self-renewal. Clin Cancer Res. 16:3113–3120.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen H, Yan Y, Davidson TL, Shinkai Y and
Costa M: Hypoxic stress induces dimethylated histone H3 lysine 9
through histone methyltransferase G9a in mammalian cells. Cancer
Res. 66:9009–9016. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brown JM and Wilson WR: Exploiting tumour
hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaudary N and Hill RP: Hypoxia and
metastasis in breast cancer. Breast Dis. 26:55–64. 2006.PubMed/NCBI
|
|
17
|
Nuyten DS, Hastie T, Chi JT, Chang HY and
van de Vijver MJ: Combining biological gene expression signatures
in predicting outcome in breast cancer: An alternative to
supervised classification. Eur J Cancer. 44:2319–2329. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Samanta D, Gilkes DM, Chaturvedi P, Xiang
L and Semenza GL: Hypoxia-inducible factors are required for
chemotherapy resistance of breast cancer stem cells. Proc Natl Acad
Sci USA. 111:E5429–E5438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Acconcia F and Marino M: The effects of
17beta-estradiol in cancer are mediated by estrogen receptor
signaling at the plasma membrane. Front Physiol. 2:302011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yager JD and Davidson NE: Estrogen
carcinogenesis in breast cancer. N Engl J Med. 354:270–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng S, Huang J, Zhou K, Zhang C, Xiang
Q, Tan Z, Wang T and Fu X: 17β-Estradiol enhances breast cancer
cell motility and invasion via extra-nuclear activation of
actin-binding protein ezrin. PLoS One. 6:e224392011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li L, Ren C, Yang G, Goltsov AA, Tabata K
and Thompson TC: Caveolin-1 promotes autoregulatory, Akt-mediated
induction of cancer-promoting growth factors in prostate cancer
cells. Mol Cancer Res. 7:1781–1791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kasagi S and Chen W: TGF-beta1 on
osteoimmunology and the bone component cells. Cell Biosci. 3:42013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prud'homme GJ: Pathobiology of
transforming growth factor beta in cancer, fibrosis and immunologic
disease, and therapeutic considerations. Lab Invest. 87:1077–1091.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akhurst RJ and Derynck R: TGF-beta
signaling in cancer - a double-edged sword. Trends Cell Biol.
11(Suppl 1): S44–S51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lv ZD, Kong B, Li JG, Qu HL, Wang XG, Cao
WH, Liu XY, Wang Y, Yang ZC, Xu HM, et al: Transforming growth
factor-β 1 enhances the invasiveness of breast cancer cells by
inducing a Smad2-dependent epithelial-to-mesenchymal transition.
Oncol Rep. 29:219–225. 2013.PubMed/NCBI
|
|
27
|
Kong D, Li Y, Wang Z and Sarkar FH: Cancer
stem cells and epithelial-to-mesenchymal transition
(EMT)-phenotypic cells: Are they cousins or twins? Cancers (Basel).
3:716–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Santisteban M, Reiman JM, Asiedu MK,
Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC,
Manjili MH, et al: Immune-induced epithelial to mesenchymal
transition in vivo generates breast cancer stem cells.
Cancer Res. 69:2887–2895. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soltanian S and Matin MM: Cancer stem
cells and cancer therapy. Tumour Biol. 32:425–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang C, Ang BT and Pervaiz S: Cancer stem
cell: Target for anti-cancer therapy. FASEB J. 21:3777–3785. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang S, Cui B, Lai H, Liu G, Ghia EM,
Widhopf GF II, Zhang Z, Wu CC, Chen L, Wu R, et al: Ovarian cancer
stem cells express ROR1, which can be targeted for
anti-cancer-stem-cell therapy. Proc Natl Acad Sci USA.
111:17266–17271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakariassen PØ, Immervoll H and Chekenya
M: Cancer stem cells as mediators of treatment resistance in brain
tumors: Status and controversies. Neoplasia. 9:882–892. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rycaj K and Tang DG: Cancer stem cells and
radioresistance. Int J Radiat Biol. 90:615–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pattarozzi A, Gatti M, Barbieri F, Würth
R, Porcile C, Lunardi G, Ratto A, Favoni R, Bajetto A, Ferrari A,
et al: 17beta-estradiol promotes breast cancer cell
proliferation-inducing stromal cell-derived factor-1-mediated
epidermal growth factor receptor transactivation: Reversal by
gefitinib pretreatment. Mol Pharmacol. 73:191–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu YF, Wu Q, Xu XM, Ren Y, Yu LN, Quan CS
and Li YL: Effects of 17β-estradiol on proliferation and migration
of MCF-7 cell by regulating expression of claudin-6. Zhonghua Bing
Li Xue Za Zhi. 39:44–47. 2010.(In Chinese). PubMed/NCBI
|
|
38
|
Fillmore CM, Gupta PB, Rudnick JA,
Caballero S, Keller PJ, Lander ES and Kuperwasser C: Estrogen
expands breast cancer stem-like cells through paracrine FGF/Tbx3
signaling. Proc Natl Acad Sci USA. 107:21737–21742. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Negulescu O, Bognar I, Lei J, Devarajan P,
Silbiger S and Neugarten J: Estradiol reverses TGF-beta1-induced
mesangial cell apoptosis by a casein kinase 2-dependent mechanism.
Kidney Int. 62:1989–1998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Silbiger S, Lei J, Ziyadeh FN and
Neugarten J: Estradiol reverses TGF-beta1-stimulated type IV
collagen gene transcription in murine mesangial cells. Am J
Physiol. 274:F1113–F1118. 1998.PubMed/NCBI
|
|
41
|
Kimura H, Braun RD, Ong ET, Hsu R, Secomb
TW, Papahadjopoulos D, Hong K and Dewhirst MW: Fluctuations in red
cell flux in tumor microvessels can lead to transient hypoxia and
reoxygenation in tumor parenchyma. Cancer Res. 56:5522–5528.
1996.PubMed/NCBI
|
|
42
|
Gillies RJ and Gatenby RA: Hypoxia and
adaptive landscapes in the evolution of carcinogenesis. Cancer
Metastasis Rev. 26:311–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Z and Rich JN: Hypoxia and hypoxia
inducible factors in cancer stem cell maintenance. Curr Top
Microbiol Immunol. 345:21–30. 2010.PubMed/NCBI
|
|
44
|
Liu J and Wang Z: Increased oxidative
stress as a selective anticancer therapy. Oxid Med Cell Longev.
2015:2943032015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liang D, Ma Y, Liu J, Trope CG, Holm R,
Nesland JM and Suo Z: The hypoxic microenvironment upgrades
stem-like properties of ovarian cancer cells. BMC Cancer.
12:2012012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Heddleston JM, Li Z, McLendon RE,
Hjelmeland AB and Rich JN: The hypoxic microenvironment maintains
glioblastoma stem cells and promotes reprogramming towards a cancer
stem cell phenotype. Cell Cycle. 8:3274–3284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
48
|
Cardoso F, Fallowfield L, Costa A,
Castiglione M and Senkus E: ESMO Guidelines Working Group: Locally
recurrent or metastatic breast cancer: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
22(Suppl 6): vi25–vi30. 2011.PubMed/NCBI
|
|
49
|
An Y and Ongkeko WM: ABCG2: The key to
chemoresistance in cancer stem cells? Expert Opin Drug Metab
Toxicol. 5:1529–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Velasco-Velázquez MA, Yu Z, Jiao X and
Pestell RG: Cancer stem cells and the cell cycle: Targeting the
drive behind breast cancer. Expert Rev Anticancer Ther. 9:275–279.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ravandi F and Estrov Z: Eradication of
leukemia stem cells as a new goal of therapy in leukemia. Clin
Cancer Res. 12:340–344. 2006. View Article : Google Scholar : PubMed/NCBI
|