Introduction
Colorectal cancer (CRC) is the third most common
cause of cancer-associated mortality worldwide. According to
mortality data from the National Center for Health Statistics
(Hyattsville, MD, USA), 71,000 men and 65,000 women are estimated
to be diagnosed with CRC in the USA each year, and approximately
26,000 men and 24,000 women succumb to disease annually (1). Treatment currently includes surgical
resection if the tumor has not progressed to an advanced stage,
cytotoxic chemotherapy and radiation. However, anticancer drugs
prolong survival without eliminating the cancer, and they
frequently have adverse effects (2).
A number of aspects of tumor function, growth and
division are affected by the pH of the tumor microenvironment
(3). The intracellular pH
(pHi) of CRC tumors has been identified to be neutral to
alkaline (7.0–7.4), whereas extracellular pH (pHe) is
typically acidic (6.9–7.0) (4).
Extracellular acidification in tumors is thought to be primarily
due to lactate secretion following anaerobic glycolysis. Lactate is
a significant regulator of cancer development and maintenance, and
stimulates tumor angiogenesis (5).
Metabolism of glucose leads to the production of high
concentrations of lactate, and this results in an acidic
microenvironment within a number of solid tumors (6).
The expression of carbonic anhydrase 9 (CA9) on the
tumor cell membrane may contribute to an acidic pHe, as CA9
is able to form H+ via catalyzing the reversible
hydration of CO2 to bicarbonate and a proton (7,8). The
active site of CA9, which is located in the extracellular space,
contributes to acidification of the extracellular environment
during hypoxia and maintains a neutral pHi within the tumor
(9). CA9 expression has been
associated with tumor progression, aggressiveness and a poor
prognosis; therefore, it has been proposed as a potential
therapeutic target (10).
Pharmacological inhibition of CA9 catalytic activity may be
achieved via the use of specific CA9 inhibitors or monoclonal
antibodies that interrupt pH regulation in cancer cells. This has
been demonstrated to decrease tumor growth and metastasis (11). Several CA9-targeted agents are in
preclinical or clinical development, and this has established a
precedent for a novel, pH-targeted therapy for the treatment of
cancer (5,12).
In order to survive in the acidic microenvironment,
the pHi-regulating system in tumor cells actively extrudes
acids via the monocarboxylate transporter (MCT) (13). MCT4 is strongly expressed in tumors,
and alterations in pHi have previously been reported to be
produced by MCT4. The transport of H+ across the plasma
membrane and the uptake of lactate markedly increases with
decreasing pHe (14). Studies
have previously shown that lactate transport in MCT4-expressing
cells is accompanied by changes in pHi. An increase in
lactate concentration generates an increase in acidification of the
pHi, indicating a concentration-dependent influx of
H+ (15,16). Therefore, MCT-targeted agents may
additionally be good candidates for cancer therapy (17).
In the present study, HCT116 human colon cancer
cells were treated with lactate calcium salt (CaLa) to investigate
the antitumor effects of artificial lactate influx via pH
regulation of the cancer cells. The combined effects of treatment
with CaLa, 5-indanesulfonamide and α-cyano-4-hydroxycinnamic acid
were additionally investigated in order to identify any enhanced
antitumor effects due to MCT4 and CA9 inhibition.
Materials and methods
Cell lines and reagents
The HCT116 colon cancer cell line was obtained from
American Type Culture Collection (Manassas, VA, USA) and maintained
in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 2 mM
L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco;
Thermo Fisher Scientific, Inc.), at 37°C in a humidified atmosphere
of 5% CO2. CaLa, α-cyano-4-hydroxycinnamic acid (CA; a
CA9 inhibitor) and 5-indanesulfonamide (IS; an MCT4 inhibitor) were
acquired from Sigma-Aldrich (St. Louis, MO, USA). Rabbit polyclonal
anti-human antibodies against poly(adenosine diphosphate ribose)
polymerase (PARP; cat no. 9542) and glyceraldehyde-3-phosphate
dehydrogenase (cat no. 2118) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Rabbit polyclonal anti-human
antibodies against CA9 (cat no. SC-25599) were acquired from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA).
Lactate assay
Lactate concentration was measured using a Lactate
Assay kit (Abcam, Cambridge, MA, USA) according to the
manufacturer's protocol (18).
Briefly, HCT116 cells were seeded into 60-mm dishes at a
concentration of 5×105 cells/well and incubated at 37°C for 24 h.
Following incubation, cells were treated with RMPI 1640 media
(control), CaLa, CA and IS for 24 h. Cells were washed using
phosphate buffered-saline (PBS) and collected. The cells were
subsequently centrifuged (694.4 × g, 5 min) and homogenized in
assay buffer, and the total protein concentration was measured with
a Bicinchoninic Acid Protein Assay kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Lactate concentration was measured by
spectrophotometry and normalized to total protein. Sample solutions
were incubated with the reaction agent for 30 min at room
temperature. Subsequently, the absorbance of samples was measured
with an Epoch microplate spectrophotometer (450 nm; cat no.
0211-3030, Bio-Tek Instruments, Inc., Winooski, VT, USA).
Measurement of pHe
Changes in pHe were measured using a pH electrode
(item no. EW-58823-36; Thermo Fisher Scientific, Inc.) (19). Briefly, 2.5×105 HCT116 cells were
seeded into 6-well plates with RPMI-1640 media and incubated for 24
h. Cells were cultured with IS, CA, CaLa and combinations of the
three for 24 h. Media was collected, and pHe was measured.
Measurement of pHi
To measure pHi, cells were seeded at 2.5×103
cells/well into 96-well plates with RPMI-1640 media and incubated
for 24 h. Cells were cultured with IS, CA, CaLa and combinations of
the three for 24 h. Subsequently, pH-sensitive pHrodoTM Red AM
(Molecular Probes; Thermo Fisher Scientific, Inc.) was incubated
with the HCT116 cells. Briefly, fluorescence was measured at an
excitation wavelength of 555 nm and emission wavelength of 580 nm,
analyzed and converted to pHi using a nigericin calibration
curve.
Colony formation assay
HCT116 cells were seeded into 6-well plates with
RPMI-1640 media at a density of 5×102 cells/well and incubated at
37°C. Following incubation and treatment with CaLa, CA and IS,
cells were allowed to grow for 7 days to form colonies. The cells
were washed twice using PBS, and stained with hematoxylin. Colony
morphology was observed with an optical microscope (Eclipse TS100;
Nikon, Tokyo, Japan).
Cell viability assay
HCT-116 cells were seeded at a density of 5,000
cells/well in 96-well plates and treated with RMPI 1640 media
(control), CaLa (5 mM), IS (1 mM) and CA (5 mM) for 7 days. The
cell viability assay was performed using
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT; 5 mg/ml; Biosesang Seongnam, Korea). MTT reduction was
assessed using an Epoch Micro-Volume Spectrophotometer System
(Bio-Tek Instruments, Inc.) at 460 nM.
Western blot
Cultured cells were washed twice using cold PBS and
lysed on ice in lysis buffer (Thermo Fisher Scientific, Inc.)
containing 1 M Tris-HCl (pH 7.4), 5 M NaCl, 0.5 mM
ethylenediaminetetraacetic acid, NP-40, NaF, deoxycholate, 0.1%
Triton X-100 and protease inhibitor cocktail tablets (Roche
Diagnostics, Indianapolis, IN, USA). Protein samples of 20 µg were
loaded on 8% polyacrylamide gels and subjected to electrophoresis.
Protein bands were transferred to nitrocellulose membranes (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and blocked with 0.1% Tween
20 and 5% skimmed milk protein in PBS for 1 h at room temperature.
Membranes were subsequently probed using primary polyclonal
antibodies (Dilutions: PARP, 1:1,000; CA9, 1:5,000; GAPDH,
1:5,000). Following incubation with goat anti-rabbit IgG antibody
(cat no. bs-0295G-HRP; 1:5000 dilution; Bioss, Inc., Woburn, MA,
USA), immunoblots were developed with western blot detection
reagents (GE Healthcare Life Sciences, Chalfont, UK) and exposed to
X-ray film (Agfa-Gevaert N.V., Leverkusen, Germany), according to
the manufacturer's protocol. Densitometry measurements were
performed using ImageJ 1.48 software (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical significance was analyzed using the
Student's t-test depending on the normality of the data. P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using SigmaStat (version
3.5; Systat Software Inc., San Jose, CA, USA).
Results
Lactate accumulation occurs via CA9
and MCT4 inhibition
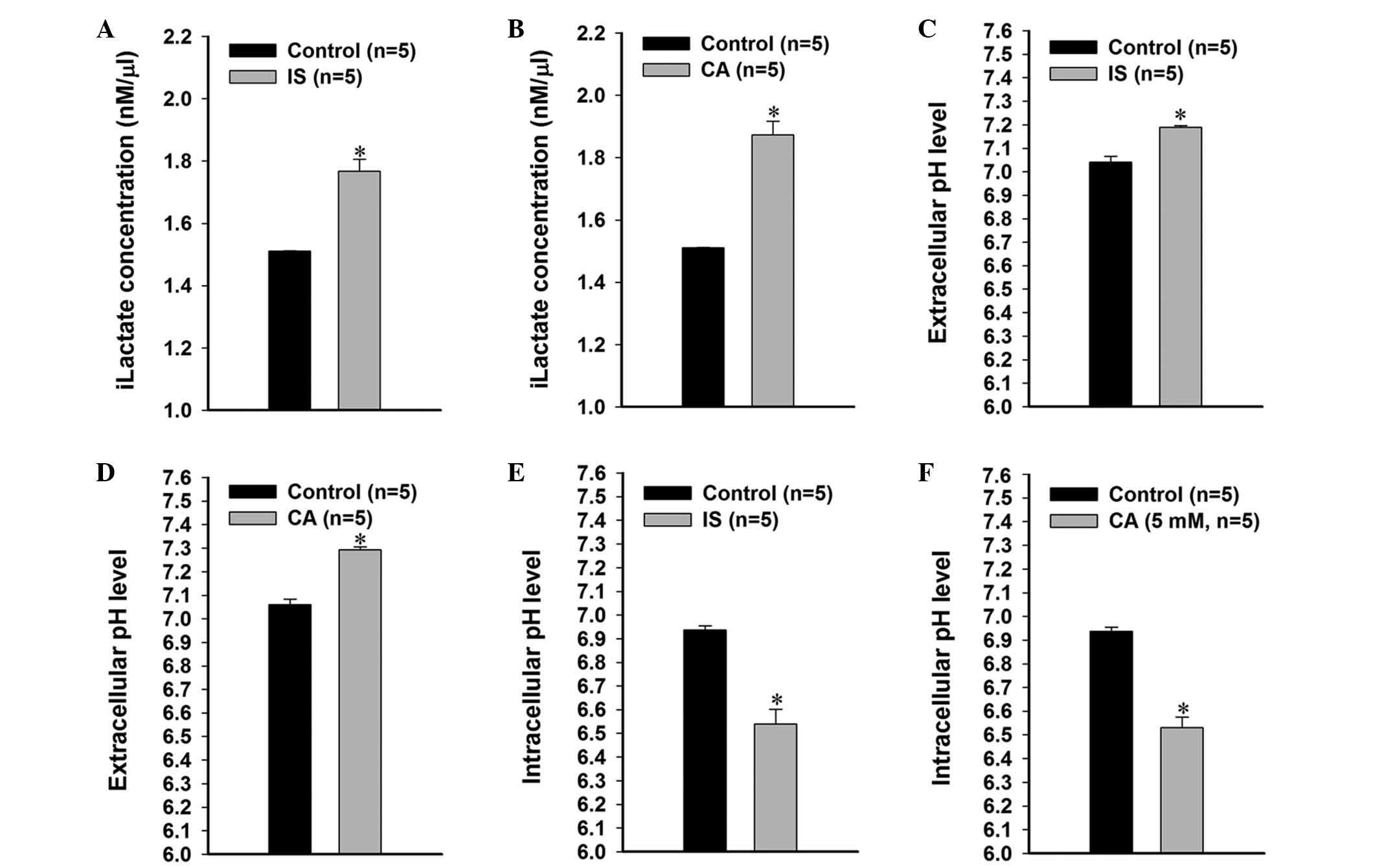
In order to investigate the role of lactate
transport via MCT4 and CA9 activity, HCT116 cells were cultured in
1 mM CA- or 5 mM IS-containing media. IS treatment led to a
significant increase (P=0.044) in the concentration of
intracellular lactate (iLactate) compared with the control group
(Fig. 1A). CA treatment also resulted
in a significant increase to 1.77 nm/µl (P=0.009) in the
concentration of iLactate compared with the control group (1.51
nm/µl; Fig. 1B).
IS and CA affect the regulation of pH
in colon cancer cells
To determine the role of CA9 and MCT4 in pH
regulation, HCT116 colon cancer cells were cultured in 1 mM CA- or
5 mM IS-containing media. pHe was observed to be significantly
increased (IS, 7.19±0.006; CA, 7.29±0.012; P=0.016) following
treatment with IS and CA (Fig. 1C and
D). By contrast, IS or CA treatment induced a significant
decrease (IS, 6.94 ± 0.017; CA, 6.21±0.045; P=0.007) in the pHi
(Fig. 1E and F).
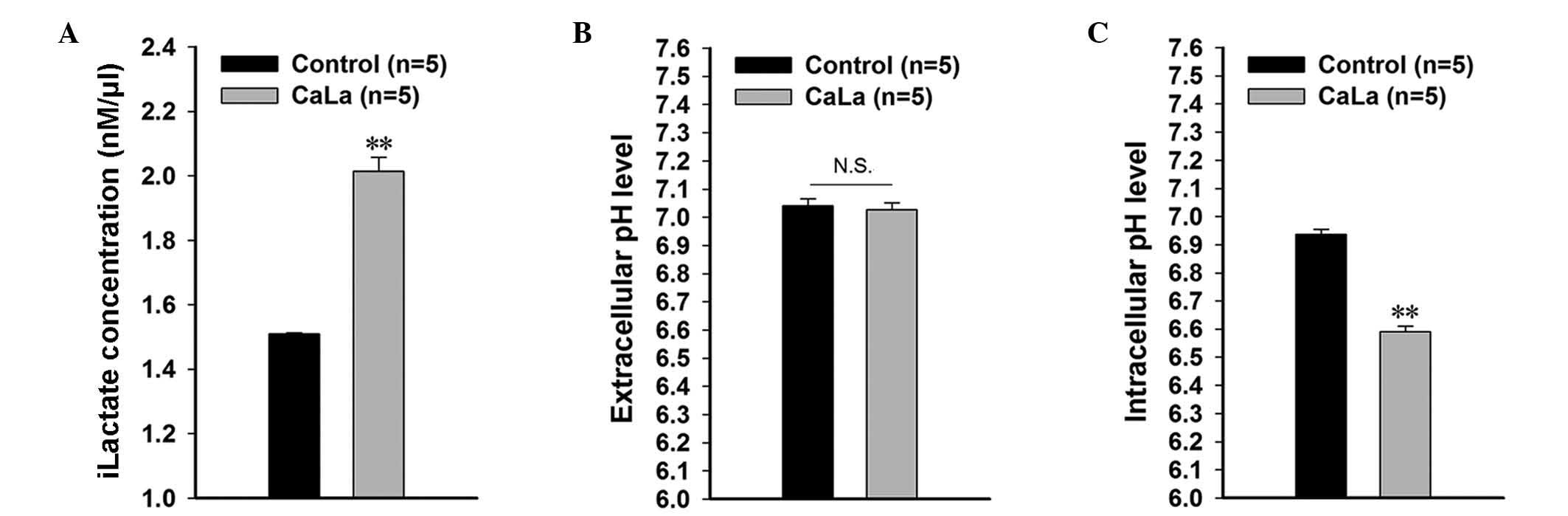
CaLa treatment affects the
pH-associated tumor microenvironment
iLactate concentration, pHe and pHi were measured in
colon cancer cells following 5 Mm CaLa treatment. iLactate was
significantly increased to 0.285 nm/µl (P=0.001) following CaLa
treatment compared with the control group (0.216 nm/µl; Fig. 2A). The pHe was similar in the control
and CaLa-treated groups (Fig. 2B).
However, pHi was significantly decreased to 6.50±0.020 (P<0.001)
in the CaLa-treated group compared with the control group
(6.94±0.017; Fig. 2C). This indicated
that lactate-induced acidification was facilitated by the addition
of CaLa to colon cancer cells.
Combined treatment with CaLa, IS and
CA affects the pH-associated tumor microenvironment of colon cancer
cells
A treatment strategy for combination treatment of
CaLa (5 mM) with IS (1 Mm) and CA (5 mM) was established.
Initially, cells were exposed to CaLa, IS and CA separately and
incubated for 12 h. Subsequently, the cells were washed using PBS
and treated to form combinations of CaLa+IS, CaLa+CA or CaLa+IS+CA.
On the final day of the experiment, the iLactate concentration, pH
and cell viability were measured (Fig.
3A). iLactate concentration was significantly increased in the
CaLa-treated (2.01 nm/µl; P=0.017), IS+CA-treated (1.85 nm/µl;
P=0.016) and CaLa+IS-treated (2.02 nm/µl; P=0.017) groups compared
with the control group (1.51 nm/µl, Fig.
3B). The CaLa+CA-treated (2.55 nm/µl) and Ca+IS+CA-treated
(2.75 nm/µl) groups demonstrated a greater increase in the iLactate
concentration, which indicated an increased effect on lactate
content (P<0.001; Fig. 3B).
According to the pHe results, the IS+CA-treated (7.32±0.02),
CaLa+IS-treated (7.26±0.02), CaLa+CA-treated (7.25±0.018) and
CaLa+IS+CA-treated (7.29±0.021) groups demonstrated an increased
pHe compared with the control group (7.00±0.026) and CaLa-treated
group (6.96±0.015; Fig. 3C). The pHi
was significantly lower (6.84±0.015; P=0.018) following CaLa
treatment (6.59±0.019) compared with the control group (6.84±0.015,
Fig. 3D). Similarly, treatment with
IS+CA (6.31±0.018), CaLa+IS (6.27±0.015) and CaLa+CA (6.33±0.018)
induced a decrease in the pHi compared with the CaLa-treated group
(6.59±0.019). The greatest decrease in pHi was observed with the
three-agent combination treatment of CaLa+IS+CA (6.19±0.015;
Fig. 3D). The decrease in pHi
signified lactate-induced intracellular acidification.
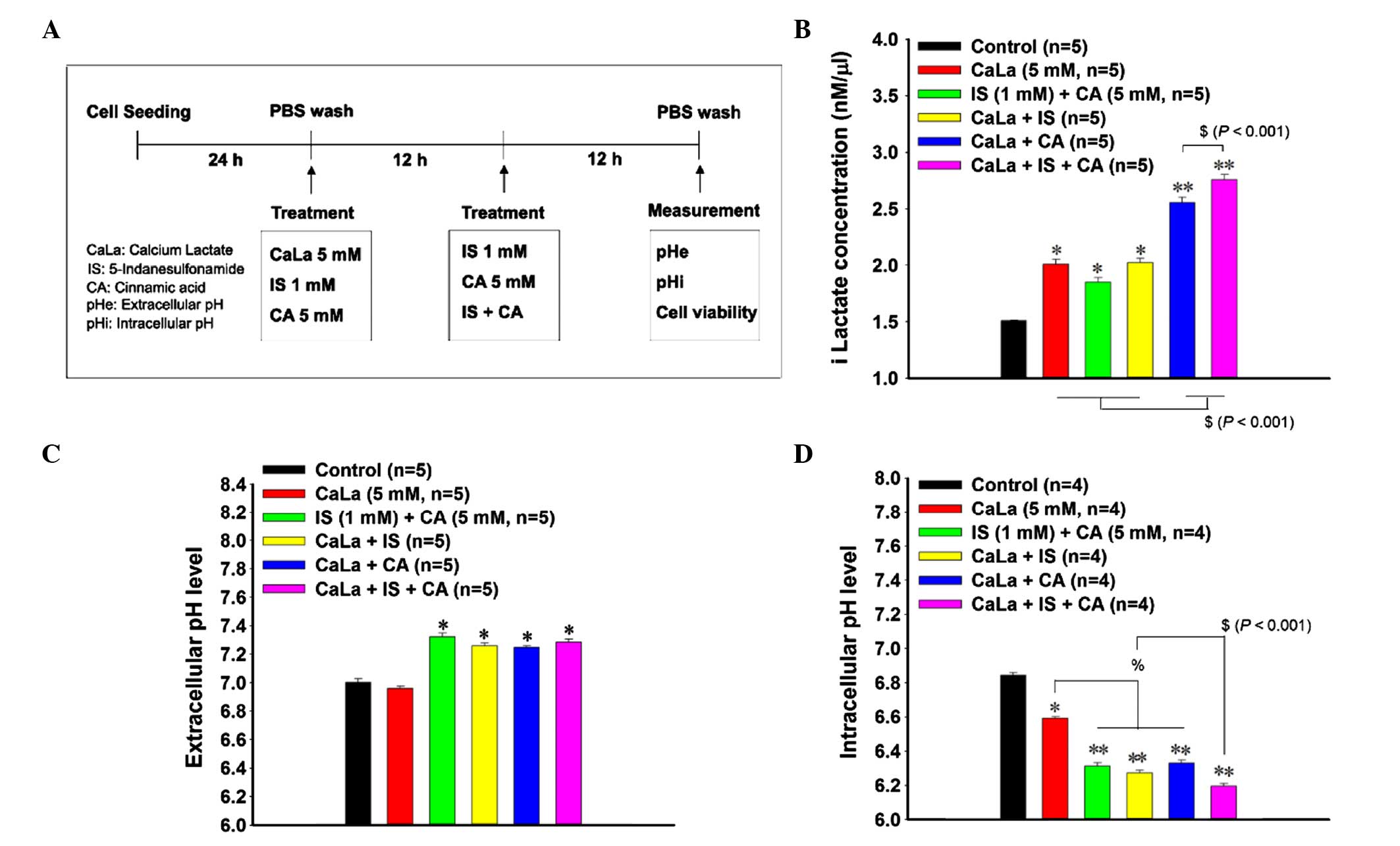 | Figure 3.Combined effects of treatment with
CaLa, IS and CA on lactate accumulation and cellular pH. (A)
Schematic representation of a strategy for combined treatment with
CaLa, IS and CA. Effect of combined treatment on (B) iLactate
accumulation, (C) extracellular pH and (D) intracellular pH. IS was
used as a carbonic anhydrase 9 inhibitor, and CA was used as a
monocarboxylate transporter 4 inhibitor. Control cells were treated
with RPMI 1640 media. Results are presented as the mean ± standard
deviation. *P<0.05 and **P<0.001 vs. control;
%P<0.05; $P<0.001. CaLa, lactate
calcium salt; IS, 5-indanesulfonamide; CA,
α-cyano-4-hydroxycinnamic acid; iLactate, intracellular lactate;
pHe, extracellular pH; pHi, intracellular pH; PBS,
phosphate-buffered saline. |
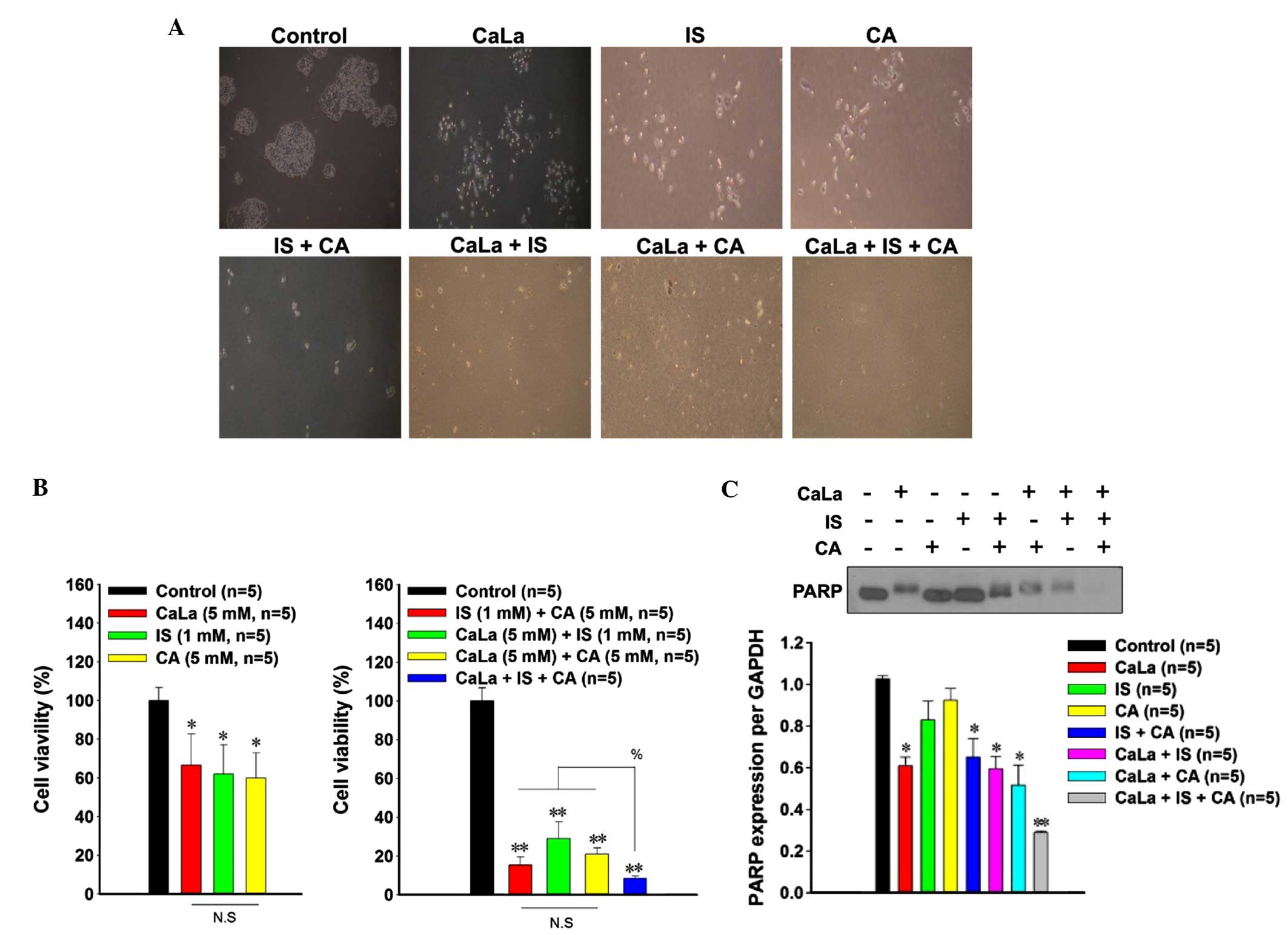
Treatment with CaLa, IS and CA has a
combined effect on the viability of colon cancer cells
Colony formation was markedly decreased following
single treatment with CaLa, IS or CA compared with controls.
Additionally, treatment with combinations of the agents (IS+CA,
CaLa+IS, CaLa+CA or CaLa+IS+CA) completely inhibited colony
formation compared with the control group (Fig. 4A). The cytotoxic effects on colon
cancer cells were compared using an MTT assay. Relative to the
control group (defined as 100% cell viability), decreased cell
viability was observed following administration of a single-agent
treatment with CaLa (66.61±16.17; P=0.029), IS (62.01±15.01;
P=0.028) or CA (59.9±13.13; P=0.023) (Fig. 4B). Administration of two different
agents (IS+CA, 15.42±4.18; CaLa+IS, 29.01±8.52; or CaLa+CA,
21.01±3.26) significantly reduced cell viability (P<0.001),
whilst the three-agent combination of CaLa+IS+CA (8.42±1.31) led to
a greater reduction in cell viability (P<0.001) compared with
the two-agent combinations.
PARP is involved in acid-induced cell
death
In order to investigate the mechanism underlying the
decrease in viability of colon cancer cells, the expression of PARP
was determined using western blot analysis (Fig. 4C). Among the single treatments, only
the CaLa-treated group (0.70±0.04) demonstrated a decrease in PARP
expression compared with the control group (1.03±0.01). Similarly,
PARP was significantly decreased (P=0.017) following treatment with
the two-agent combinations (IS+CA, 0.65±0.08; CaLa+IS, 0.79±0.06;
and CaLa+CA, 0.72±0.09). The three-agent combination of CaLa+IS+CA
resulted in a greater reduction (0.38±0.008) in the expression of
PARP compared with CaLa-only treatment and two-agent combination
treatments.
Discussion
In the present study, the effects of CaLa on the pH
microenvironment of colon cancer cells were investigated. The
findings revealed that lactate was able to induce intracellular
acidification via MCT4 and CA9 inhibition. CaLa increased the
intracellular lactate concentration, which led to a more acidic
pHi. These conditions may contribute to the inhibition of
cancer cell survival. Combination treatment of CaLa with CA and IS
led to a marked decrease in the viability of colon cancer cells.
The current findings demonstrate that CaLa may potentiate the
activity of CA9 and MCT4 inhibitors, providing in vitro
evidence for the utility of CaLa as a potential pharmacological
agent for the treatment of colon cancer.
The majority of cancer cells produce lactate under
aerobic conditions, which indicates a state of active glycolysis
(20). The intracellular lactate
shuttle is important in maintaining the redox balance within cells
(21). Lactate overproduction by
tumors may be due to exaggerated glycolysis and a decreased
clearance capacity caused by an impaired capacity for oxidative
phosphorylation (22). Lactate
accumulation has been proposed as a marker of malignancy in certain
types of human cancer (23).
CA is known to inhibit MCT4, preventing
monocarboxylate uptake and therefore inhibiting extracellular
lactate transport (24). In addition,
IS may block lactate transport via inhibition of CA9 activity
(4). Therefore, intracellular lactate
is increased following treatment with CA and IS. Lactate
accumulation in tumor cells is accompanied by changes in the
cytosolic pH, leading to acidification (25).
The primary function of CA9 in cancer is maintenance
of pH homeostasis, which is associated with acidification of the
tumor microenvironment and promotion of cancer cell migration and
invasiveness. An additional function of CA9 is to maintain the
intracellular pH under hypoxic conditions (9). Expression of CA9 has been proposed to be
a marker of hypoxia and an indicator of poor prognosis (25). MCT offers a mechanism for achieving
low pHe, while maintaining an alkaline pHi (4). This mechanism has been well-demonstrated
using an MCT4 inhibitor, which led to an alkaline pHe and an
acidic pHi (5,26). Previous reports have demonstrated that
lactate transport in MCT4-expressing cells was accompanied by
changes in the cytosolic pH (16).
The high levels of lactate produced by cancer cells are usually
removed by MCTs. MCT transport depends on pH, intracellular vs.
extracellular lactate concentration and the levels of additional
MCT substrates. The cotransport of protons with lactate prevents
the toxic buildup of lactate and the acidification of the
intracellular environment. Accordingly, lactate transport by MCTs
is a therapeutic vulnerability of cancer cells, as intracellular
acidosis poses a threat to cell survival (5).
In the present study, CaLa was used to induce
iLactate accumulation in colon cancer cells. CaLa was observed to
pass easily through tumor cell membranes. As a number of cancer
cells are able to utilize calcium, a calcium-bound form of lactate
was used. MCT4 channels were able to facilitate the transport of
CaLa out of tumor cells. Therefore, inhibition of MCT4 led to
increased intracellular acidification following CaLa treatment.
PARP is important in the maintenance of genetic
integrity in response to DNA damage, and has been implicated in
cell death induced by DNA damage (27). PARP is the substrate for the majority
of caspases in vitro, and it has been reported that
intracellular acidification may lead to apoptotic cell death
(28). Apoptotic stimuli, including
cancer chemotherapies, are capable of inducing intracellular
acidification (29). Direct induction
of an acidic pHi reportedly triggers the classic hallmarks
of apoptosis, including nuclear condensation, cytoplasmic
vacuolization and endonuclease-mediated DNA degradation (30). These observations suggest a direct
association between acidification and a decrease in the viability
of colon cancer cells. In the present study, downregulation of PARP
activity was significant in the CaLa-treated group. Furthermore,
combination treatment with CaLa+IS+CA led to enhanced inhibition of
PARP activity.
In conclusion, the present study demonstrated that
combination treatment with CaLa+IS+CA is able to induce
intracellular acidification in colon cancer cells via maintenance
of lactate accumulation, and to inhibit the viability of colon
cancer cells. Therefore, it is proposed that combined treatment
with CaLa+IS+CA may enhance antitumor activity compared with
single-agent treatment, and this may provide a potential approach
for the treatment of colon cancer via regulation of pHi.
Acknowledgements
The present study was supported by the Gachon
Institute of Pharmaceutical Sciences Research Fund 2013 (Gachon
University, Incheon, Republic of Korea).
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer, pHi,
intracellular pH
|
|
pHe
|
extracellular pH
|
|
CA9
|
carbonic anhydrase 9
|
|
MCT
|
monocarboxylate transporter
|
|
CaLa
|
lactate calcium salt
|
|
CA
|
α-cyano-4-hydroxycinnamic acid
|
|
IS
|
5-indanesulfonamide
|
|
PARP
|
poly(adenosine diphosphate ribose)
polymerase
|
|
iLactate
|
intracellular lactate
|
|
PBS
|
phosphate-buffered saline
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrasolium bromide
|
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benito M and Díaz-Rubio E: Molecular
biology in colorectal cancer. Clin Transl Oncol. 8:391–398. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parks SK, Chiche J and Pouyssegur J: pH
control mechanisms of tumor survival and growth. J Cell Physiol.
226:299–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swietach P, Patiar S, Supuran CT, Harris
AL and Vaughan-Jones RD: The role of carbonic anhydrase 9 in
regulating extracellular and intracellular pH in three-dimensional
tumor cell growths. J Biol Chem. 284:20299–20310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doherty JR and Cleveland JL: Targeting
lactate metabolism for cancer therapeutics. J Clin Invest.
123:3685–3692. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Semenza GL: Tumor metabolism: Cancer cells
give and take lactate. J Clin Invest. 118:3835–3837.
2008.PubMed/NCBI
|
|
7
|
Kato Y, Ozawa S, Miyamoto C, Maehata Y,
Suzuki A, Maeda T and Baba Y: Acidic extracellular microenvironment
and cancer. Cancer Cell Int. 13:892013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Swietach P, Vaughan-Jones RD and Harris
AL: Regulation of tumor pH and the role of carbonic anhydrase 9.
Cancer Metastasis Rev. 26:299–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McDonald PC, Winum JY, Supuran CT and
Dedhar S: Recent developments in targeting carbonic anhydrase IX
for cancer therapeutics. Oncotarget. 3:84–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gu MJ and Kwon KW: Carbonic anhydrase IX
expression is associated with favorable prognostic factors in small
intestinal carcinoma. J Histochem Cytochem. 62:205–210. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McIntyre A, Patiar S, Wigfield S, Li JL,
Ledaki I, Turley H, Leek R, Snell C, Gatter K, Sly WS, et al:
Carbonic anhydrase IX promotes tumor growth and necrosis in
vivo and inhibition enhances anti-VEGF therapy. Clin Cancer
Res. 18:3100–3111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dubois L, Peeters S, Lieuwes NG, Geusens
N, Thiry A, Wigfield S, Carta F, McIntyre A, Scozzafava A, Dogné
JM, et al: Specific inhibition of carbonic anhydrase IX activity
enhances the in vivo therapeutic effect of tumor
irradiation. Radiother Oncol. 99:424–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiche J, Ilc K, Laferrière J, Trottier E,
Dayan F, Mazure NM, Brahimi-Horn MC and Pouysségur J:
Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell
growth by counteracting acidosis through the regulation of the
intracellular pH. Cancer Res. 69:358–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nguyen TT and Bonanno JA: Bicarbonate,
NBCe1, NHE, and carbonic anhydrase activity enhance lactate-H+
transport in bovine corneal endothelium. Invest Ophthalmol Vis Sci.
52:8086–8093. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakayama Y, Torigoe T, Inoue Y, Minagawa
N, Izumi H, Kohno K and Yamaguchi K: Prognostic significance of
monocarboxylate transporter 4 expression in patients with
colorectal cancer. Exp Ther Med. 3:25–30. 2012.PubMed/NCBI
|
|
16
|
Dimmer KS, Friedrich B, Lang F, Deitmer JW
and Bröer S: The low-affinity monocarboxylate transporter MCT4 is
adapted to the export of lactate in highly glycolytic cells.
Biochem J. 350:219–227. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gotanda Y, Akagi Y, Kawahara A, Kinugasa
T, Yoshida T, Ryu Y, Shiratsuchi I, Kage M and Shirouzu K:
Expression of monocarboxylate transporter (MCT)-4 in colorectal
cancer and its role: MCT4 contributes to the growth of colorectal
cancer with vascular endothelial growth factor. Anticancer Res.
33:2941–2947. 2013.PubMed/NCBI
|
|
18
|
Hussien R and Brooks GA: Mitochondrial and
plasma membrane lactate transporter and lactate dehydrogenase
isoform expression in breast cancer cell lines. Physiol Genomics.
43:255–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lou Y, McDonald PC, Oloumi A, Chia S,
Ostlund C, Ahmadi A, Kyle A, dem Keller Auf U, Leung S, Huntsman D,
et al: Targeting tumor hypoxia: Suppression of breast tumor growth
and metastasis by novel carbonic anhydrase IX inhibitors. Cancer
Res. 71:3364–3376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pacchiano F, Carta F, McDonald PC, Lou Y,
Vullo D, Scozzafava A, Dedhar S and Supuran CT: Ureido-substituted
benzenesulfonamides potently inhibit carbonic anhydrase IX and show
antimetastatic activity in a model of breast cancer metastasis. J
Med Chem. 54:1896–1902. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Draoui N and Feron O: Lactate shuttles at
a glance: From physiological paradigms to anti-cancer treatments.
Dis Model Mech. 4:727–732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng J: Energy metabolism of cancer:
Glycolysis versus oxidative phosphorylation (Review). Oncol Lett.
4:1151–1157. 2012.PubMed/NCBI
|
|
23
|
Touisni N, Maresca A, McDonald PC, Lou Y,
Scozzafava A, Dedhar S, Winum JY and Supuran CT: Glycosyl coumarin
carbonic anhydrase IX and XII inhibitors strongly attenuate the
growth of primary breast tumors. J Med Chem. 54:8271–8277. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klier M, Andes FT, Deitmer JW and Becker
HM: Intracellular and extracellular carbonic anhydrases cooperate
non-enzymatically to enhance activity of monocarboxylate
transporters. J Biol Chem. 289:2765–2775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ditte Z, Ditte P, Labudova M, Simko V,
Iuliano F, Zatovicova M, Csaderova L, Pastorekova S and Pastorek J:
Carnosine inhibits carbonic anhydrase IX-mediated extracellular
acidosis and suppresses growth of HeLa tumor xenografts. BMC
Cancer. 14:3582014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lim KS, Lim KJ, Price AC, Orr BA, Eberhart
CG and Bar EE: Inhibition of monocarboxylate transporter-4 depletes
stem-like glioblastoma cells and inhibits HIF transcriptional
response in a lactate-independent manner. Oncogene. 33:4433–4441.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim MY, Zhang T and Kraus WL:
Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear
signal. Genes Dev. 19:1951–1967. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zanke BW, Lee C, Arab S and Tannock IF:
Death of tumor cells after intracellular acidification is dependent
on stress-activated protein kinases (SAPK/JNK) pathway activation
and cannot be inhibited by Bcl-2 expression or interleukin
1beta-converting enzyme inhibition. Cancer Res. 58:2801–2808.
1998.PubMed/NCBI
|
|
29
|
Lagadic-Gossmann D, Huc L and Lecureur V:
Alterations of intracellular pH homeostasis in apoptosis: Origins
and roles. Cell Death Diff. 11:953–961. 2004. View Article : Google Scholar
|
|
30
|
Thammasit P, Sangboonruang S, Suwanpairoj
S, Khamaikawin W, Intasai N, Kasinrerk W, Tayapiwatana C and
Tragoolpua K: Intracellular Acidosis promotes mitochondrial
apoptosis pathway: Role of EMMPRIN down-regulation via specific
single-chain Fv intrabody. J Cancer. 6:276–286. 2015. View Article : Google Scholar : PubMed/NCBI
|