Introduction
Inflammatory myofibroblastic tumors (IMTs) belong to
an intermediate group of soft-tissue tumors, and were first
reported in 1990 by Pettinato et al (1). IMTs are associated with a number of
non-specific symptoms, including fever, pain and weight-loss, which
are common to many diseases. IMTs are therefore difficult to
differentiate clinically from malignant tumors and infections. IMTs
develop in the soft tissues of patients of a wide age-range, from
infants to adults, and are most commonly detected in the lung,
mesentery, and omentum (2).
Histological findings and pathological analysis are indispensable
for diagnosing IMTs, and they are characterized by proliferation of
spindle-shaped cells, including fibroblasts and myofibroblasts that
stain positive for alpha-smooth muscle actin (α-SMA), accompanied
by inflammatory cell infiltration (3). Furthermore, an increased level of the
pro-inflammatory cytokine interleukin (IL)-6 in peripheral blood,
and an increase in nuclear factor kappa B activation in peripheral
blood mononuclear cells are both commonly detected in clinical
manifestations of IMTs (4). IMTs are
frequently removed by surgery, and complete resection often results
in a good prognosis (5).
In recent years, associations with anaplastic
lymphoma kinase 1 (ALK1, commonly referred to as ALK) gene
rearrangements and the presence of immunoglobulin subclass G4
(IgG4)-expressing plasma cells have been highlighted as important
factors for differential diagnosis of IMTs. ALK expression has been
observed in ~50% of IMTs (6), and
ALK-positive IMTs are reported to have high rates of rapid
progression and recurrence, associated with a high grade of
malignancy (7,8). Associations between IgG4-positive IMTs
and autoimmune diseases appear to have been suggested (9). Pathologically, infiltration by
lymphocytes and plasma cells, in addition to spindle-shaped cells,
is observed, and they are considered to be inflammatory
pseudotumors (IPTs) (10). IgG4 is
detected in IPTs but seldom in IMTs, whereas ALK expression has not
been detected in IPTs. Thus, ALK and IgG4 expression may be
regarded as important for the classification of IMTs (11,12). The
present study reports the first case of pulmonary IMT without ALK
and IgG4 to show an improvement in the patient's condition with use
of clarithromycin as a long-term macrolide therapy.
Case report
A 73-year-old female with a past medical history of
hystero-oophorectomy for uterine cancer at 60 years of age, who had
never smoked, was examined in our department (Respiratory Medicine
and Infection Control, Tokai University Hachioji Hospital, Tokyo,
Japan) on November 12, 2012 after abnormal chest shadows were
observed during a health check-up with a radiographer. The patient
had no fever or cough, and no particular clinical manifestations
were observed. Additionally, no superficial lymph nodes were
palpable, and no skin rashes were noted. Chest radiography and
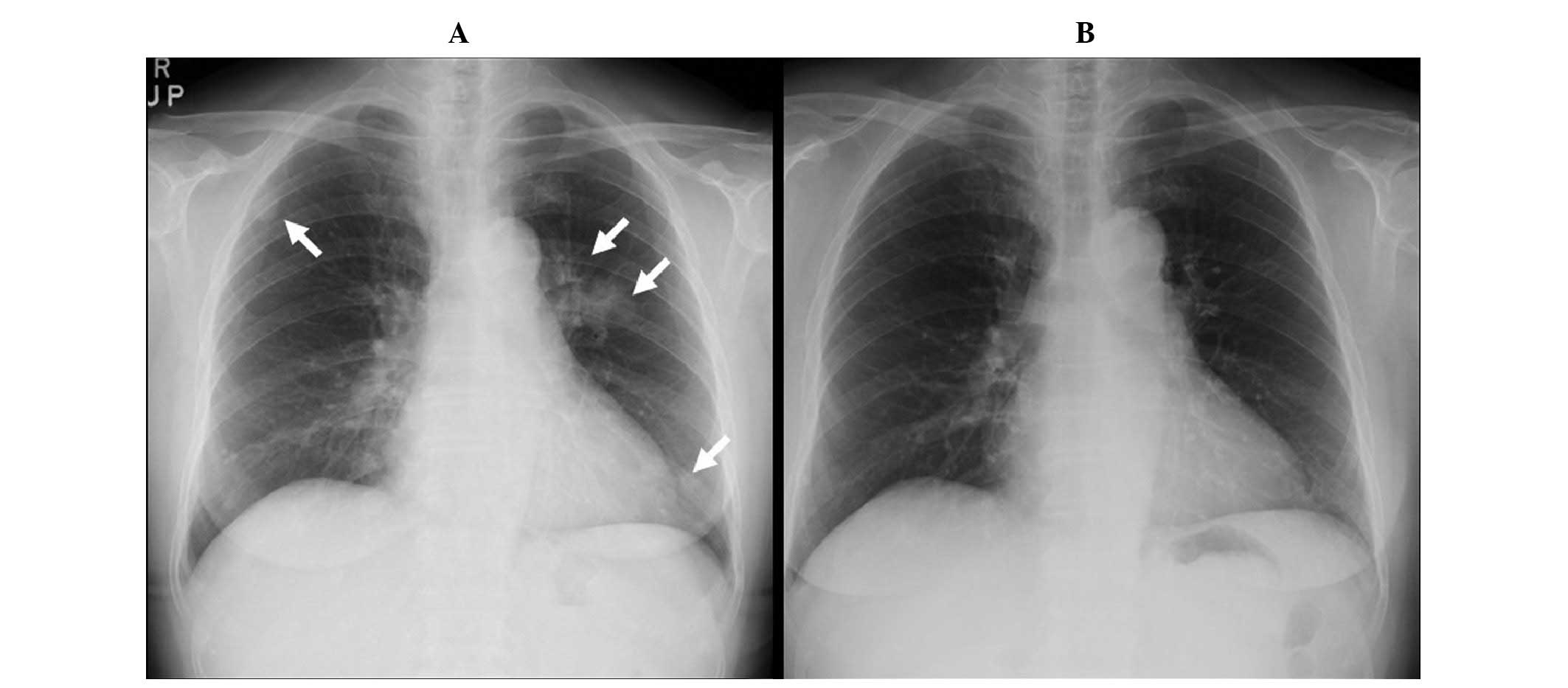
computed tomography (CT) revealed infiltrative shadows primarily in
the left pulmonary hilum, and small nodular shadows in both the
right and left lung fields (Figs. 1
and 2). The blood examination and
tumor marker results were within normal limits (CEA 2.0 ng/mL,
CYFRA 1.1 ng/mL, RProGRP 31.6 pg/mL, NSE 8.7 ng/mL, sIL-2R 327 U/mL
and KL-6 410 IU/mL); the interferon-γ release assay for active
tuberculosis (IGRA: T-SPOT.TB) was negative, and neither sputum
cultures or cytology yielded any significant findings. Two weeks
following the first relevant examination (FRE), a transbronchial
lung biopsy (TBLB) was performed on the patient on November 26,
2012, who was admitted to the Tokai University Hachioji Hospital
overnight. Histopathological assessment of the biopsy tissue
revealed nonspecific organized tissue with no evidence of
malignancy. No clinical manifestations were observed during that
time; however, a definitive diagnosis was deemed necessary, and
after consulting with the patient and obtaining consent,
video-assisted thoracic surgery (VATS) and excision of the right
upper nodule was performed on December 18, 2012, three weeks after
the TBLB, for which the patient was admitted until December 28,
2012. Histological evaluation of the frozen material demonstrated
the presence of an organizing pneumonia with no malignancy. A
detailed pathological evaluation of fixed tissue led to the
preliminary diagnosis of an IgG4-related, solitary, fibrous IMT,
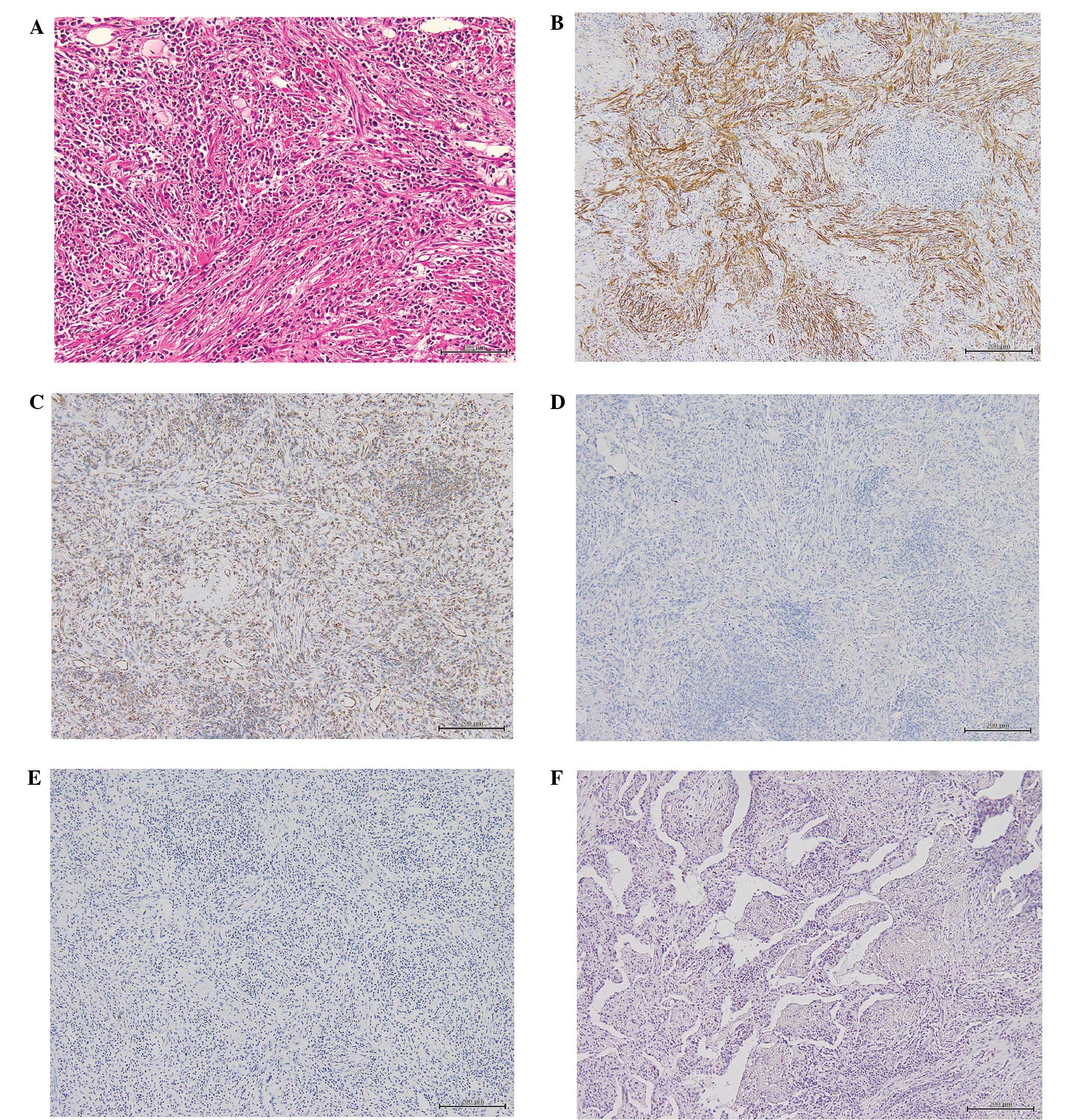
which required further immunohistochemical investigation. The
immunostaining revealed that the nodule was vimentin-positive,
strongly α-SMA-positive, CD34-negative, CD68-positive,
Congo-red-negative, ALK-negative and IgG4-negative (Fig. 3). No evidence of malignancy, including
evidence of cancer or spindle-cell carcinoma, was detected. The
macroscopic findings showed a clearly demarcated mass. Based on the
above findings, a final diagnosis of IMT was made. As there were no
clinical manifestations, we consulted with the patient and decided
to carefully follow her course. The chronic inflammation in the
ALK-negative tissue was considered to be a chronic respiratory
tract inflammation at that time. Clarithromycin was administered as
a long-term macrolide antibiotic therapy, three months following
the FRE, as we expected it would be effective in controlling the
inflammation. The chest shadows slowly resolved on follow-up
imaging examinations, performed every two months. The chest shadows
had almost completely disappeared on the images obtained eight
months following clarithromycin therapy (Figs. 1 and 2).
Clarithromycin therapy was performed for ten months in total, and
during this time there were no clinical manifestations and no
impairments to the patient's everyday life. The patient has
experienced no relapses, and appears to have followed a favorable
course almost one year following the FRE.
Discussion
IMTs are characterized histologically by the
proliferation of spindle-shaped cells (fibroblasts and
myofibroblasts) and an accompanying infiltration by inflammatory
cells, including lymphocytes, plasma cells, and histiocytes. The
histological findings in the present case revealed relatively
uniform spindle-shaped cells, and immunohistochemical staining
demonstrated that the cells were vimentin-positive, strongly
α-SMA-positive, CD68-positive, CD34-negative, IgG4-negative, and
both Congo-red-negative and Direct-fast-scarlet (DFS)-negative,
showing the absence of amyloid (Fig.
3). Our findings were consistent with the description of
earlier IMT cases by Makhlouf et al (13) and Coffin et al (6), and we therefore made a diagnosis of IMT.
The behavior of IMTs range from benign to malignant, and they have
been treated using a number of different methods, including
surgery, anticancer drug therapy, and steroids (14–16).
However, it has been reported that some cases are made worse by the
use of steroids (17). To date, no
standard treatment has been established. Furthermore, there are a
number of reports that ALK-positive IMTs have a poor prognosis
(7,8,18),
contrasting with relatively favorable reports for ALK-negative
cases (19). However, Coffin et
al (6) reported that
ALK-reactivity was associated with local recurrence, but not
distant metastasis (which was confined to ALK-negative lesions).
This was based on the analysis of 59 cases of IMTs, of which 56%
were ALK-positive. The absence of ALK expression was associated
with a greater age overall, and death from disease or distant
metastases. Thus, ALK reactivity may be a favorable prognostic
indicator in IMT.
There have been several previous reports of
successful treatment of ALK-positive IMTs with the ALK inhibitor
crizotinib (20–22). Gene- and immunological-classifications
according to ALK, IgG4 and other markers, as well as histological
classifications, appear necessary for selecting among treatment
options for IMT. Crizotinib may become the mainstay of treatment
for ALK-positive cases.
The etiology of IMT implies that an inflammatory
response is important to the pathology, and IMT cases have often
been presented clinically as a fever of unknown origin (23,24). Local
proinflammatory cytokine production has been previously
demonstrated to have an impact on the pathology (4). Kuppe et al (25) reported that macrophage activation
syndrome was seen in IMT of the lung and thoracic spine.
Accumulation and activation of macrophages by the inflammatory
processes of IMT led to phagocytosis of hematopoietic stem cells in
the bone marrow and caused an increase in proinflammatory
cytokines, including IL-1β, IL-6, and sIL-2R, in the blood.
In the present case, the patient's IMT was
ALK-negative, and controlling the inflammatory response appeared to
lead to improvement of the pathology. Chaves et al (26) investigated the anti-inflammatory
action of non-steroidal anti-inflammatory drugs (NSAIDs). The
authors analyzed reports of ALK-negative cases receiving NSAID
therapy and reported the striking result that NSAIDs were effective
in treating 10 of 11 cases of IMT.
Some macrolide antibiotics are known to possess
unique anti-inflammatory activities (27). Siddiqui et al (28) reported the ability of macrolides to
reduce the secretion of proinflammatory cytokines, ameliorate the
infiltration of inflammatory cells into the airways, and to reduce
mucus secretion, enables them to improve pulmonary function and the
quality of life for patients with chronic inflammatory diseases of
the airways (cystic fibrosis, asthma, bronchiectasis,
panbronchiolitis and cryptogenic organizing pneumonia), as well as
diffuse panbronchiolitis. Furthermore, macrolide antibiotics
decrease levels of IL-1β, IL-6, IL-10, tumor necrosis factor-alpha,
the chemokine ligands (CCLs) 33, 5, 20 and 22, the CXC chemokine
ligands CXCL1, CXCL5, and granulocyte-colony stimulating factor in
the sputum cells from patients with chronic obstructive lung
disease, and have a local anti-inflammatory effect (29–31).
Macrolide antibiotics have been effective in other inflammatory
diseases aside from respiratory diseases, including adult Still's
disease (32), rheumatoid arthritis
(33), and Crohn's disease (34).
Since tissue inflammation appears to be the central
pathology when an IMT is both ALK-negative and IgG4-negative,
controlling the inflammation seems to be an important factor in
improving the pathology. The suppression of inflammatory cell
accumulation and suppression of the production of cytokines,
including Il-6 and IL-8, by macrolide antibiotics confers
anti-inflammatory immunosuppressive effects that differ from the
effects of NSAIDs, and furthermore, macrolides appear to be
effective against chronic tissue inflammation.
To the best of our knowledge, there have been no
previous reports of the treatment of ALK-negative, IgG4-negative
IMTs with macrolide drugs, including clarithromycin, and the
present case appears to be the first reported case of IMT treated
with a macrolide. It may therefore be worth performing a trial of
anti-inflammatory treatment of a series of ALK-negative,
IgG4-negative IMTs in a future study
In conclusion, IMTs are rare disease entity,
sometimes with malignant pathology, and careful attention to the
diagnosis and treatment remains essential. In cases where IMTs are
ALK-negative and IgG4-negative, the
anti-inflammatory-immunomodulating effects of macrolide antibiotics
may have a potential benefit. It is possible that ALK- and,
IgG4-negative IMTs will be reclassified in the future, based on
more detailed genetic analysis and immunohistochemical studies of
the lesion.
References
|
1
|
Pettinato G, Manivel JC, De Rosa N and
Dehner LP: Inflammatory myofibroblastic tumor (plasma cell
granuloma). Clinicopathologic study of 20 cases with
immunohistochemical and ultrastructural observations. Am J Clin
Pathol. 94:538–546. 1990.PubMed/NCBI
|
|
2
|
Zhao JJ, Ling JQ, Fang Y, Gao XD, Shu P,
Shen KT, Qin J, Sun YH and Qin XY: Intra-abdominal inflammatory
myofibroblastic tumor: Spontaneous regression. World J
Gastroenterol. 20:13625–13631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
World Health Organization Classification
of Tumors: Inflammatory myofibroblastic tumor. Pathology and
Genetics of Tumors of Soft Tissue and Bone (Lyon). IARC Press.
91–93. 2002.
|
|
4
|
Fukano R, Matsubara T, Inoue T, Gondo T,
Ichiyama T and Furukawa S: Time lag between the increase of IL-6
with fever and NF-kappaB activation in the peripheral blood in
inflammatory myofibroblastic tumor. Cytokine. 44:293–297. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakurai H, Hasegawa T, Watanabe S, Suzuki
K, Asamura H and Tsuchiya R: Inflammatory myofibroblastic tumor of
the lung. Eur J Cardiothorac Surg. 25:155–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coffin CM, Hornick JL and Fletcher CD:
Inflammatory myofibroblastic tumor: Comparison of
clinicopathologic, histologic, and immunohistochemical features
including ALK expression in atypical and aggressive cases. Am J
Surg Pathol. 31:509–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sokai A, Enaka M, Sokai R, Mori S, Mori S,
Gunji M, Fujino M and Ito M: Pulmonary inflammatory myofibroblastic
tumor harboring EML4-ALK fusion gene. Jpn J Clin Oncol. 44:93–96.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Denis DJ, Elayoubi K, Weil AG, Berthelet F
and Bojanowski MW: Inflammatory myofibroblastic tumors of the
central nervous system that express anaplastic lymphoma kinase have
a high recurrence rate. Surg Neurol Int. 4:702013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karashima T, Taniguchi Y, Shimamoto T, Nao
T, Nishikawa H, Fukata S, Kamada M, Inoue K, Oko K, Nakajima H, et
al: IgG4-related disease of the paratestis in a patient with Wells
syndrome: A case report. Diagn Pathol. 9:2252014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto H, Yamaguchi H, Aishima S, Oda Y,
Kohashi K, Oshiro Y and Tsuneyoshi M: Inflammatory myofibroblastic
tumor versus IgG4-related sclerosing disease and inflammatory
pseudotumor: A comparative clinicopathologic study. Am J Surg
Pathol. 33:1330–1340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zen Y, Kitagawa S, Minato H, Kurumaya H,
Katayanagi K, Masuda S, Niwa H, Fujimura M and Nakanuma Y:
IgG4-positive plasma cells in inflammatory pseudotumor (plasma cell
granuloma) of the lung. Hum Pathol. 36:710–717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bhagat P, Bal A, Das A, Singh N and Singh
H: Pulmonary inflammatory myofibroblastic tumor and IgG4-related
inflammatory pseudotumor: A diagnostic dilemma. Virchows Arch.
463:743–747. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Makhlouf HR and Sobin LH: Inflammatory
myofibroblastic tumors (inflammatory pseudotumors) of the
gastrointestinal tract: How closely are they related to
inflammatory fibroid polyps? Hum Pathol. 33:307–315. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dishop MK, Warner BW, Dehner LP, Kriss VM,
Greenwood MF, Geil JD and Moscow JA: Successful treatment of
inflammatory myofibroblastic tumor with malignant transformation by
surgical resection and chemotherapy. J Pediatr Hematol Oncol.
25:153–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee MH, Lee HB, Lee YC, Rhee YK, Lee EJ,
Chung MJ, Jin GY, Kweon EY and Park SJ: Bilateral multiple
inflammatory myofibroblastic tumors of the lung successfully
treated with corticosteroids. Lung. 189:433–435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diop B, Konate I, Ka S, Sall I, Fall D,
Dieng M and Wone Y: Mesenteric myofibroblastic tumor: NSAID therapy
after incomplete resection. J Visc Surg. 148:e311–e314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schaeffer CJ, Minai OA, Sharma N, Kanne JP
and Mohammed TL: Inflammatory myofibroblastic tumor of the lung:
Recurrence after steroid treatment. J Thorac Imaging. 23:191–193.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He CY, Dong GH and Liu HG: Recurrent
laryngeal inflammatory myofibroblastic tumor with positive
anaplastic lymphoma kinase mimicking recurrent respiratory
papillomatosis: A case report. World J Surg Oncol. 12:542014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Satomi T, Watanabe M, Matsubayashi J,
Nagao T and Chiba H: A successfully treated inflammatory
myofibroblastic tumor of the mandible with long-term follow-up and
review of the literature. Med Mol Morphol. 43:185–191. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lovly CM, Gupta A, Lipson D, Otto G,
Brennan T, Chung CT, Borinstein SC, Ross JS, Stephens PJ, Miller
VA, et al: Inflammatory myofibroblastic tumors harbor multiple
potentially actionable kinase fusions. Cancer Discov. 4:889–895.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jacob SV, Reith JD, Kojima AY, Williams
WD, Liu C and Duckworth Vila L: An unusual case of systemic
inflammatory myofibroblastic tumor with successful treatment with
ALK-Inhibitor. Case Rep Pathol. 2014:4703402014.PubMed/NCBI
|
|
22
|
Tothova Z and Wagner AJ: Anaplastic
lymphoma kinase-directed therapy in inflammatory myofibroblastic
tumors. Curr Opin Oncol. 24:409–413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiu JF, Shi YJ, Fang L, Wang HF and Zhang
MC: High fever as an initial symptom of primary gastric
inflammatory myofibroblastic tumor in an adult woman. Int J Clin
Exp Med. 7:1468–1473. 2014.PubMed/NCBI
|
|
24
|
Zhou R, Xiang J, Chen Z, Li Z and Hong J:
Fever of unknown origin as a presentation of colonic inflammatory
myofibroblastic tumor in a 36-year-old female: A case report. Oncol
Lett. 7:1566–1568. 2014.PubMed/NCBI
|
|
25
|
Kuppe C, Westphal S, Bücher E, Moeller MJ,
Heintz B, Schneider ME and Floege J: Macrophage activation syndrome
in a patient with pulmonary inflammatory myofibroblastic tumour.
Allergy Asthma Clin Immunol. 8:62012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chavez C and Hoffman MA: Complete
remission of ALK-negative plasma cell granuloma (inflammatory
myofibroblastic tumor) of the lung induced by celecoxib: A case
report and review of the literature. Oncol Lett. 5:1672–1676.
2013.PubMed/NCBI
|
|
27
|
Tamaoki J, Kadota J and Takizawa H:
Clinical implications of the immunomodulatory effects of
macrolides. Am J Med. 117(Suppl 9A): 5S–11S. 2004.PubMed/NCBI
|
|
28
|
Siddiqui J: Immunomodulatory effects of
macrolides: Implications for practicing clinicians. Am J Med.
117(Suppl 9A): 26S–29S. 2004.PubMed/NCBI
|
|
29
|
Giamarellos-Bourboulis EJ: Macrolides
beyond the conventional antimicrobials: A class of potent
immunomodulators. Int J Antimicrob Agents. 31:12–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zarogoulidis P, Papanas N, Kioumis I,
Chatzaki E, Maltezos E and Zarogoulidis K: Macrolides: From in
vitro anti-inflammatory and immunomodulatory properties to
clinical practice in respiratory diseases. Eur J Clin Pharmacol.
68:479–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marjanović N, Bosnar M, Michielin F, Willé
DR, Anić-Milić T, Culić O, Popović-Grle S, Bogdan M, Parnham MJ and
Haber Eraković V: Macrolide antibiotics broadly and distinctively
inhibit cytokine and chemokine production by COPD sputum cells
in vitro. Pharmacol Res. 63:389–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thanou-Stavraki A, Aberle T, Aksentijevich
I, Bane BL and Harley JB: Clarithromycin in adult-onset still's
disease: A potentially useful therapeutic. J Clin Rheumatol.
17:373–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Urasaki Y, Nori M, Iwata S, Sasaki T,
Hosono O, Kawasaki H, Tanaka H, Dang NH, Ikeda E and Morimoto C:
Roxithromycin specifically inhibits development of collagen induced
arthritis and production of proinflammatory cytokines by human T
cells and macrophages. J Rheumatol. 32:1765–1774. 2005.PubMed/NCBI
|
|
34
|
Gui GP, Thomas PR, Tizard ML, Lake J,
Sanderson JD and Hermon-Taylor J: Two-year-outcomes analysis of
Crohn's disease treated with rifabutin and macrolide antibiotics. J
Antimicrob Chemother. 39:393–400. 1997. View Article : Google Scholar : PubMed/NCBI
|