Introduction
Gastric cancer is one of the most common types of
cancer, and the second leading cause of cancer-associated mortality
worldwide (1). Almost half of the
patients with gastric cancer are Chinese, the majority of whom are
diagnosed when the disease has progressed to advanced stages, due
to the non-specific symptoms, including epigastric pain, anorexia
and vomiting, that present during the early stages of the disease
(2). As a result, the overall 5-year
survival rate of gastric cancer is ~20% (3). Numerous studies have investigated the
etiology of gastric cancer (4–6). However,
the molecular mechanisms that underlie the pathogenesis and
progression of gastric cancer remain undefined. Therefore,
additional studies are required to elucidate the molecular
mechanisms that lead to the metastasis and progression of gastric
cancer, and to identify novel markers for the diagnosis, prognosis
and treatment of patients with gastric cancer.
MicroRNAs (miRNAs) are short non-coding RNAs of
19–24 nucleotides in length that control the translation and
stability of their target messenger (m) RNA by binding to
regulatory sites located in the 3′-untranslated region of the
transcripts (7). It is widely
accepted that miRNAs play pivotal roles in various biological
processes, including development, metabolism, cell proliferation,
differentiation and apoptosis (8).
Numerous studies suggest that there is an association between
altered miRNA expression and cancer, since aberrant expression of
miRNAs appears to be involved in several important processes that
occur during carcinogenesis. Previous studies have investigated the
role of miRNA-524-5p (miR-524-5p) in various types of cancer. Chen
et al (9) demonstrated that
miR-524-5p was associated with overall survival rate and
pathological grade of patients with glioma, and Liu et al
(10) revealed that the expression of
miR-524-5p was reduced in human melanoma, while overexpression of
miR-524-5p effectively inhibited melanoma cell proliferation and
migration. Furthermore, Liu et al (10) demonstrated that tumors overexpressing
miR-524-5p were significantly smaller than those displayed by
negative control mice. However, the role of miR-524-5p in gastric
cancer remains unclear.
The present study investigated the expression levels
of miR-524-5p in human gastric cancer tissues and cell lines,
including MKN-45, SGC-7901 and MGC-803 cell lines and gastric
epithelial mucosa GES-1 cells. In addition, cell proliferation and
migration assays, as well as a cell apoptosis analysis, were
performed using human gastric cancer SGC-7901 and MGC-803 cell
lines to explore the in vitro effects of miR-524-5p in
gastric cancer cells.
Materials and methods
Tissue samples
A total of 15 gastric cancer and adjacent
non-cancerous tissue samples were obtained from patients that had
undergone surgical treatment for gastric cancer at The First
Affiliated Hospital of Zhengzhou University (Zhengzhou, China)
between March 2011 and March 2013. The patients were diagnosed
independently by two experienced pathologists from The First
Affiliated Hospital of Xinxiang Medical University, according to
the Cancer Staging Manual published by the American Joint Committee
on Cancer. The present study was approved by the Ethics Committee
of the Medical College of Zhengzhou University and informed consent
was obtained from the patients prior to sample collection,
conforming to the Declaration of Helsinki and the local
legislation. Written informed consent was obtained from all
patients prior to the start of the study.
Cell culture
Human gastric cancer MKN-45, SGC-7901 and MGC-803
cell lines were obtained from the American Type Culture Collection
(Manassas, VA, USA), while the human gastric epithelial mucosa
GES-1 cell line was purchased from the Shanghai Institutes for
Biological Sciences of the Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in RPMI-1640 medium (BioTeke
Corporation, Beijing, China) supplemented with 10% heat-inactivated
Invitrogen fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich, St. Louis, MO, USA) in a humidified cell incubator
with 5% CO2 at 37°C.
Plasmids and cell transfection
A miR-524-5p mimic and inhibitor, alongside their
corresponding negative controls (scramble miRNA), were purchased
from Shanghai GenePharma Co., Ltd. (Shanghai, China). The SGC-7901
and MGC-803 cells were seeded in 6-well plates at 30% confluence
one day prior to transfection. The cells were transfected with
miR-524-5p mimic, miR-524-5p inhibitor and control miRNA using
Invitrogen Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cell lines and the
tissue samples using Invitrogen TRIzol reagent (Thermo Fisher
Scientific, Inc.) and DNAse (catalog no. ab32124; Abcam, Cambridge,
MA, USA). RT was performed using the PrimeScript™ RT reagent Kit
with gDNA Eraser (Takara Biotechnology Co., Ltd., Dalian, China),
according to the manufacturer's protocol. The expression of
miR-524-5p was verified by stem-loop RT-qPCR with specific RT and
PCR primers. The primer sequences (Sangon, Shanghai, China) were:
Matrix metallopeptidase (MMP)-2, sense, 5′-CCCCAGACAGGTGATCTTGAC-3′
and antisense, 5′-GCTTGCGAGGGAAGAAGTTG-3′; and MMP-9, sense,
5′-CGCTGGGCTTAGATCATTCC-3′ and antisense,
5′-AGGTTGGATACATCACTGCATTAGG-3′. U6 small nuclear RNA was used as
an internal control. RT-qPCR for MMP-2 and MMP-9 was performed
using the following conditions: 95°C for 2 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 30 sec. qPCR was performed
on an Applied Biosystems® 7500 thermocycler (Thermo Fisher
Scientific, Inc.) using SYBR® Premix Ex Taq™ (Tli RNaseH Plus)
(Takara Biotechnology Co., Ltd.), according to the manufacturer's
protocol. The comparative CT method, ∆∆Ct, was used to quantify the
data. Briefly, ΔCt was calculated by subtracting the CT of U6 or
GAPDH mRNA from the mRNA of interest, and ΔΔCt was calculated by
subtracting the ΔCt of the negative control from the ΔCt of the
samples. The data was normalized according to the study conducted
by Schmittgen and Livak (11).
Western blot analysis
Transfected cells were washed with ice-cold
phosphate-buffered saline (PBS) and lysed using a cell lysis buffer
consisting of 50 mM Tris (pH 8.0), 120 mM NaCl, 0.5% NP-40, 50 mM
NaF and 1 mM phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology, Haimen, China). The cells were centrifuged at 10,000
× g for 15 min at 4°C. The protein concentration was measured by
BCA Protein Assay Kit (Beyotime Institute of Biotechnology). Cell
protein lysates were separated on a 12% sodium dodecyl
sulfate-polyacrylamide gel with the Mini-PROTEAN® II
Electrophoresis System (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) using Mini-Protean precast gels (Bio-Rad Laboratories, Inc.)
at 200 V for 1 h, and subsequently transferred onto a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). The membranes were next blocked with 5% skimmed milk in
Tris-buffered saline (pH 7.4) containing 0.05% Tween 20 (TBST), and
incubated with primary antibodies, including anti-MMP-2 (mouse;
monoclonal; dilution, 1:10; catalog no. ab80737; Abcam, Cambridge,
UK), anti-MMP-9 (mouse; monoclonal; dilution, 1:25; catalog no.
ab51203; Abcam) and anti-β-actin (mouse; monoclonal; dilution,
1:5000; catalog no. ab75373; Abcam) overnight at 4°C. The membranes
were washed three times in TBST for 10 min, and subsequently
incubated with HRP conjugated goat anti-rabbit IgG secondary
antibodies (catalog no. MBS560261; Mybiosource, San Diego, CA,
USA). Chemiluminescence detection was performed using Pierce™ Fast
Western Blot kit (Thermo Fisher Scientific, Inc.) and ECL Substrate
(GE Healthcare Life Sciences, Chalfont, UK). The densitometry
analysis was analyzed using Image-Pro Plus software, version
1.61.
Cell proliferation assays
The transfected cells were seeded onto 96-well
plates (Corning Incorporated, Corning, NY, USA) at a density of
1×104 cells/well. In total, 20 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT;
Sigma-Aldrich) at a concentration of 5 mg/ml was added to the
transfected cells, and incubated at 37°C for 4 h. Following the
removal of the culture medium, the remaining crystals were
dissolved in dimethyl sulfoxide (Sigma-Aldrich), and the absorbance
at 570 nm was measured. All experiments were performed in
triplicate.
Cell apoptosis
Cell apoptosis analyses were performed as previously
described, with certain modifications (12). Briefly, SGC-7901 and MGC-803 cells
were detached using trypsin (Sigma-Aldrich), washed twice in PBS,
centrifuged at 1,000 × g for 5 min and resuspended in 195 µl
Annexin V-fluorescein isothiocyanate (FITC) binding buffer
(Sigma-Aldrich). Subsequently, 5 µl Annexin V-FITC (BD Biosciences,
Erembodegem, Belgium) was added to the cells. Following an
incubation period of 15 min in the dark at room temperature, 400 µl
binding buffer was added. The percentage of apoptotic cells was
analyzed using flow cytometry (FACSCalibur™; BD Biosciences,
Franklin Lakes, NJ, USA) and CellQuest Pro™ Software (version
5.2.1; BD Biosciences).
Cell invasion assays
Cell invasion was determined using 24-well Transwell
chambers coated with Matrigel™ (BD Biosciences, Bedford, MD, USA)
in order to determine the effect of miR-524-5p on gastric cancer
cell invasion. Briefly, 8-µm pore size filters (Sigma-Aldrich) were
coated with 100 µl Matrigel™ (1 mg/ml; dissolved in serum-free
RPMI-1640). A total of 600 µl RPMI-1640 containing 10% Invitrogen
FBS (Thermo Fisher Scientific) was added to the lower chamber,
while single cell suspensions (1×105 cells/well) were
added to the upper chamber. Cells were incubated for 48 h at 37°C,
and non-invading cells were removed using cotton swabs (Takara
Biotechnology Co. Ltd.). Invaded cells were stained with 0.1%
crystal violet (Takara Biotechnology Co., Ltd.) for 10 min at room
temperature and examined using the Olympus BX51 fluorescence
microscope (Olympus Optical Co., Tokyo, Japan).
Statistical analysis
The results are presented as the mean ± standard
deviation. The Student's t-test was used to assess the statistical
significance of differences between groups. The data were analyzed
by SPSS version 11.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of miR-524-5p in gastric
cancer tissues and cell lines
In order to determine the expression levels of
miR-524-5p in gastric cancer, the present study evaluated the
expression levels of miR-524-5p in human gastric cancer tissues and
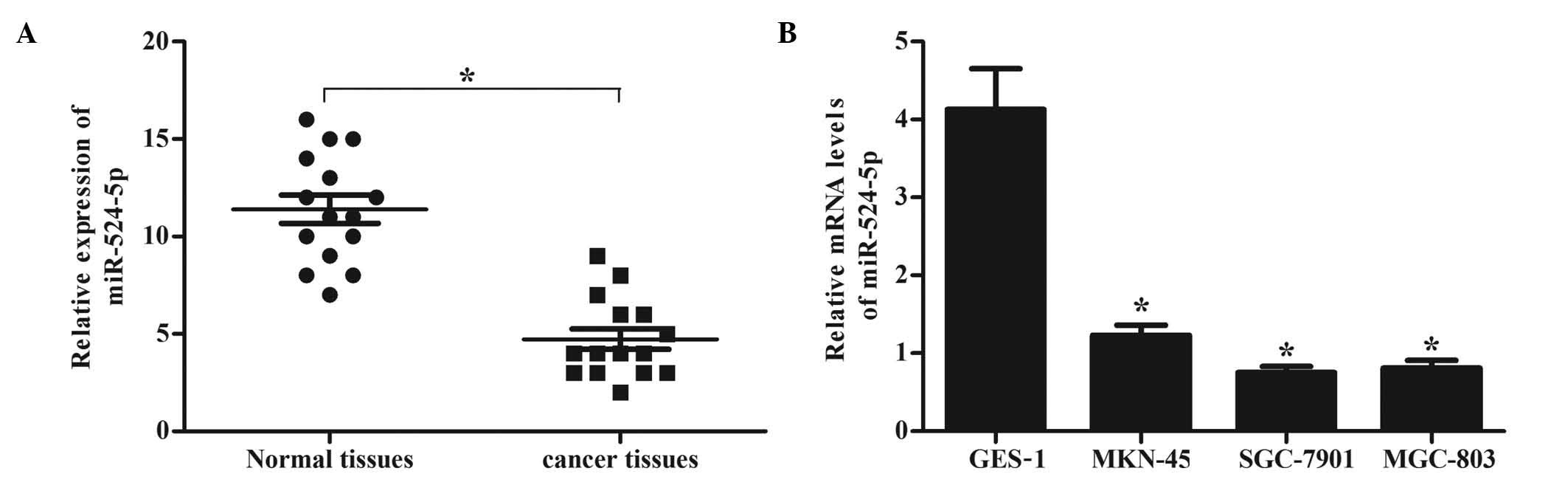
cell lines using RT-qPCR. As demonstrated by Fig. 1A, the expression levels of miR-524-5p
were markedly lower in gastric cancer tissues, compared with normal
gastric mucosa tissue. Similarly, the expression levels of
miR-524-5p were significantly decreased in MKN-45, SGC-7901 and
MGC-803 cells, compared with human normal gastric epithelial mucosa
GES-1 cells (Fig. 1B). These results
demonstrate that miR-524-5p is downregulated in gastric cancer
tissues and cell lines. SGC-7901 and MGC-803 cells were selected
for the subsequent in vitro experiments, since the mRNA
expression levels of miR-524-5p were lower in these cells than in
MKN-45 cells.
Effects of miR-524-5p on gastric
cancer cell proliferation
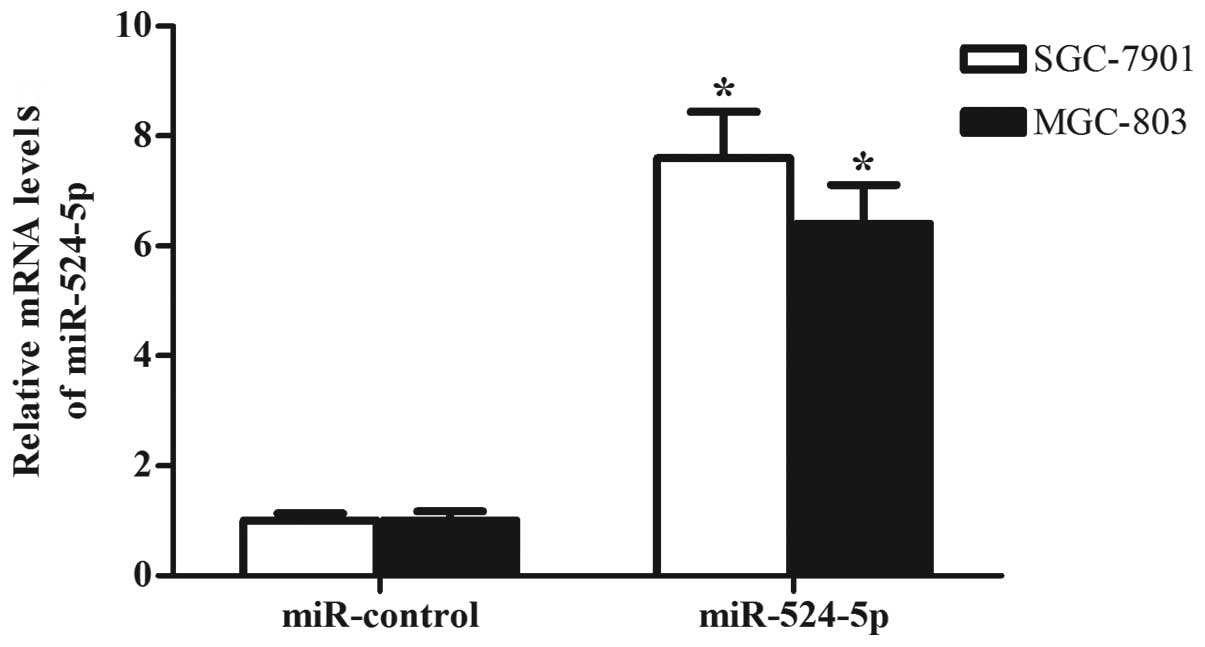
Since miR-524-5p was significantly downregulated in
gastric cancer, the present study concluded that miR-524-5p may
inhibit growth, promote apoptosis and enhance invasion in gastric
cancer cells. To verify this hypothesis, the expression levels of
miR-524-5p in SGC-7901 and MGC-803 cells were upregulated by
transfecting the cells with miR-524-5p mimics, as detected by
RT-qPCR (Fig. 2). Subsequently, an
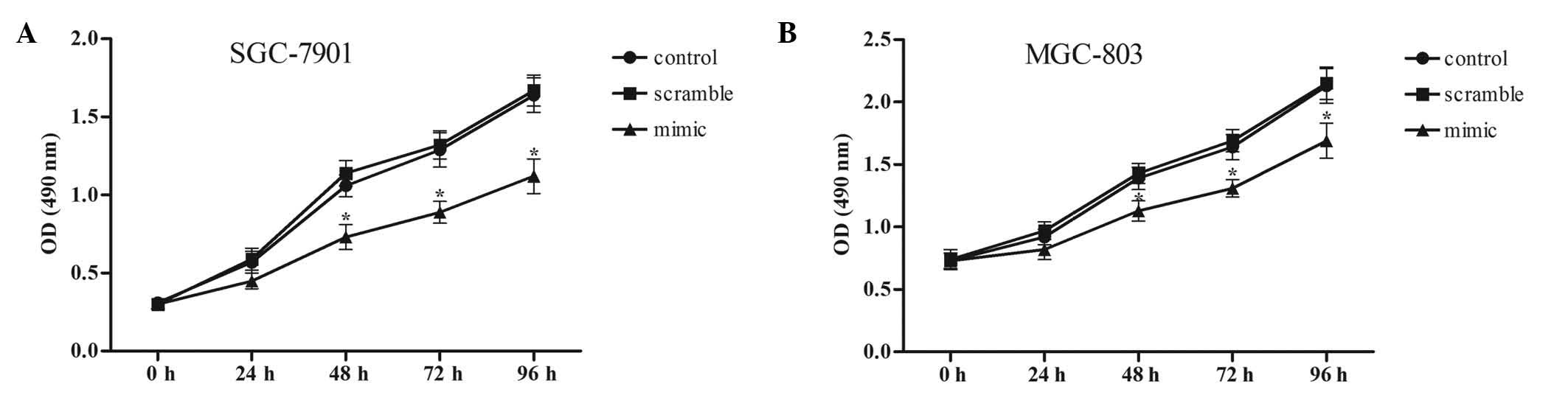
MTT assay was performed to evaluate the effects of miR-524-5p on
the growth of SGC-7901 and MGC-803 cells. As demonstrated by
Fig. 3, miR-524-5p overexpression
inhibited the growth of SGC-7901 and MGC-803 cells, and the
inhibitory effect of miR-524-5p was markedly increased with
increasing transfection times.
Effect of miR-524-5p on gastric cancer
cell apoptosis
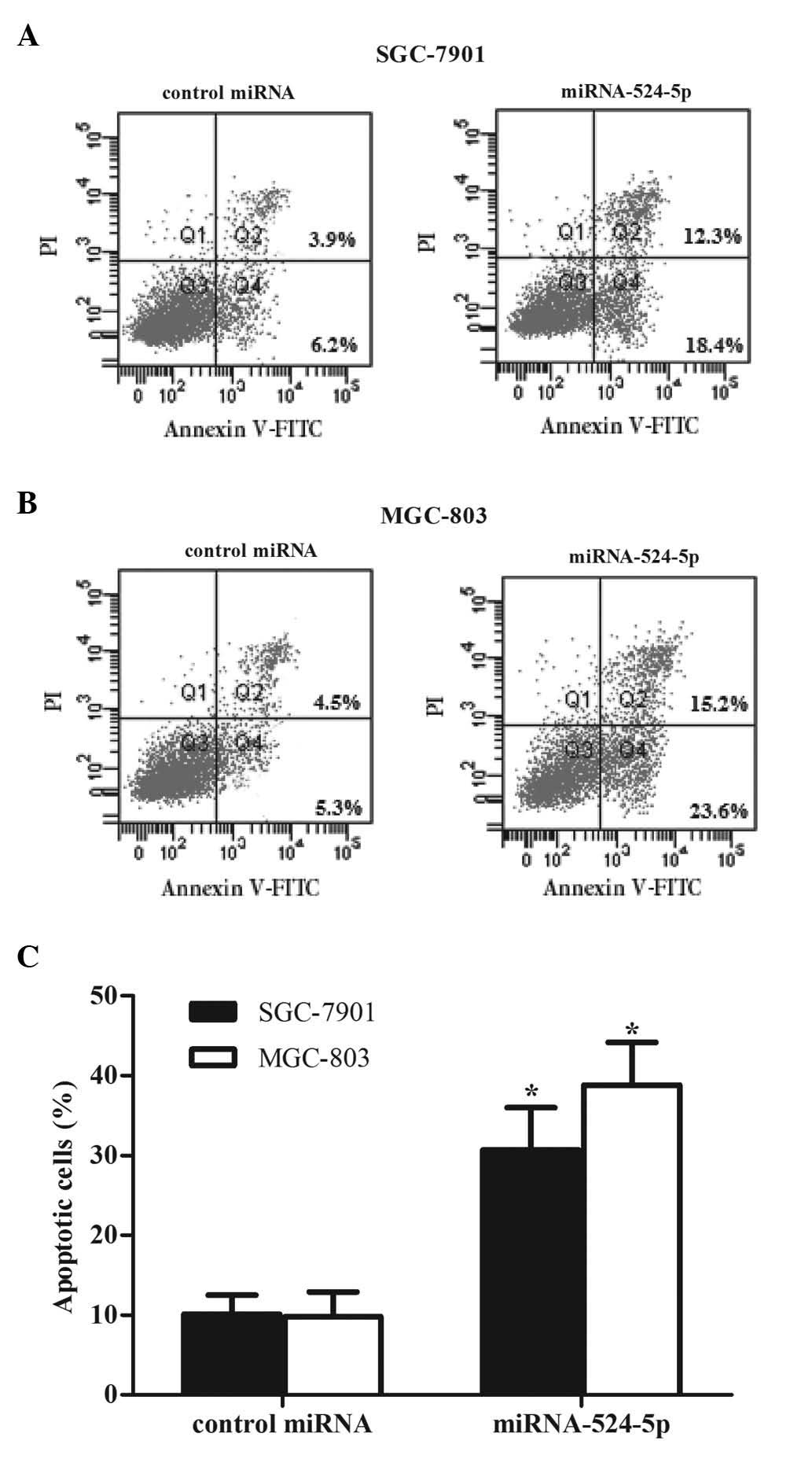
Cell apoptosis was detected using propidium iodide
and Annexin V-FITC staining following 48 h of miR-524-5p mimic
transfection. The percentage of cells undergoing apoptosis was
measured using flow cytometry. Following transfection with
miR-524-5p mimics, the percentage of apoptosis significantly
increased to 30.7% in SGC-7901 cells (Fig. 4A) and 38.8% in MGC-803 cells (Fig. 4B), compared with 10.1 and 9.8%,
respectively, in the control groups. These results demonstrate that
miR-524-5p is critical in promoting the apoptosis of gastric cancer
cells.
Effects of miR-524-5p on gastric
cancer cell invasion
The present study examined the effect of miR-524-5p
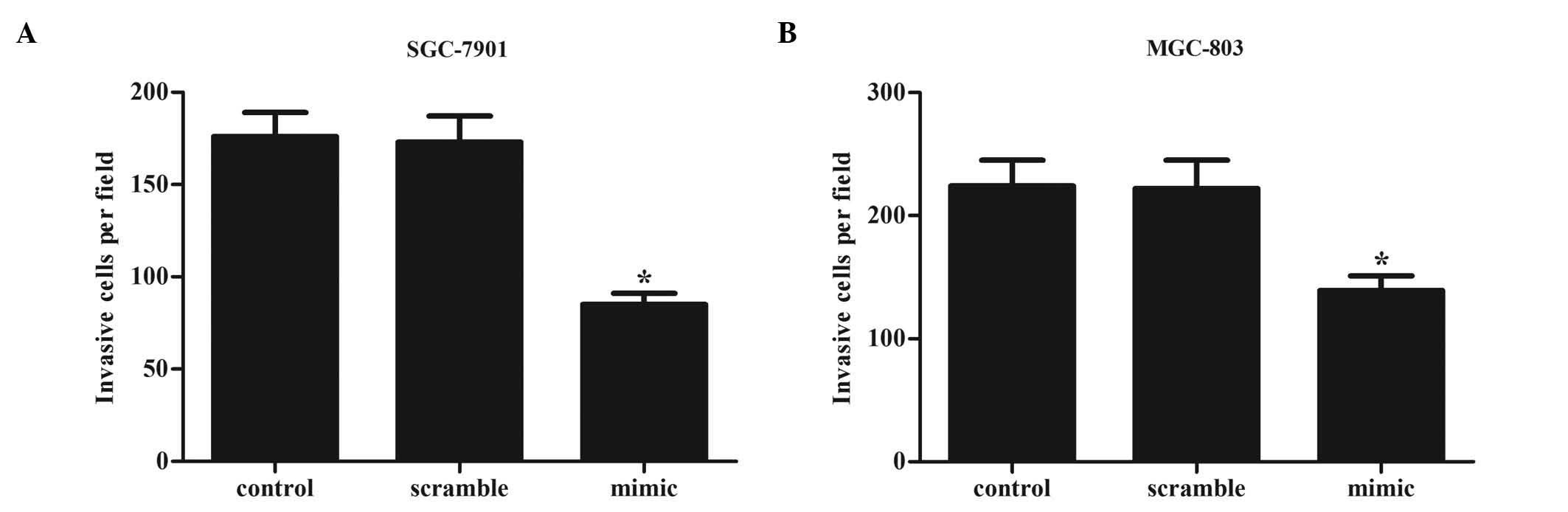
on gastric cancer cell invasion. As demonstrated by Fig. 5, the number of invasive cells was
significantly decreased in SGC-7901 and MGC-803 cells transfected
with miR-524-5p mimics, compared with miR-control-transfected cells
(SGC-7901 miR-524-5p-transfected vs. control cells, 85 vs. 173
cells; MGC-803 miR-524-5p-transfected vs. control cells, 139 vs.
222 cells). These results indicate that overexpression of
miR-524-5p inhibits the invasion ability of gastric cancer cells
in vitro.
miR-524-5p transfection attenuates
cell invasion by inhibiting the expression of MMP-2 and MMP-9
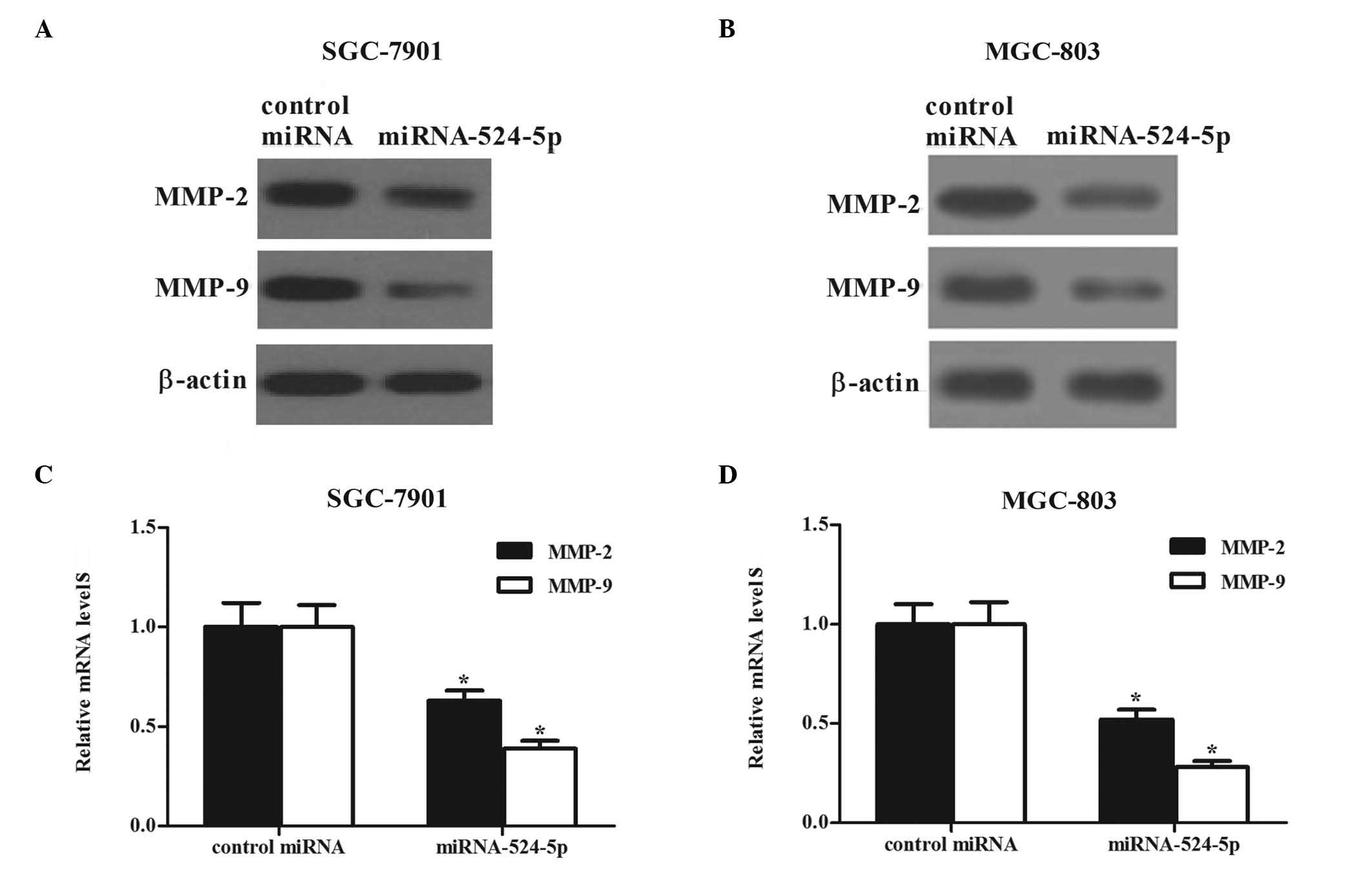
Numerous studies have demonstrated that
extracellular MMP-2 and MMP-9 are overexpressed in various types of
cancer (13–15). As demonstrated in Fig. 6, the protein expression levels of
MMP-2 and MMP-9 were significantly decreased in SGC-7901 (Fig. 6A) and MGC-803 (Fig. 6B) cells transfected with miR-524-5p
mimics. Similarly to the results of western blot analysis, a
decrease in the mRNA levels of MMP-2 and MMP-9 was observed using
RT-qPCR (Fig. 6C and D). These
results demonstrate that transfection with miR-524-5p attenuates
gastric cancer cell invasion by inhibiting the expression of MMP-2
and MMP-9.
Discussion
Previous studies have demonstrated that the aberrant
expression of miRNAs is associated with the development of various
types of human cancer. These studies have also indicated that
miRNAs may function as oncogenes or tumor-suppressor genes
(16,17). In addition, previous studies have
identified numerous dysregulated miRNAs, including miR-30b,
miR-372, miR-126 and miR-21, that modulate growth, apoptosis,
migration and invasion in gastric cancer cells (18–23). The
present study revealed that the expression levels of miR-524-5p
were downregulated in gastric cancer tissues and cell lines, and
overexpression of miR-524-5p inhibited gastric cancer cell
proliferation and invasion and promoted cell apoptosis. Therefore,
the present study suggests that miR-524-5p functions as a tumor
suppressor in human gastric cancer.
Dysregulation of miR-524-5p is a frequent event in
several types of cancer, including a reduction in the expression of
miR-524-5p in melanoma (10) and
glioma cells (9). The present study
demonstrated that the expression levels of miR-524-5p were
downregulated in gastric cancer tissues and cell lines, suggesting
that miR-524-5p functions as a tumor suppressor in human gastric
cancer, which is consistent with previous studies (24).
Apoptosis is programmed cell-death that occurs in
physiological and pathological conditions (25). A disruption in cell apoptosis may
result in the development of cancer (26). The current study revealed that ectopic
expression of miR-524-5p induced a greater percentage of gastric
cancer cells to undergo apoptosis, compared with control cells
in vitro. These results suggest that miR-524-5p may be
involved in gastric cancer by promoting the apoptosis of cancer
cells.
In patients with gastric cancer, metastasis is one
of the leading causes of mortality (27). Since miR-524-5p was observed to be
downregulated in gastric cancer tissues and cell lines, the present
study hypothesized that overexpressing miR-524-5p may suppress the
metastasis of gastric cancer cells. The results of the present
study demonstrated that overexpression of miR-524-5p in human
gastric cancer SGC-7901 and MGC-803 cells significantly inhibited
metastasis. These findings suggest an inhibitory role for
miR-524-5p in the development of metastasis in gastric cancer,
which is, to the best of our knowledge, a novel result. In
addition, the results of the present study indicate that decreased
expression of miR-524-5p in gastric cells may contribute to the
development of gastric cancer.
Degradation of the extracellular matrix (ECM) in
blood or lymph vessels is critical to develop metastasis, since the
loss of the ECM allows the cancer cells to invade the blood or
lymphatic system and spread to distant tissues and organs (28). MMPs are a family of zinc-dependent
enzymes that are essential for the progression of cancer (29). MMP-2 is expressed on the surface of
tumor cells, and is involved in tumor metastasis by activating
pro-MMP-2, which exacerbates the malignancy (30). The activation of pro-MMP-2 by
membrane-type 1 matrix metalloproteinase (MT1-MMP) is considered to
be a critical event in cancer cell invasion. In the activation
step, TIMP metallopeptidase inhibitor 2 bound to MT1-MMP on the
cell surface acts as a receptor for pro-MMP-2. MT1-MMP is expressed
on the cancer cell surface as an invasion-promoting proteinase. By
localizing at the leading edge of invasive cancer cells, MT1-MMP
degrades components of the tissue barriers. One of the major
targets is type I collagen, the most abundant ECM component.
Although MT1-MMP itself cannot degrade type IV collagen in the
basement membrane, it binds to and activates proMMP-2, one of the
type IV collagenases. However, degradation of the ECM is not the
sole function of MT1-MMP (31). MMP-2
is considered an important marker for the presence of distant
metastasis in cancer (32). MMP-9 is
also responsible for the degradation of the ECM, and is pivotal in
tumor invasion and metastasis (33).
The activated MMP-9 enhances the invasive phenotype of the cultured
cells as their ability to both degrade extracellular matrix and
transverse basement membrane is significantly increased following
zymogen activation.
Liao et al (34) reported that miRNA-196 (miR-196) is
highly expressed in gastric cancer, and overexpression of miR-196
significantly induced migration and invasion of gastric cancer
cells by decreasing the levels of MMP-2 and MMP-9. By contrast, the
present study investigated whether the inhibitory role of
miR-524-5p on cell invasion occurs through regulating the
expression of MMP-2 and MMP-9. The present study revealed that
miR-524-5p downregulated the expression levels of MMP-2 and MMP-9,
which explains the inhibition of invasion caused by miR-524-5p in
gastric cells during the invasion assay.
In summary, the results of the present study
indicate that miR-524-5p is a tumor suppressor gene that is
involved in the development of gastric cancer. Overexpression of
miR-524-5p inhibits cell proliferation and invasion, and promotes
apoptosis in gastric cancer cells. Therefore, miR-524-5p may be a
novel therapeutic target for the treatment of gastric cancer.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer
in: 2008 GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan B, Yau EX, Omar Bte SS, Ong CW, Pang
B, Yeoh KG and Salto-Tellez M: A study of HER2 gene amplification
and protein expression in gastric cancer. J Clin Pathol.
63:839–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamanoi K, Fukuma M, Uchida H, Kushima R,
Yamazaki K, Katai H, Kanai Y and Sakamoto M: Overexpression of
leucine-rich repeat-containing G protein-coupled receptor 5 in
gastric cancer. Pathol Int. 63:13–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ning X, Sun S, Zhang K, Liang J, Chuai Y,
Li Y and Wang X: S100A6 protein negatively regulates
CacyBP/SIP-mediated inhibition of gastric cancer cell proliferation
and tumorigenesis. PLoS One. 7:e301852012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baek D, Villén J, Shin C, Camargo FD, Gygi
SP and Bartel DP: The impact of microRNAs on protein output.
Nature. 455:64–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Zhang W, Yan W, Han L, Zhang K,
Shi Z, Zhang J, Wang Y, Li Y, Yu S, et al: The putative tumor
suppressor miR-524-5p directly targets Jagged-1 and Hes-1 in
glioma. Carcinogenesis. 33:2276–2282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu SM, Lu J, Lee HC, Chung FH and Ma N:
miR-524-5p suppresses the growth of oncogenic BRAF melanoma by
targeting BRAF and ERK2. Oncotarget. 5:9444–9459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative CT method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hou T, Ou J, Zhao X, Huang X, Huang Y and
Zhang Y: MicroRNA-196a promotes cervical cancer proliferation
through the regulation of FOXO1 and p27Kip1. Br J Cancer.
110:1260–1268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao J, Ding F, Liu Q and Yao Y: Knockdown
of MACC1 expression suppressed hepatocellular carcinoma cell
migration and invasion and inhibited expression of MMP2 and MMP9.
Mol Cell Biochem. 376:21–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Q, Yang P, Tu K, Zhang H, Zheng X, Yao
Y and Liu Q: TPX2 knockdown suppressed hepatocellular carcinoma
cell invasion via inactivating AKT signaling and inhibiting MMP2
and MMP9 expression. Chin J Cancer Res. 26:410–417. 2014.PubMed/NCBI
|
|
15
|
Huang Q, Lan F, Wang X, Yu Y, Ouyang X,
Zheng F, Han J, Lin Y, Xie Y, Xie F, et al: IL-1β-induced
activation of p38 promotes metastasis in gastric adenocarcinoma via
upregulation of AP-1/c-fos, MMP2 and MMP9. Mol Cancer. 13:182014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao Y, Suo AL, Li ZF, Liu LY, Tian T, Ni
L, Zhang WG, Nan KJ, Song TS and Huang C: MicroRNA profiling of
human gastric cancer. Mol Med Rep. 2:963–970. 2009.PubMed/NCBI
|
|
18
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu ED, Li N, Li BS, Li W, Zhang WJ, Mao
XH, Guo G, Zou QM and Xiao B: miR-30b, down-regulated in gastric
cancer, promotes apoptosis and suppresses tumor growth by targeting
plasminogen activator inhibitor-1. PLoS One. 9:e1060492014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho WJ, Shin JM, Kim JS, Lee MR, Hong KS,
Lee JH, Koo KH, Park JW and Kim KS: miR-372 regulates cell cycle
and apoptosis of ags human gastric cancer cell line through direct
regulation of LATS2. Mol Cells. 28:521–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng R, Chen X, Yu Y, Su L, Yu B, Li J,
Cai Q, Yan M, Liu B and Zhu Z: miR-126 functions as a tumour
suppressor in human gastric cancer. Cancer Lett. 298:50–63. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu
W, Xiao S and Lu H: miR-21 plays a pivotal role in gastric cancer
pathogenesis and progression. Lab Invest. 88:1358–1366. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu SM, Lu J, Lee HC, Chung FH and Ma N:
miR-524-5p suppresses the growth of oncogenic BRAF melanoma by
targeting BRAF and ERK2. Oncotarget. 5:9444–9459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cotter TG: Apoptosis and cancer: The
genesis of a research field. Nat Rev Cancer. 9:501–507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bang YJ, Kim YW, Yang HK, Chung HC, Park
YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, et al: CLASSIC trial
investigators: Adjuvant capecitabine and oxaliplatin for gastric
cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label,
randomised controlled trial. Lancet. 379:315–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen K, Zhang S, Ji Y, Li J, An P, Ren H,
Liang R, Yang J and Li Z: Baicalein inhibits the invasion and
metastatic capabilities of hepatocellular carcinoma cells via
down-regulation of the ERK pathway. PLoS One. 8:e729272013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee SJ, Kim WJ and Moon SK: Role of the
p38 MAPK signaling pathway in mediating interleukin-28A-induced
migration of UMUC-3 cells. Int J Mol Med. 30:945–952.
2012.PubMed/NCBI
|
|
30
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seiki M: Membrane-type 1 matrix
metalloproteinase: A key enzyme for tumor invasion. Cancer Lett.
194:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ueda J, Kajita M, Suenaga N, Fujii K and
Seiki M: Sequence-specific silencing of MT1-MMP expression
suppresses tumor cell migration and invasion: Importance of MT1-MMP
as a therapeutic target for invasive tumors. Oncogene.
22:8716–8722. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liao YL, Hu LY, Tsai KW, Wu CW, Chan WC,
Li SC, Lai CH, Ho MR, Fang WL, Huang KH and Lin WC: Transcriptional
regulation of miR-196b by ETS2 in gastric cancer cells.
Carcinogenesis. 33:760–769. 2012. View Article : Google Scholar : PubMed/NCBI
|