Introduction
Lung cancer is the leading cause of cancer mortality
worldwide and is also associated with a poor prognosis (1). The tumor microenvironment has been
demonstrated to be an important factor in cancer progression and
drug resistance, as it may lead to dysregulated immune responses
during tumor progression and the facilitation of tumor invasion
(2). Tumor-associated dendritic cells
(TADCs) are important in the tumor microenvironment, as they
secrete numerous factors that promote lung cancer growth,
migration, invasion and epithelial-to-mesenchymal transition
(3). One factor, lung
tumor-associated dendritic cell-derived resistin, has been
indicated to promote cancer progression (4). Other lung TADC factors that have
synergistic effects on cancer progression include heparin-binding
epidermal growth factor-like growth factor and chemokine CXCL5
(5).
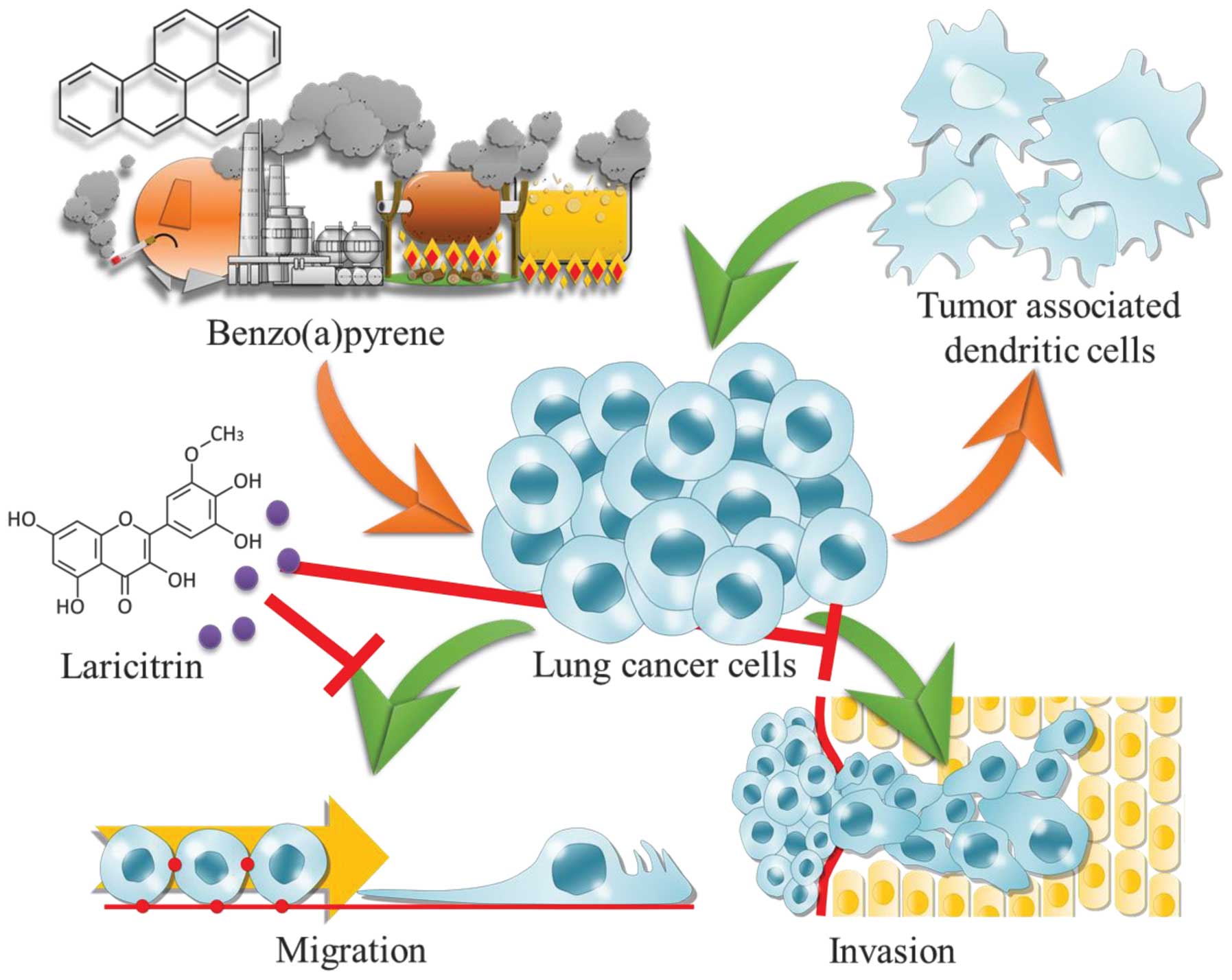
Benzo(a)pyrene (BaP) is a carcinogenic polycyclic
aromatic hydrocarbon that has been associated with lung cancer. BaP
is found in cigarettes, food and automobile exhausts (6). BaP causes DNA adduct formation, which is
the initiating event in carcinogenesis (7). BaP has also been demonstrated to promote
A549 cell migration and invasion by upregulating Twist (8).
Flavonols are usually present in glycosidic forms
and are synthesized in grape skin; therefore, they are also present
in red wine (9). Flavonols are a
subclass of flavonoid that have antioxidant properties and have a
potential role in the prevention of cardiovascular disease
(10). Flavonoids have also been
demonstrated to have the potential to induce colorectal cancer cell
apoptosis via the mitochondrial-mediated pathway (11). Flavonoids also suppress the growth of
H460 and A549 cells by inducing cell cycle arrest in the S and G2/M
phases. Additionally, flavonoids also induce apoptosis in H460 and
A549 cells (12). Laricitrin is a
flavonol that is present predominantly as 3-glucoside (13). The present study investigated the
association between laricitrin and the BaP-associated lung cancer
tumor microenvironment.
Materials and methods
Chemicals
Laricitrin (Extrasynthese, Genay, France) was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis,
MO, USA) and stored at −20°C. Control cultures contained the
carrier solvent 0.1% DMSO.
Cell cultures and conditioned media
(CM)
The human lung adenocarcinoma H1395, H1975, H2087
and HCC2935 cell lines (catalog nos. ATCC CRL-5868, ATCC CRL-5908,
ATCC CRL-5922 and ATCC CRL-2869, respectively) were purchased from
the American Type Culture Collection (Manassas, VA, USA). The
characteristics of the cell lines are reported in Table I. The cells were cultured in Gibco
Roswell Park Memorial Institute (RPMI)-1640 medium (Thermo Fisher
Scientific, Waltham, MA, USA) that contained 10% Gibco fetal bovine
serum (Thermo Fisher Scientific). In order to obtain the various
CM, the H1395, H1975, H2087 and HCC2935 cells (2×106
cells/100 mm dish) were treated with or without BaP (Sigma-Aldrich)
at a concentration of 10 µM for 6 h. Subsequent to washing and
culturing for 24 h, the CM of BaP-treated H1395, H1975, H2087 and
HCC2935 cells (BaP-H1395-CM, BaP-H1975-CM, BaP-H2087-CM and
BaP-HCC2935-CM, respectively) were harvested (Fig. 1A).
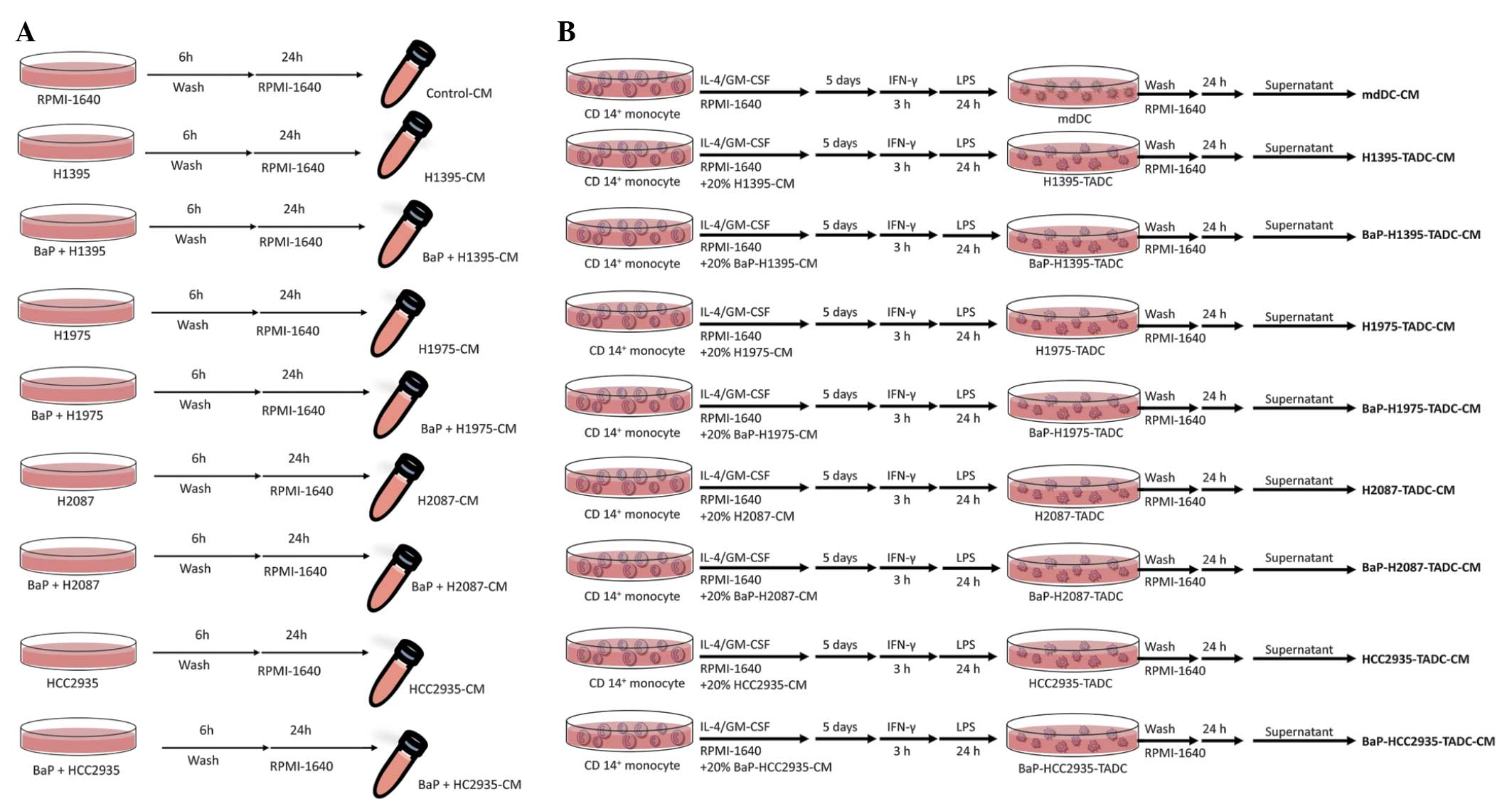 | Figure 1.Flow chart of the production of
various CM. (A) Flow chart of the production of control-CM,
H1395-CM, BaP-H1395-CM, H1975-CM, BaP-H1975-CM, H2087-CM,
BaP-H2087-CM, HCC2935-CM, and BaP-HCC2935-CM. (B) Flow chart of the
production of mdDC-CM, H1395-TADC-CM, BaP-H1395-TADC-CM,
H1975-TADC-CM, BaP-H1975-TADC-CM, H2087-TADC-CM, BaP-H2087-TADC-CM,
HCC2935-TADC-CM and BaP-HCC2935-TADC-CM. CM, conditioned media;
BaP, benzo(a)pyrene; mcDC, monocyte-derived dendritic cell; TADC,
tumor-associated dendritic cell; IL-4, interleukin 4; GM-CSF,
granulocyte macrophage-colony-stimulating factor; IFN-γ,
interferon-γ; LPS, lipopolysaccharide. |
 | Table I.Background of the human cell lines
used in the present study. |
Table I.
Background of the human cell lines
used in the present study.
| Cell line | Tissue | Mutation | Disease | Gender | Smoker status | Morphology | References |
|---|
| H1395 | Lung | BRAF | Stage 2
adenocarcinoma | Female | Smoker, 15
pack-years | Epithelial | (14–18) |
| HCC2935 | Lung, pleural
effusion | EGFR | Adenocarcinoma | Male | Non-smoker | Epithelial | (18–22) |
| H2087 | Lung, derived from
the lymph node metastatic site | BRAF | Stage 1
adenocarcinoma | Male | Smoker, 60
pack-years | Epithelial-like
and/or rounded | (14,18,23–25) |
| H1975 | Lung | EGFR | Adenocarcinoma | Female | Non-smoker | Epithelial | (14,18,26–28) |
Isolation of CD14+
monocytes and differentiation of monocyte-derived dendritic cells
(mdDCs)
Monocytes were obtained from peripheral blood
mononuclear cells (PBMCs) obtained from healthy, consenting donors.
Mononuclear cells were isolated from the blood using the
Ficoll-Hypaque gradient (GE Healthcare Life Sciences, Little
Chalfont, UK). CD14+ monocytes were purified using MACS
MicroBeads CD14+ monoclonal antibody-conjugated magnetic
beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), according
to the manufacturer's protocol. mdDCs were generated by culturing
CD14+ monocytes in RPMI-1640 containing FBS and 20 ng/ml
granulocyte macrophage-colony-stimulating factor (GM-CSF) and 10
ng/ml interleukin-4 (IL-4) (R&D Systems, Inc., Minneapolis, MN,
USA) for 5 days. The medium was replaced with fresh medium
containing GM-CSF and IL-4 on day 3. For the maturation of DCs,
immature mdDCs were stimulated with lipopolysaccharide (LPS; 100
ng/ml; Sigma-Aldrich) subsequent to priming with interferon-γ
(IFN-γ; EMD Millipore, Billerica, MA, USA) for 3 h.
H1395-TADCs, Bap-H1395-TADCs, H1975-TADCs,
BaP-H1975-TADCs, H2087-TADCs, BaP-H2087-TADCs, HCC2935-TADCs or
BaP-HCC2935-TADCs were generated by culturing CD14+
monocytes in RPMI-1640 medium containing FBS, IL-4 and GM-CSF, with
20% H1395-CM, BaP-H1395-CM, H1975-CM, BaP-H1975-CM, H2087-CM,
BaP-H2087-CM, HCC2935-CM or BaP-HCC2935-CM. The cell culture was
then stimulated with LPS, subsequent to priming with IFN-γ for 3 h.
Subsequent to washing, the supernatants were collected and
identified as H1395-TADC-CM BaP-H1395-TADC-CM, H1975-TADC-CM,
BaP-H1975-TADC-CM, H2087-TADC-CM, BaP-H2087-TADC-CM,
HCC2935-TADC-CM or BaP-HCC2935-TADC-CM (Fig. 1B). The Institutional Review Board
(IRB) of Kaohsiung Medical University Hospital (Kaohsiung, Taiwan)
approved the protocol of the present study (IRB nos.,
KMUH-IRB-990345, KMUH-IRB-20110377 and KMUH-IRB-20130054), and all
participants provided written informed consent in accordance with
the Declaration of Helsinki (5).
Cell proliferation
The cells were plated in 96-well culture plates.
Following 24 h incubation, the cells were treated with vehicle
mdDC-CM or various CM for 72 h. The effects of H1395-TADC-CM and
BaP-H1395-TADC-CM on the proliferation of H1395 cells,
H1975-TADC-CM and BaP-H1975-TADC-CM on the proliferation of H1975
cells, H2087-TADC-CM and BaP-H2087-TADC-CM on the proliferation of
H2087 cells, and HCC2935-TADC-CM and BaP-HCC2935-TADC-CM on the
proliferation of HCC2935 cells were assessed using a water-soluble
tetrazolium salt-1 (WST-1) assay (Clontech Laboratories, Inc.,
Mountain View, CA, USA) subsequent to 72 h incubation. Cell
proliferation was determined by Premixed WST-1 Cell Proliferation
reagent (Clontech Laboratories, Inc.) in accordance with the
manufacturer's protocol. Then, the H1395, H1975, H2087 or HCC2935
cells were treated with the vehicle control or 10 µM of BaP for 6
h.
Cell migration and invasion
assays
The cell migration and invasion assays were
conducted using QCM™ 24-well Cell Migration and Invasion Assay kits
(EMD Millipore). The effects of H1395-TADC-CM and BaP-H1395-TADC-CM
on the migration of H1395 cells, H1975-TADC-CM and
BaP-H1975-TADC-CM on the migration of H1975 cells, H2087-TADC-CM
and BaP-H2087-TADC-CM on the migration of H2087 cells, and
HCC2935-TADC-CM and BaP-HCC2935-TADC-CM on the migration of HCC2935
cells were quantified using the QCM 24-well cell migration assay.
Briefly, the cells were seeded onto the migration chamber and
mdDC-CM or a 20% concentration of the various media was added to
the bottom wells to act as a chemoattractant for 24 h.
The QCM 24-well cell invasion assay was used to
quantify the effect of H1395-TADC-CM and BaP-H1395-TADC-CM on the
invasion of H1395 cells, H1975-TADC-CM and BaP-H1975-TADC-CM on the
invasion of H1975 cells, H2087-TADC-CM and BaP-H2087-TADC-CM on the
invasion of H2087 cells, and HCC2935-TADC-CM and
BaP-HCC2935-TADC-CM on the invasion of HCC2935 cells. A 20%
concentration of the aforementioned media acted as a
chemoattractant for 48 h.
At the end of treatment in the two assays, the cells
were stained with CyQuant GR dye (part of the QCM 24-well Cell
Migration and Invasion Assay kit; EMD Millipore) in cell lysis
buffer for 15 min at room temperature. The fluorescence of the
migrated and invaded cells was read using a fluorescence plate
reader (FLx800; Bio-Tek Instruments, Inc., Winooski, VT, USA) at
excitation/emission wavelengths of 485/520 nm.
Laricitrin treatments
The protocol for assessing the effect of laricitrin
involved the aforementioned isolation, proliferation, migration and
invasion steps, with a slight modification. Briefly, the mdDCs,
H1395-TADCs, Bap-H1395-TADCs, HCC2935-TADCs or BaP-HCC2935-TADCs
were derived by culturing CD14+ monocytes in RPMI-1640
medium containing FBS, IL-4 and GM-CSF, with or without a 20%
concentration of H1395-CM, BaP-H1395-CM, HCC2935-CM or
BaP-HCC2935-CM, for 72 h. For the maturation of DCs, immature mdDCs
were stimulated with 100 ng/ml LPS subsequent to priming with IFN-γ
for 3 h. mdDCs, H1395-TADCs, Bap-H1395-TADCs, HCC2935-TADCs or
BaP-HCC2935-TADCs were then pretreated with or without 2 µM
laricitrin for 1 h. Subsequently, mdDC-CM, H1395-TADC-CM,
BaP-H1395-TADC-CM, HCC2935-TADC-CM or BaP-HCC2935-TADC-CM, with or
without laricitrin, were added to H1395 or HCC2935 cells, as
appropriate, for another 72 h. Cell proliferation was assessed
using a WST-1 assay. H1395 and HCC2935 cells were seeded into the
top Transwell insert, and the various CMs of mdDCs,
laricitrin-treated mdDCs, H1395-TADCs, laricitrin-treated
H1395-TADCs, BaP-H1395-TADCs, laricitrin-treated Bap-H1395-TADCs,
HCC2935-TADCs, laricitrin-treated HCC2935-TADCs, BaP-H2935-TADCs or
laricitrin-treated Bap-HCC2935-TADCs were added to the bottom
chamber as chemoattractants for 24 h (for migration) or 48 h (for
invasion). The migratory and invasive abilities of the H1395 and
HCC2935 cells were quantified by QCM 24-well Cell Migration and
Invasion assay.
Statistical analysis
The data were expressed as the mean ± standard
error. Statistical comparisons were made using one-way analysis of
variance with post-hoc Tukey's test. Significant differences
between the means of the two test groups were analyzed by Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Subsequent to exposure to BaP, lung
cancer cells affect mdDCs, which then contributes to lung cancer
progression by increasing cancer cell migration and invasion. To
investigate the effect of BaP on tumor progression in the lung
cancer tumor microenvironment, the effects of BaP-H1395-TADC-CM,
BaP-H1975-TADC-CM, BaP-H2087-TADC-CM and BaP-HCC2935-CM on the
proliferation, migration and invasion of lung cancer cells were
examined
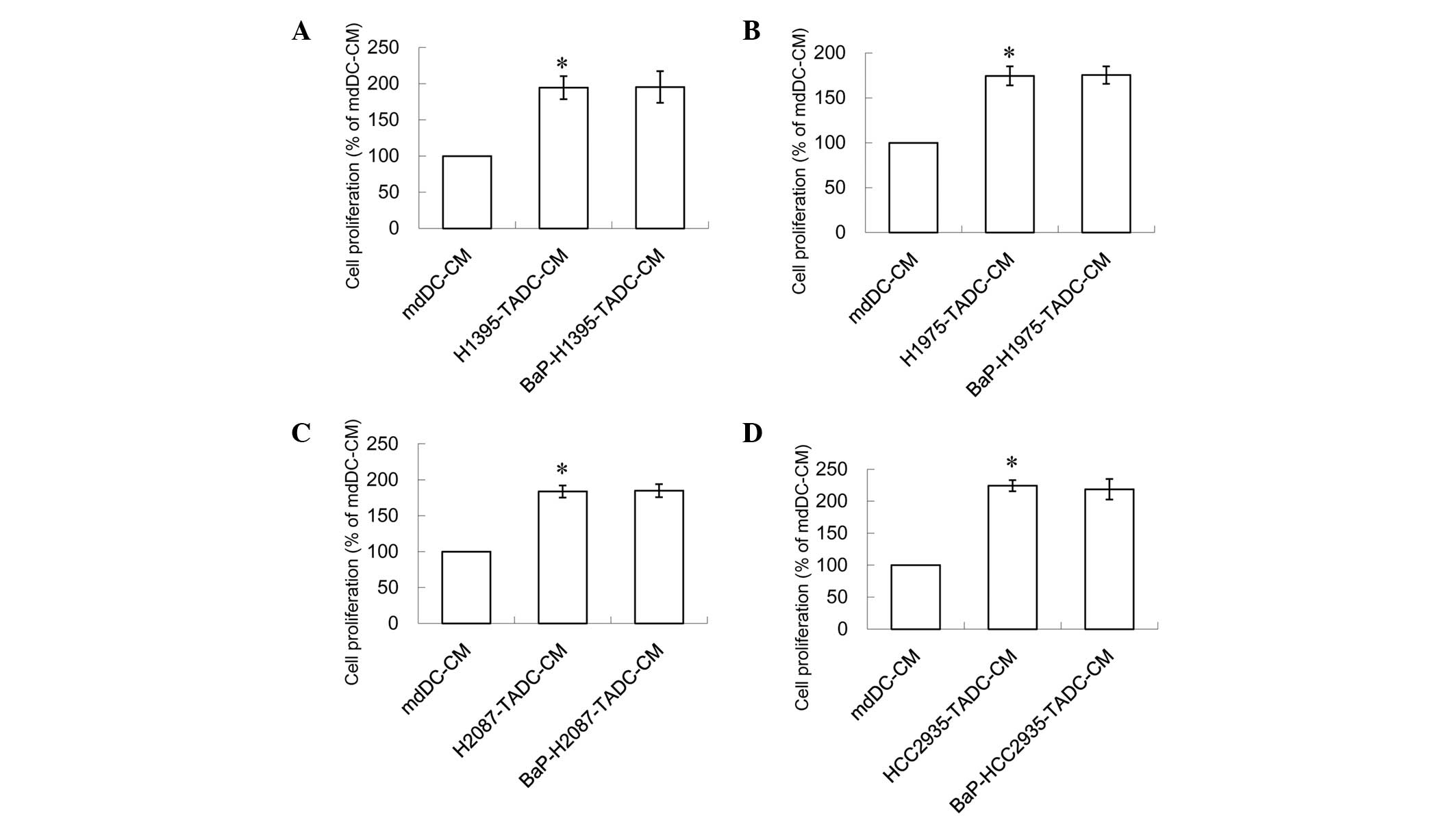
A concentration of 20% of the aforementioned media
increased the proliferation of lung cancer cells. This effect is
similar to that in H1395, H1975, H2087 or HCC2935 cells treated
with 10 µM BaP. Notably, the proliferation effect was not increased
when these cells were treated with BaP (Fig. 2). Compared with H1395-TADC-CM,
BaP-H1395-TADC-CM did not enhance H1395 cell proliferation
(Fig. 2A). Compared with
H1975-TADC-CM, BaP-H1975-TADC-CM did not enhance H1975 cell
proliferation (Fig. 2B). Compared
with H2087-TADC-CM, BaP-H2087-TADC-CM did not enhance H2087 cell
proliferation (Fig. 2C). Compared
with HCC2935-TADC-CM, BaP-HCC2935-TADC-CM did not enhance HCC2935
cell proliferation (Fig. 2D).
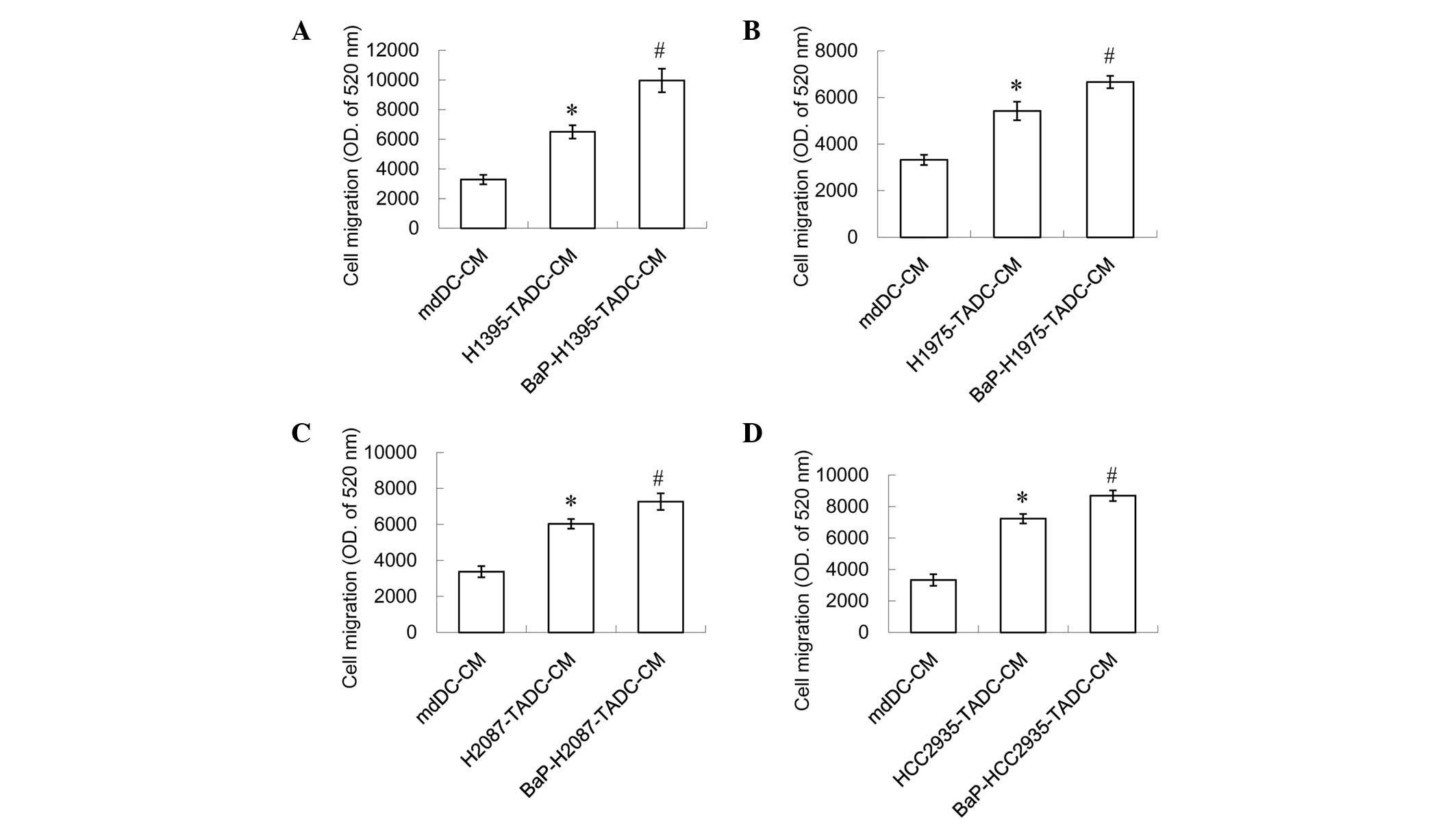
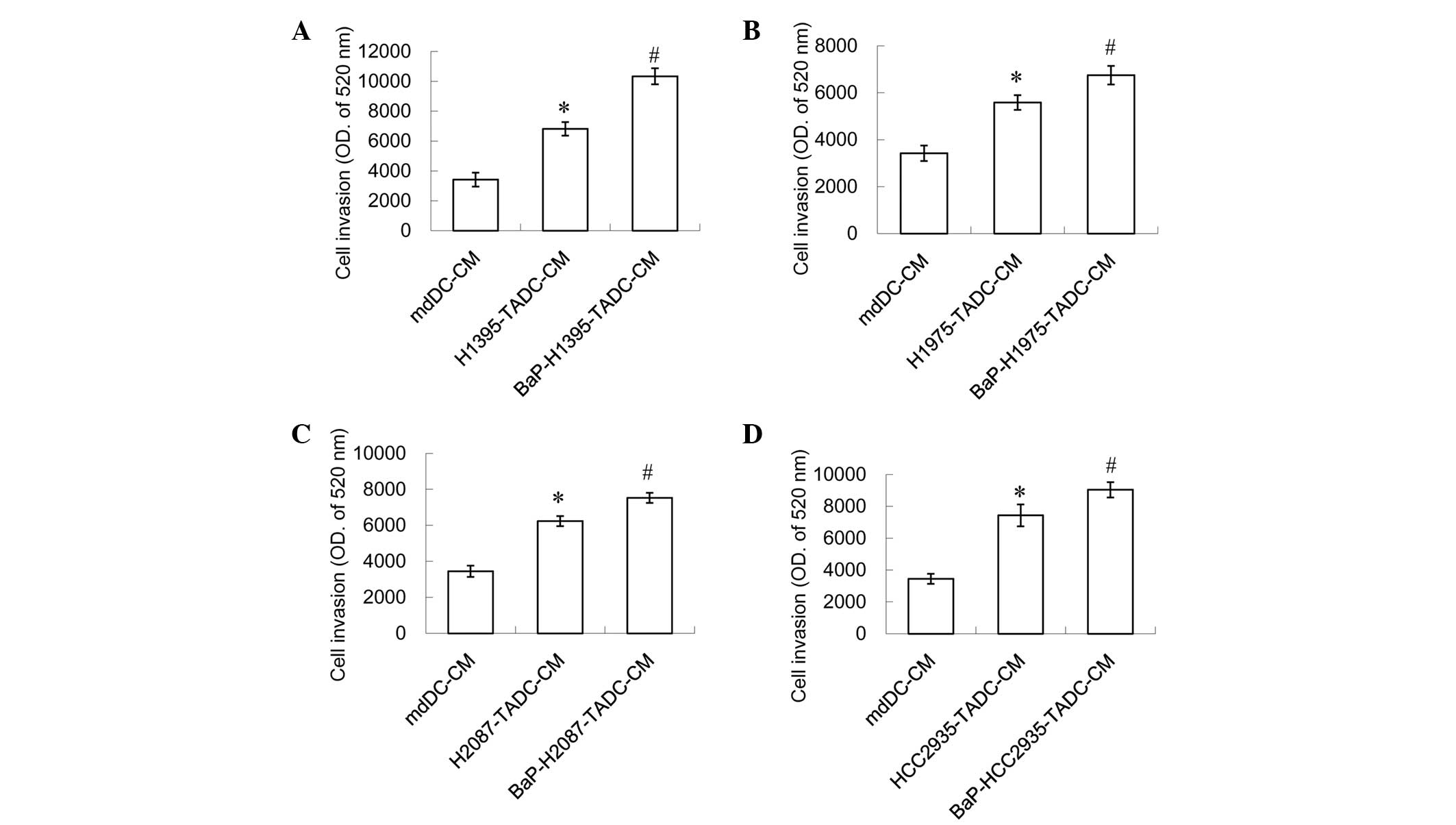
However, a 20% concentration of H1395-TADC-CM, H1975-TADC-CM,
H2087-TADC-CM or HCC2935-TADC-CM was indicated to induce lung
cancer cell migration and invasion, and a 20% concentration of the
aforementioned media may additionally increase this effect
(Figs. 3 and 4). It was found that BaP-H1395-TADC-CM
enhanced H1395 cell migration and invasion compared with
H1395-TADC-CM (Figs. 3A and 4A). BaP-H1975-TADC-CM enhanced H1975 cell
migration and invasion compared with H1975-TADC-CM (Figs. 3B and 4B). BaP-H2087-TADC-CM enhanced H2087 cell
migration and invasion compared with H2087-TADC-CM (Figs. 3C and 4C). In addition, BaP-HCC2935-TADC-CM
enhanced HCC2935 cell migration and invasion compared with
HCC2935-TADC-CM (Figs. 3D and
4D). Therefore, the proliferation
effect may not be increased; however, the migration and invasion
effects increase in cancer cells with BaP-treatment. Comparing each
TADC-CM with mdDC-CM, HCC2935-TADC-CM revealed that had the most
marked effect on cancer proliferation, migration and invasion.
Comparison of the BaP-treated TADC-CMs with each TADC-CM revealed
that BaP-H1395-TADC-CM demonstrated the most marked effect on
promoting cancer cell migration and invasion.
Laricitrin suppresses BaP-induced lung
tumor-associated monocyte-derived dendritic cell-increased cancer
progression in the lung cancer tumor microenvironment
Compared with cell lines not treated with BaP,
HCC2935-TADC-CM demonstrated the most marked effect on the
proliferation, migration and invasion of lung cancer cells.
However, BaP-H1395-TADC-CM exerted the strongest effect on the
migration and invasion of lung cancer cells (Figs. 3 and 4).
Therefore, the HCC2935 and H1395 cell lines were selected as models
for developing antidotes against BaP-associated cancer aggravation
in the lung cancer tumor microenvironment.
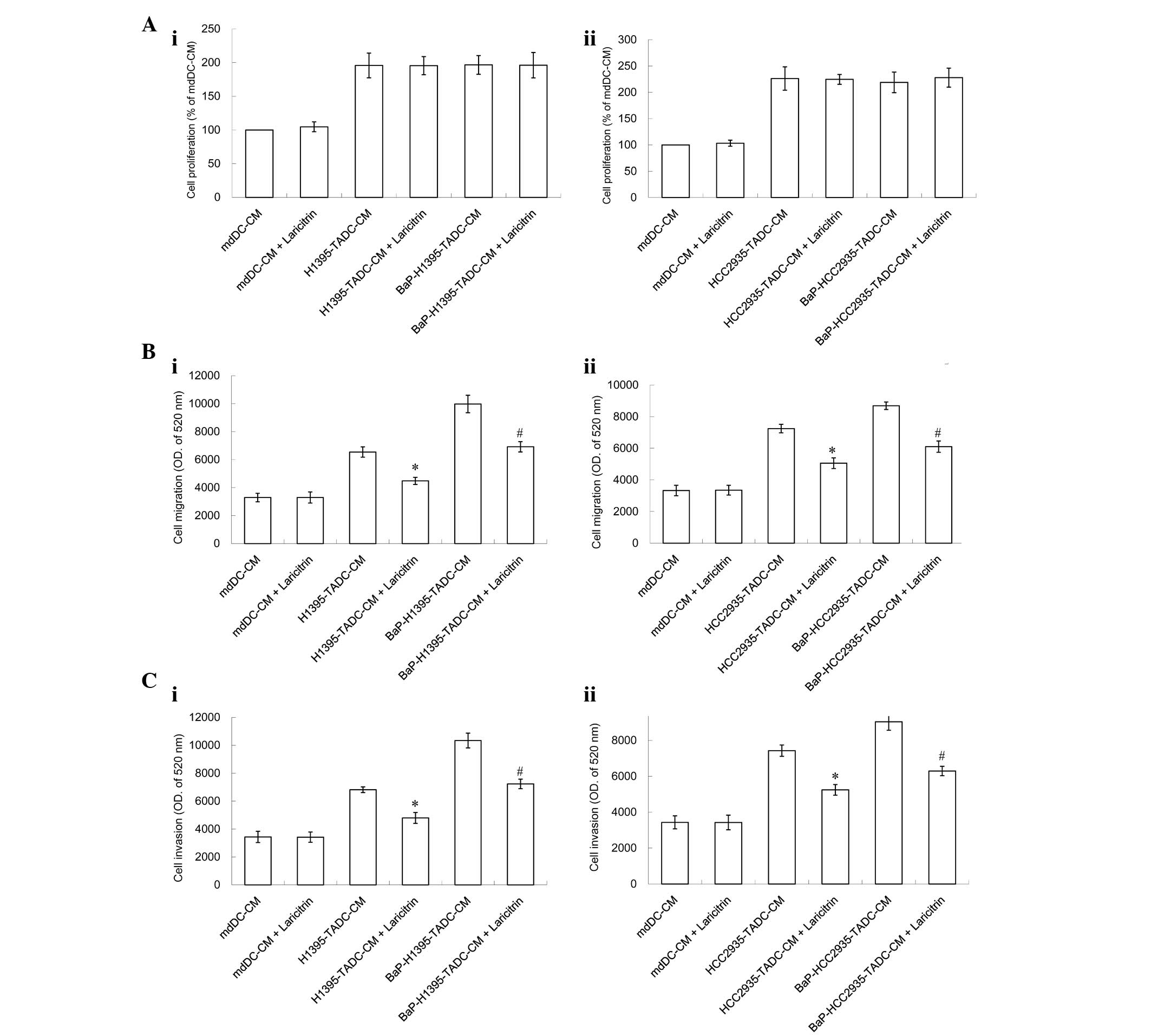
The effect of laricitrin, a dietary flavonoid
derivative present in grapes and red wine, on BaP-induced cancer
progression was then assessed. The results in Fig. 5 revealed that, although laricitrin did
not inhibit the proliferation of lung cancer cells in the lung
cancer tumor microenvironment (Fig.
5A), it did inhibit the lung cancer cell migration induced by
H1395-TADC-CM and HCC2935-TADC-CM (Fig.
5B). In addition, laricitrin suppressed the lung cancer cell
invasion that was induced by BaP-H1395-TADC-CM and
BaP-HCC2935-TADC-CM (Fig. 5C).
Discussion
Lung cancer is currently the leading cause of
cancer-associated mortality worldwide. BaP has been demonstrated to
induce lung cancer development; however, the role of BaP in the
lung cancer tumor microenvironment remains unclear. Exposure to BaP
may not be completely avoided; however, numerous factors may affect
the deleterious effects of BaP, such as gender, epidermal growth
factor receptor (EGFR) mutation and proto-oncogene B-Raf (BRAF)
mutation. The present study investigated the association between
certain factors and the effect of BaP on lung cancer progression.
Table I shows the background of the
cell lines used in the present study (14–28). A
previous study using CD-1 mice indicated that female mice are more
susceptible to the carcinogenic effects of BaP compared with males
(29). Therefore, being female may
increase the risk of BaP-induced lung cancer. BRAF mutation is
another risk factor for non-small cell lung cancer and
adenocarcinoma of the lungs (30). A
previous study performed using BRAF V600E mutant mice revealed that
the expression of the BRAF mutation leads to adenocarcinoma
development and is essential for tumor maintenance (31). EGFR mutation is also a risk factor for
adenocarcinoma, as EGFR maintains the malignant phenotype of lung
cancer cells (32). Comparison
between invasive adenocarcinoma and preinvasive adenocarcinoma
tissue samples indicated that there is an increased frequency of
EGFR mutation in invasive adenocarcinoma, and an increased
frequency of the BRAF mutation in preinvasive adenocarcinoma
(33). Although EGFR mutation is not
an independent prognostic factor (34), BRAF mutation is associated with poor
disease-free survival and poor overall survival rates (35). Female gender, late-stage lung cancer
and EGFR and BRAF mutations are risk factors for BaP-induced lung
cancer progression. In addition, BRAF mutation is a greater risk
factor compared with EGFR mutation.
Comparison between the BaP-treated TADC-CMs and each
TADC-CM indicated that BaP-H1395-TADC-CM had the most marked effect
on promoting cancer cell migration and invasion, with an increase
of 1.5 fold, compared with the other cell lines, which increased
~1.2 fold. This result may be due to H1395 cells possessing the
greatest number of risk factors, as they were obtained from a
female smoker with BRAF mutation (14–18).
Comparison between each TADC-CM and mdDC-CM indicated that
HCC2935-TADC-CM had the most marked effect on the proliferation,
migration and invasion of cancer cells. The HCC2935 cell line was
established from the pleural effusion cells of an adenocarcinoma
patient (18–22). Lung cancer with malignant pleural
effusion indicates terminal-stage disease (36). Therefore, HCC2935 cells may possess
increased potential for metastasis compared with other cell
lines.
To the best of our knowledge, the present study is
the first to investigate the association between BaP and lung
tumor-associated monocyte-derived dendritic cell-increased cancer
aggravation in various cell lines. The present study has two novel
findings: i) That lung tumor-associated monocyte-derived dendritic
cells, following exposure to BaP, contribute to cancer progression
by increasing cancer cell migration and invasion; and ii) that
laricitrin, a dietary flavonoid derivative present in grapes and
red wine, reverses BaP-mediated lung cancer aggravation (Fig. 6). BaP exacerbates cancer cell
migration and invasion in the lung cancer tumor microenvironment.
Laricitrin may reverse this effect. Notably, the H1395 cell line
demonstrated the most marked enhancement of migration and invasion
following exposure to BaP-H1395-TADC-CM, and possessed the greatest
number of risk factors. This finding suggests that BaP may be able
to induce cancer cell migration and invasion without promoting
proliferation in patients with the highest number of risk factors.
Therefore, patients with lung cancer that possess numerous risk
factors and are in contact with BaP may demonstrate an increased
possibility of metastasis and invasion. In conclusion, the
development of a drug with the potential for treating BaP-induced
cancer progression is vitally important.
Acknowledgements
The present study was supported by the National
Science Council (grant nos., NSC 101-2628-B-037-001-MY3 and NSC
101-2320-B-037-043-MY3), Ministry of Science and Technology (grant
nos., MOST 104-2320-B-037-014-MY3, MOST 103-2314-B-037-052 and MOST
103-2320-B-037-032), Kaohsiung Medical University ‘Aim for the Top
500 Universities Grant’ (grant no., KMU-DT103008), Kaohsiung
Medical University ‘Aim for the Top Universities Grant’ (grant
nos., KMU-TP103A19 and KMU-TP103A20) and the Kaohsiung Medical
University Hospital Research Foundation (grant no., KMUH103-3M04).
The authors thank the Center for Research Resources and Development
of Kaohsiung Medical University for support with the
instrumentation.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsai MJ, Chang WA, Huang MS and Kuo PL:
Tumor microenvironment: A new treatment target for cancer. ISRN
Biochem. 2014:3519592014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsu YL, Huang MS, Cheng DE, Hung JY, Yang
CJ, Chou SH and Kuo PL: Lung tumor-associated dendritic
cell-derived amphiregulin increased cancer progression. J Immunol.
187:1733–1744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuo CH, Chen KF, Chou SH, Huang YF, Wu CY,
Cheng DE, Chen YW, Yang CJ, Hung JY and Huang MS: Lung
tumor-associated dendritic cell-derived resistin promoted cancer
progression by increasing Wolf-Hirschhorn syndrome candidate
1/Twist pathway. Carcinogenesis. 34:2600–2609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuo PL, Huang MS, Hung JY, Chou SH, Chiang
SY, Huang YF, Yang CJ, Tsai MJ, Chang WA and Hsu YL: Synergistic
effect of lung tumor-associated dendritic cell-derived HB-EGF and
CXCL5 on cancer progression. Int J Cancer. 135:96–108. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alexandrov K, Rojas M and Satarug S: The
critical DNA damage by benzo(a)pyrene in lung tissues of smokers
and approaches to preventing its formation. Toxicol Lett.
198:63–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zuo J, Brewer DS, Arlt VM, Cooper CS and
Phillips DH: Benzo pyrene-induced DNA adducts and gene expression
profiles in target and non-target organs for carcinogenesis in
mice. BMC Genomics. 15:8802014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Zhai W, Wang H, Xia X and Zhang C:
Benzo(a)pyrene promotes A549 cell migration and invasion through
up-regulating Twist. Arch Toxicol. 89:451–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mattivi F, Guzzon R, Vrhovsek U, Stefanini
M and Velasco R: Metabolite profiling of grape: Flavonols and
anthocyanins. J Agric Food Chem. 54:7692–7702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kozłowska A and Szostak-Wegierek D:
Flavonoids - food sources and health benefits. Rocz Panstw Zakl
Hig. 65:79–85. 2014.PubMed/NCBI
|
|
11
|
Wang CZ, Calway TD, Wen XD, Smith J, Yu C,
Wang Y, Mehendale SR and Yuan CS: Hydrophobic flavonoids from
Scutellaria baicalensis induce colorectal cancer cell apoptosis
through a mitochondrial-mediated pathway. Int J Oncol.
42:1018–1026. 2013.PubMed/NCBI
|
|
12
|
Tsui KC, Chiang TH, Wang JS, Lin LJ, Chao
WC, Chen BH and Lu JF: Flavonoids from Gynostemma pentaphyllum
exhibit differential induction of cell cycle arrest in H460 and
A549 cancer cells. Molecules. 19:17663–17681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Castillo-Muñoz N, Gómez-Alonso S,
García-Romero E and Hermosín-Gutiérrez I: Flavonol profiles of
Vitis vinifera red grapes and their single-cultivar wines. J Agric
Food Chem. 55:992–1002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
NCI-Navy Medical Oncology Branch cell line
supplement. J Cell Biochem. (Suppl)24:1–291. 1996.
|
|
15
|
Naoki K, Chen TH, Richards WG, Sugarbaker
DJ and Meyerson M: Missense mutations of the BRAF gene in human
lung adenocarcinoma. Cancer Res. 62:7001–7003. 2002.PubMed/NCBI
|
|
16
|
Koivunen JP, Kim J, Lee J, et al:
Mutations in the LKB1 tumour suppressor are frequently detected in
tumours from Caucasian but not Asian lung cancer patients. Br J
Cancer. 99:245–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jala VR, Radde BN, Haribabu B and Klinge
CM: Enhanced expression of G-protein coupled estrogen receptor
(GPER/GPR30) in lung cancer. BMC Cancer. 12:6242012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sunaga N, Kaira K, Imai H, et al:
Oncogenic KRAS-induced epiregulin overexpression contributes to
aggressive phenotype and is a promising therapeutic target in
non-small-cell lung cancer. Oncogene. 32:4034–4042. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou BB, Peyton M, He B, et al: Targeting
ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in
non-small cell lung cancer. Cancer Cell. 10:39–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomshine JC, Severson SR, Wigle DA, Sun Z,
Beleford DA, Shridhar V and Horazdovsky BF: Cell proliferation and
epidermal growth factor signaling in non-small cell lung
adenocarcinoma cell lines are dependent on Rin1. J Biol Chem.
284:26331–26339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zito CR, Jilaveanu LB, Anagnostou V, et
al: Multi-level targeting of the phosphatidylinositol-3-kinase
pathway in non-small cell lung cancer cells. PLoS One.
7:e313312012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khode R, Larsen DA, Culbreath BC, et al:
Comparative study of epidermal growth factor receptor mutation
analysis on cytology smears and surgical pathology specimens from
primary and metastatic lung carcinomas. Cancer Cytopathol.
121:361–369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uchiyama M, Usami N, Kondo M, et al: Loss
of heterozygosity of chromosome 12p does not correlate with KRAS
mutation in non-small cell lung cancer. Int J Cancer. 107:962–969.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Futreal PA, Wooster R and Stratton MR:
Somatic mutations in human cancer: Insights from resequencing the
protein kinase gene family. Cold Spring Harb Symp Quant Biol.
70:43–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Medina PP, Romero OA, Kohno T, et al:
Frequent BRG1/SMARCA4-inactivating mutations in human lung cancer
cell lines. Hum Mutat. 29:617–622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sordella R, Bell DW, Haber DA and
Settleman J: Gefitinib-sensitizing EGFR mutations in lung cancer
activate anti-apoptotic pathways. Science. 305:1163–1167. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobayashi S, Boggon TJ, Dayaram T, et al:
EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shibata T, Hanada S, Kokubu A, et al: Gene
expression profiling of epidermal growth factor receptor/KRAS
pathway activation in lung adenocarcinoma. Cancer Sci. 98:985–991.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharma R, Haque AK, Awasthi S, Singh SV,
Piper JT and Awasthi YC: Differential carcinogenicity of
benzo[a]pyrene in male and female CD-1 mouse lung. J Toxicol
Environ Health. 52:45–62. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paik PK, Arcila ME, Fara M, et al:
Clinical characteristics of patients with lung adenocarcinomas
harboring BRAF mutations. J Clin Oncol. 29:2046–2051. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji H, Wang Z, Perera SA, et al: Mutations
in BRAF and KRAS converge on activation of the mitogen-activated
protein kinase pathway in lung cancer mouse models. Cancer Res.
67:4933–4939. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang X, Shigematsu H, Bekele BN, Roth JA,
Minna JD, Hong WK, Gazdar AF and Wistuba II: EGFR tyrosine kinase
domain mutations are detected in histologically normal respiratory
epithelium in lung cancer patients. Cancer Res. 65:7568–7572.
2005.PubMed/NCBI
|
|
33
|
Hu H, Pan Y, Li Y, Wang L, Wang R, Zhang
Y, Li H, Ye T, Zhang Y, Luo X, et al: Oncogenic mutations are
associated with histological subtypes but do not have an
independent prognostic value in lung adenocarcinoma. Onco Targets
Ther. 7:1423–1437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bonanno L, Schiavon M, Nardo G, Bertorelle
R, Bonaldi L, Galligioni A, Indraccolo S, Pasello G, Rea F and
Favaretto A: Prognostic and predictive implications of EGFR
mutations, EGFR copy number and KRAS mutations in advanced stage
lung adenocarcinoma. Anticancer Res. 30:5121–5128. 2010.PubMed/NCBI
|
|
35
|
Marchetti A, Felicioni L, Malatesta S, et
al: Clinical features and outcome of patients with non-small-cell
lung cancer harboring BRAF mutations. J Clin Oncol. 29:3574–3579.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Froudarakis ME: Pleural effusion in lung
cancer: more questions than answers. Respiration. 83:367–376. 2012.
View Article : Google Scholar : PubMed/NCBI
|