Introduction
HCC is one of the most prevalent types of human
cancer worldwide, particularly in Southeast Asia (1). The 5-year survival rate of patients with
HCC remains poor, with ~600,000 mortalities each year, despite
recent advances in surgical techniques and medical treatment
(2). There is a lack of typical
symptoms during the early stages of HCC. However, a common clinical
manifestation is dull or tingling pain, as well as distension, in
the hepatic area, which is the initial symptom in 50% of HCC
patients. Patients may demonstrate additional symptoms, including
weakness, weight loss, anorexia, abdominal distension, nausea,
vomiting, fever and diarrhea. Hepatomegaly frequently occurs in
terminal-stage HCC patients. There are a number of methods via
which HCC may be diagnosed, including blood concentration of
α-fetoprotein, color Doppler ultrasound, x-ray, computed tomography
and magnetic resonance imaging (MRI) (3,4).
Metastasis is one of the primary causes of the poor prognosis
associated with the disease. Tumor metastasis is a difficulty that
must frequently be faced when treating hepatocellular carcinoma.
Decreased antitumor immunity in patients with cancer has been
established to be a major factor associated with the development,
progression and metastasis of several types of cancer, including
hepatocellular carcinoma (HCC) (5–7). Natural
killer (NK) cells serve an important role in the suppression of
carcinogenesis (5). Several previous
studies have demonstrated that NK cells inhibit tumor growth and
that interleukin (IL)-2 is an essential cytokine for NK cell
proliferation and activation (5,8). Thus, NK
cells may serve as a promising treatment for HCC metastasis.
However, the role of NK cells in the inhibition of hepatocellular
carcinoma metastasis to the lungs has not been well characterized.
In the present study, it was assessed whether NK cells, activated
by IL-2, may inhibit the metastasis of hepatocellular carcinoma
in vitro and in vivo.
Materials and methods
Isolation and culture of NK cells
The study protocol conformed to the ethical
guidelines of the Declaration of Helsinki and was approved by the
Institute Research Ethics Committee of the Xiamen Traditional
Hospital (Xiamen, China). Blood samples obtained from healthy
volunteers were used to separate peripheral blood mononuclear cells
(PBMCs). The PBMCs were then used to isolate the NK cells using the
Human NK Cell Isolation kit (Miltenyi Biotec GmbH, Bergisch
Gladbach, Germany), following the manufacturer's protocols. The NK
cells were cultured in RPMI-1640 medium (HyClone; GE Healthcare
Life Sciences, Logan, Utah, USA), containing 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Subsequently, the NK cells were activated using recombinant human
IL-2 (PeproTech, Inc., Rocky Hill, NJ, USA) at a concentration of 2
pg/ml (9). The MHCC-97H cells were
cultured in RPMI-1640 medium containing 10% FBS.
Detection of NK cell receptors and
activated NK cell markers
Flow cytometry was used to examine the expression of
the following cell surface proteins: Cluster of differentiation
(CD)56 [phycoerythrin (PE)-labeled mouse anti-human CD56 clone
B159; BD Biosciences, San Diego, CA, USA], CD3 [allophycocyanin
(APC)-labeled mouse anti-human CD3 clone UCHTI; BD Pharmingen, San
Diego, CA, USA], NKG2D [fluorescein isothiocyanate (FITC)-labeled
anti-human NKG2D clone 1D11; eBioscience, Inc., San Diego, CA,
USA], NKB1 (FITC-labeled anti-human NKB1 clone DX9; BD Pharmingen),
perforin (FITC Perforin Reagent set containing FITC-labeled mouse
anti-human perforin clone δG9 and FITC-mouse immunoglobulin
(Ig)G2bκ clone 27–35; BD Pharmingen) and granzyme (FITC-labeled
mouse anti-human granzyme clone GB11; BD Pharmingen). The isotype
control included PE-labeled mouse IgG1κ (clone MOPC-21),
APC-labeled mouse IgG1κ (clone MOPC-21), FITC-labeled mouse IgG1κ
(clone MOPC-21) and PerCP-CyTM5.5 mouse IgG1κ (clone MOPC-21) (all
BD Pharmingen). The dilution ratio for all antibodies was
1:100.
Proliferation assay
The NK cells were stimulated with IL-2 (2 pg/ml) for
48 h at 37°C and were then cocultured with the MHCC97-H cells,
which were obtained from the Liver Cancer Institute and Zhongshan
Hospital (Shanghai, China). The cells were cultured at a
concentration of 2×106 cells/plate (1×106
cells/ml), using varying amounts of RPMI-1640 medium that contained
10% FBS. Following a total of 48 h, the culture medium was removed
and the MHCC97-H cells were washed with phosphate-buffered saline
(PBS). Subsequently, 5-bromo-2′-deoxyuridine (BrdU; Roche
Diagnostics, Basel, Switzerland) was added to the MHCC97-H cells,
and the cell cultures were reincubated in the RPMI-1640 medium
containing 10% FBS at 37°C. Following a total of 4 h, the culture
medium was removed and the cells were fixed and permeabilized, and
the genomic DNA was denatured in one step through the addition of
FixDenat (Roche Diagnostics) according the manufacturer's
protocols. The MHCC97-H cells were collected and the BrdU-positive
cells were detected with anti-BrdU antibody (PerCP-CyTM5.5-labeled
mouse anti-BrdU clone 3D4; 1:100; BD Pharmingen) using flow
cytometry.
Wound healing assays
Migration of the NK cells was determined by
performing wound healing assays. The MHCC97-H cells were cocultured
with the NK cells in 6-well plates (106 cells/well),
with RPMI-1640 medium containing 10% FBS. Confluent monolayers were
wounded in a straight line with a sterile plastic pipette tip, and
were subsequently incubated for 72 h with the NK cells
(106 cells/well), in 0.2% FBS medium containing IL-2 (2
pg/ml). In the control group, the monolayers were incubated with
medium instead of NK cells. The NK cell migration was evaluated by
comparing the remaining cell-free area with that of the initial
wound.
Matrigel invasion assays
Cell invasion was determined using BD BioCoat
Matrigel Invasion Chambers (BD Biosciences, Franklin Lakes, NJ,
USA). In the NK group, 200 µl of 0.2% FBS medium, containing
5×104 MHCC97-H cells and 5×104 NK cells that
had been activated with IL-2 (2 pg/ml), were added to the upper
compartment of the Transwell chamber, and 600 µl of 10%
FBS-containing medium was added to the lower chamber. In the
control group, medium was used instead of the NK cells. Following
48 h of incubation at 37°C in a humidified CO2
incubator, non-invaded cells in the upper chamber were removed with
a cotton swab, and the invaded cells were stained and counted using
a microscope (×200 magnification) along the longitudinal or
transverse axis, including five fields, without any overlap in the
fields assessed. The mean value of the cells per field was
calculated.
In vivo assessment of NK cell
survival
All experimental procedures involving animals were
approved by the Animal Care and Use Committee of Xiamen University
(Fujian, China). Healthy nude mice were injected through the portal
vein with 2×106 NK cells that were stained with
carboxyfluorescein diacetate-succinimidyl ester (CFDA-SE; EnoGene
Biotech, Co., Ltd., Nanjing, China), a non-fluorescent dye that
passively diffuses into the cell cytoplasm. Once inside the cell,
the non-fluorescent dye is cleaved by intracellular esterase to
become fluorescent and can be detected under a fluorescence
microscope. The NK cells were detected 2 weeks after the cell
injection using the IX73-U Fluorescence Microscope (Olympus
Corporation, Tokyo, Japan).
Tumor growth and metastasis assays in
vivo
The study protocol was approved by the Institutional
Animal Care and Use Committee of the Xiamen University (Xiamen,
China), and the mice were kept in specific pathogen-free conditions
(an environment without specific disease-causing microbe and
parasites, including the hepatitis A and E virus; certain
non-pathogenic microbes and parasites, including yeasts, were
allowed to exist). A hind leg xenograft was established by
subcutaneously injecting 2×106 MHCC97-H cells into
4-week-old nude mice (BALB/c nu/nu: Shanghai SLAC Laboratory Animal
Co., Ltd., Shanghai, China). Following a total of 14 days, the
subcutaneous tumors were removed and dissected into
1-mm3 sections, which were inoculated into the left
hepatic lobe of the nude mice to establish orthotopic implantation
models. Subsequently, 2×106 NK cells, which had been
activated with IL-2, were injected into the orthotopic implantation
models through the portal vein, prior to closing of the
enterocoelia in the experimental group. In order to continuously
activate the NK cells in vivo, IL-2 (1 pg/mg of body weight)
was injected into the enterocelia of the orthotopic implantation
models from the experimental and control groups on the 5th and 30th
days post-surgery. In general, the nude mice exhibited lung
metastasis 8 weeks after orthotopic implantation. Tumor growth was
monitored, and the nude mice were sacrificed (anesthesia with a 5%
chloral hydrate intraperitoneal injection) on the 56th day
post-surgery. The control group was administered PBS instead of NK
cells. The mice were sacrificed and the tumors, livers and lungs
were removed, weighed, and 5-µm sections were fixed in formalin and
embedded in paraffin. The liver tumors were detected through MRI,
with the tumor volume estimated using the MRI data. The lung
metastases were assayed according to methods described by Yang
et al (10). The grading
system was described as follows: Grade I, <20 tumor cells; grade
II, 20–50 tumor cells; grade III, 50–100 tumor cells; and grade IV,
>100 tumor cells.
Statistical analysis
Data were analyzed using commercially available
software (SPSS version 13.0; SPSS, Inc., Chicago, IL, USA). Mean ±
standard deviation values are used to represent replicate
experimental data. Student's t-test was used for bivariate
analysis, and the rank sum test was used for the assessment of
multivariable data. P<0.05 was considered to indicate a
statistically significant difference.
Results
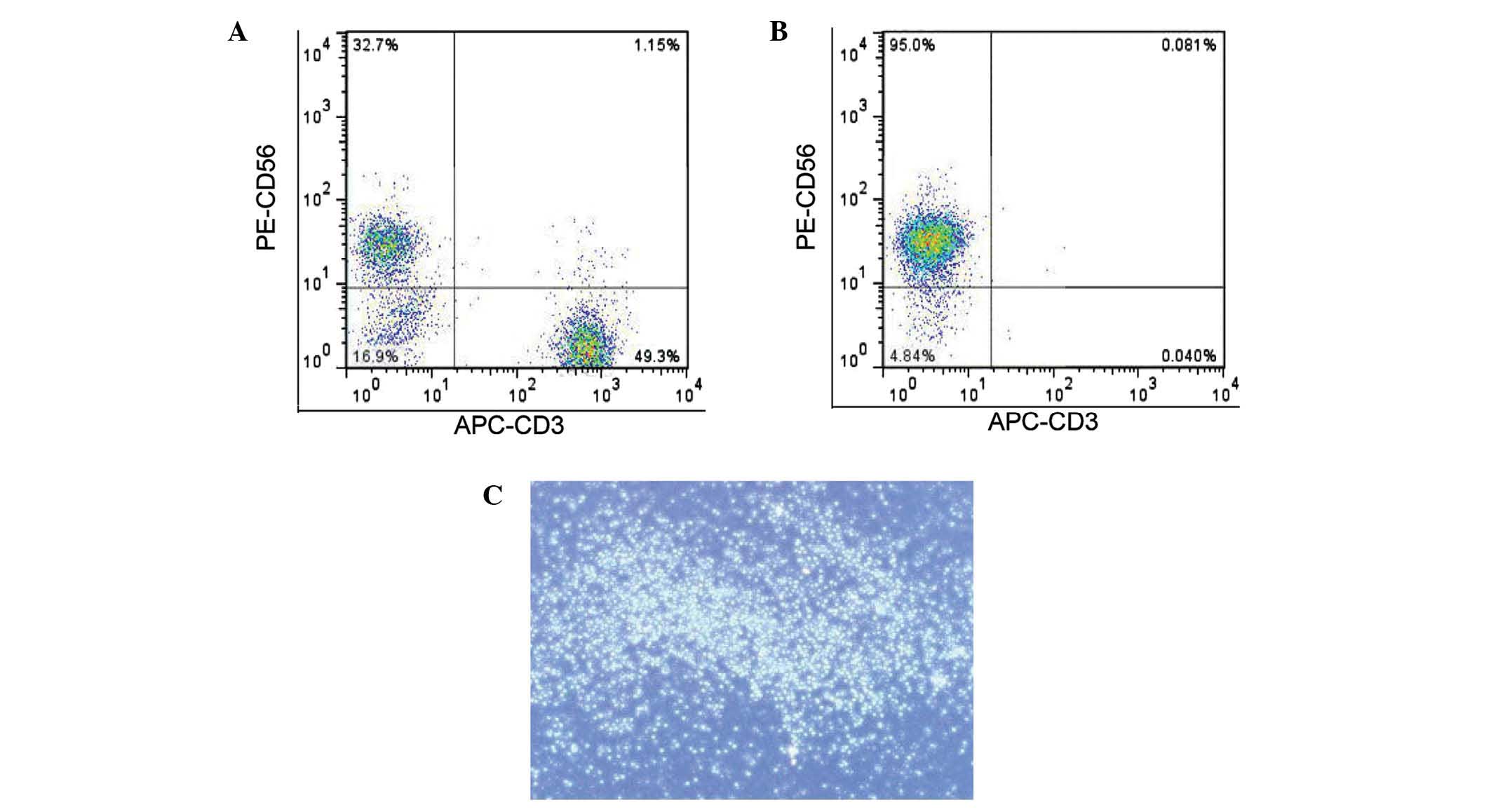
Culture and purity of NK cells
A previous study demonstrated that NK cells isolated
from PBMCs express high levels of CD56 and do not express CD3
(5). Therefore, the
CD56+CD3− cells obtained from PBMCs were
considered to be NK cells. The percentage of NK cells in the PBMCs
was 10–30% (Fig. 1A) and the purity
of the isolated NK cells was determined to be >95% (Fig. 1B). The isolated NK cells exhibited
high activity and propagated in clusters in suspension cultures
(Fig. 1C).
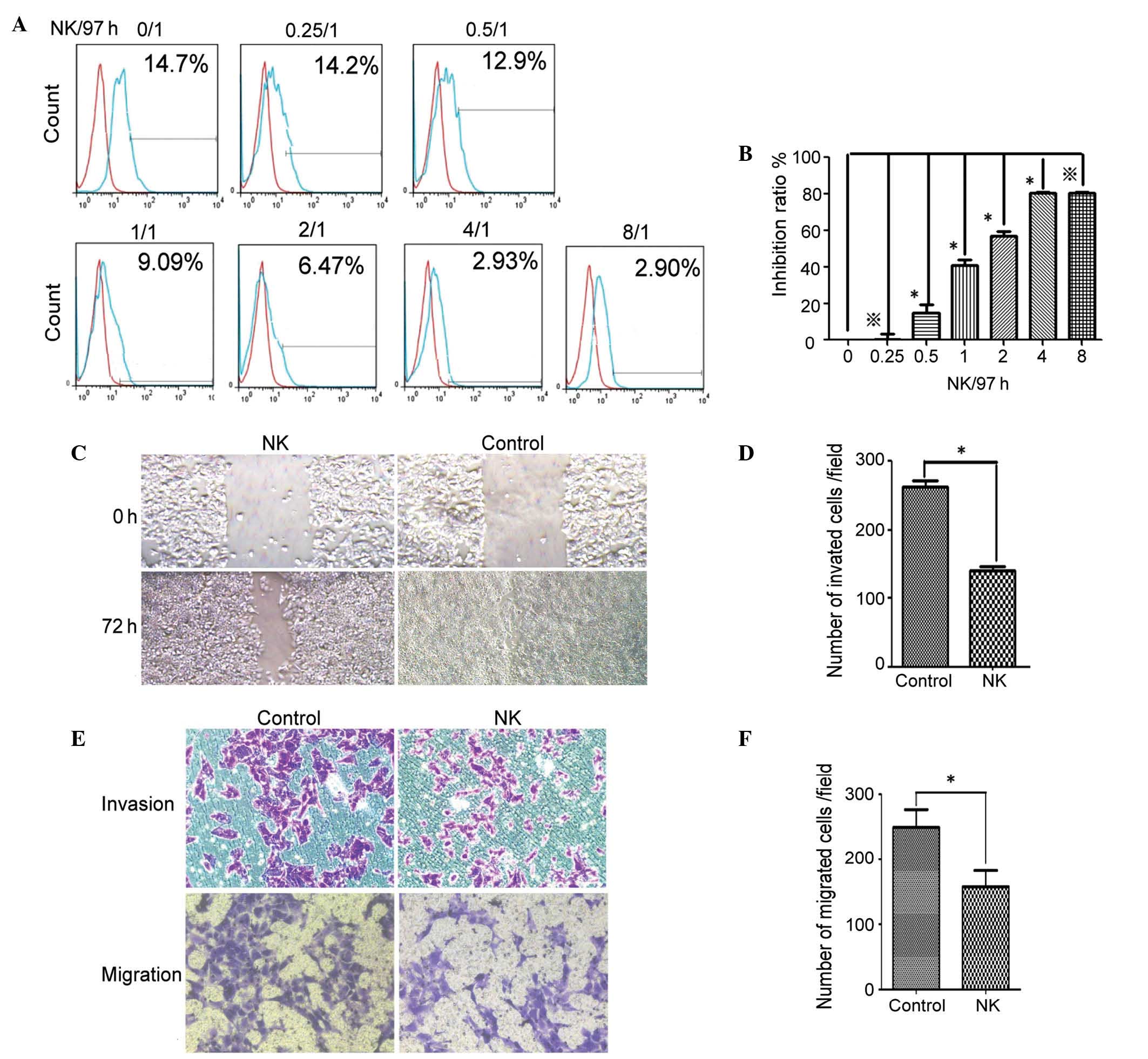
Role of NK cells in inhibiting
MHCC97-H cell mobility in vitro
In order to examine the role of NK cells in the
inhibition of hepatocellular carcinoma in vitro, the
MHCC97-H cells were cocultured with or without NK cells. The BrdU
stain was used to enumerate progenitive MHCC97-H cells in the
presence and absence of the NK cells (Fig. 2A and B). The data demonstrated that
the NK cells inhibited the MHCC97-H cell proliferation when the
ratio of NK/MHCC97-H was >0.25/1, and the inhibitory effect
peaked at an NK/MHCC97-H ratio of 4/1 (P<0.05). Therefore, the
data indicated that NK cells inhibited the proliferation of the
MHCC97-H cells in vitro.
The data also demonstrated that the mobility of the
MHCC97-H cells in the wound healing assays was significantly
decreased in the presence of the NK cells (Fig. 2C and D; P<0.05). Similarly, the
Matrigel invasion assay data indicated that the NK cells
significantly decreased migration and invasion of the MHCC97-H
cells (Fig. 2E and F; P<0.05).
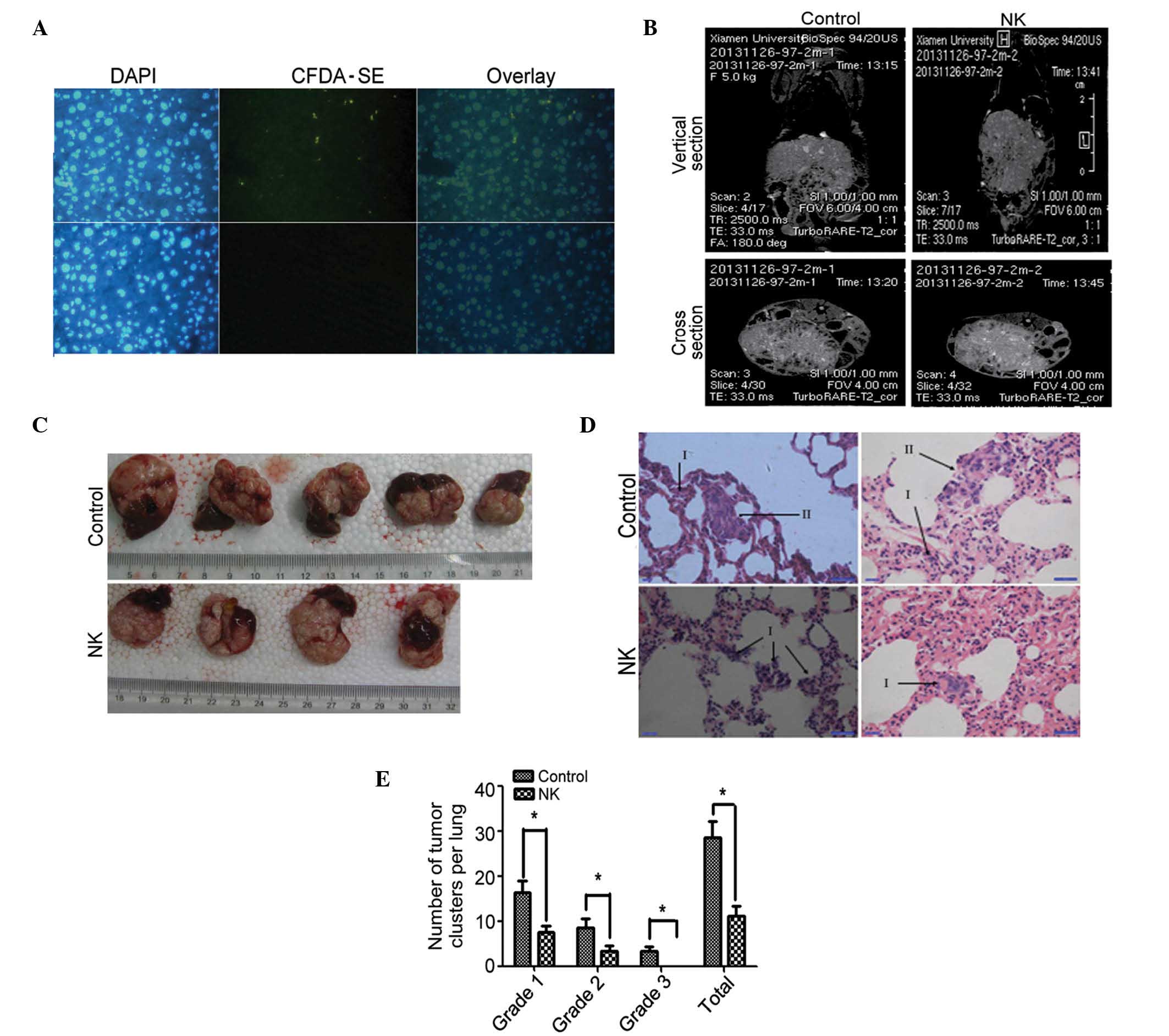
Role of NK cells in inhibiting
MHCC97-H cell survival in vivo
In vivo cell survival experiments
demonstrated that NK cells stained with CFDA-SE were localized in
the liver tissue. Thus, NK cells may survive in the liver of nude
mice (Fig. 3A). To examine the role
of NK cells in the inhibition of hepatocellular carcinoma in
vivo, the orthotopic implantation models (n=5) were injected
with NK cells, and control models (n=5) were injected with PBS. The
general state of health, tumor burden in the liver and cancer
metastasis to the lungs were examined.
It was observed that the experimental and control
animals survived for longer than 6 weeks. The appetite and activity
of the two groups were not significantly different.
The volume and weight of the liver tumors of the
experimental and control mice were not significantly different
(data not shown). The metastatic lesions in the lungs were too
small to be detected with MRI (Fig. 3B
and C). Metastatic lesions in the lungs of the orthotopic
implantation models were measured on the 56th day
post-implantation. The total numbers and grades of lung metastatic
lesions of the experimental group were considerably lower than
those of the control group (Fig. 3D and
E; P<0.05).
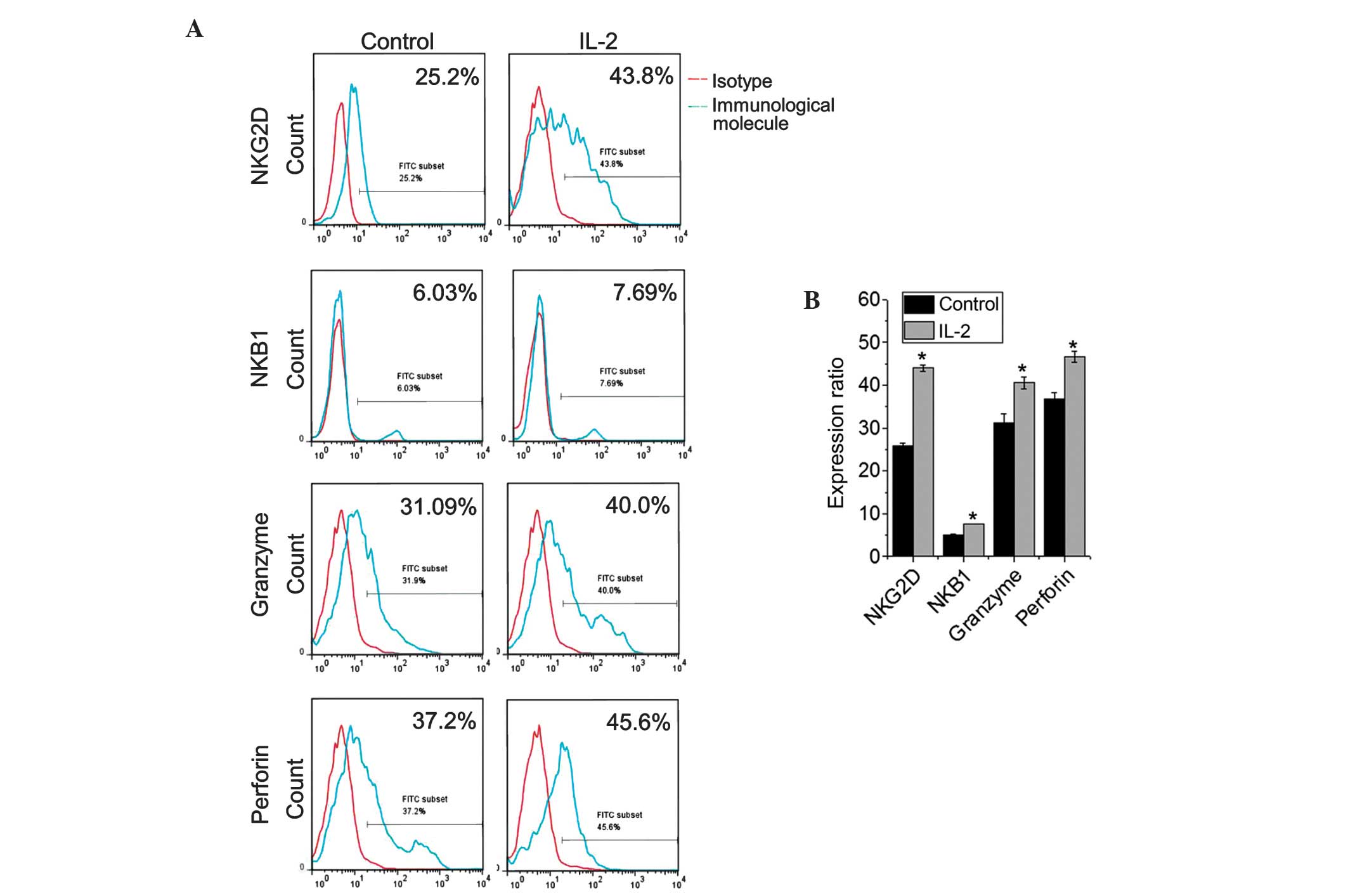
Effect of IL-2 stimulation on NK cell
function
Previous studies have indicated that IL-2 is
required for NK cell growth, proliferation and activation (11). In the present study, NK cells were
cultured with or without IL-2 for 48 h using a previously described
protocol (8). The expression of NK
group 2, member D (NKG2D; an activating receptor for NK cells) and
killer cell immunoglobulin-like receptor, three domains, long
cytoplasmic tail, 1 (KIR3DL1; NKB1; an inhibitory receptor) was
assessed. Additionally, the expression of perforin and granzyme was
also evaluated (P<0.05). The data indicated that the NK cells
exhibited moderate expression levels of NKG2D, perforin and
granzyme, and that the expression of the aforementioned molecules
was considerably upregulated following IL-2 stimulation
(P<0.05). However, the NK cells expressed low levels of KIR3DL
and the KIR3DL expression was slightly upregulated following IL-2
treatment (Fig. 4; P=0.023).
Discussion
NK cells are important mediators of the innate
immune system, and the number of tumor-infiltrating NK cells may be
used as a predictor of liver cancer prognosis (12). A previous study indicated that the
function of NK cells is repressed in the liver tumor
microenvironment (13). Therefore,
strategies to enable NK cell function and activity are required to
develop an effective treatment approach.
In the present study, the antitumor activity of NK
cells was assessed. The PBMCs separated from blood consisted of
20–30% CD56+CD3− cells, which were
characterized as the NK cells. The isolated
CD56+CD3− cell purity was determined to be
95%. In the presence of IL-2, the NK cells demonstrated robust
growth and maintained vitality. In vivo, the NK cells
exhibited long-term survival in the liver of the nude mice. This
finding was consistent with the findings of previous studies
(8,10). NK cell dysfunction in patients with
hepatocellular carcinoma has been associated with several factors
(14,15), including the suppression of cytokine
expression in the tumor environment (16,17).
Cytokines, such as IL-12, serve an important role in the activation
of NK cells in the tumor microenvironment (18). As aforementioned, IL-2 is required for
the growth, proliferation and activation of NK cells. Furthermore,
NK cells with repressed effector function regain cytolytic function
following stimulation with IL-2 (19).
Overall, several studies have demonstrated that NK
cells inhibit tumor growth. Maat et al (20) demonstrated that NK cells were involved
in the prevention of uveal melanoma metastases. However, few
studies have investigated the treatment efficacy of NK cell
administration in the prevention of hepatocellular carcinoma
metastases. Barkholt et al (8)
reported that NK cells, along with IL-2, may be safely administered
to patients with hepatocellular carcinoma. In the present in
vitro study, the data indicated that IL-2 activated the NK
cells, and therefore inhibited MHCC97-H proliferation, migration
and invasion. Furthermore, the in vivo data demonstrated
that the NK cells inhibited pulmonary metastases of hepatocellular
cancer. However, the tumor burden in the livers obtained from the
control and NK groups was not significantly different. The
similarity in tumor burden may be due to low levels of tumor
necrosis and the poor nutritional intake by the nude mice in the
last weeks of the experiments.
The mechanisms underlying the NK-mediated inhibition
of tumor invasion and tumor metastasis have not been well
characterized. As aforementioned, the cytokines secreted by NK
cells may be crucial in activating antitumor immunity. For example,
NK cells are a major producer of interferon-γ, which is critical
for the IL-12-induced antitumor effect (21). Furthermore, NK cells may mediate
antitumor responses via direct contact with tumor cells or other
immune cells. The contact between NK cells and tumor cells may lead
to tumor cell immunoediting through cell surface molecular
interactions (22,23).
Previous studies have demonstrated that NK cell
receptors and their corresponding ligands serve an important role
in liver diseases (24,25). NKG2D is an important activating
receptor of NK cells, and NKG2D expression has been directly
associated with hepatocellular carcinoma prognosis (26). Ul16 binding protein is an NKG2D ligand
and is expressed on tumor cells. The absence of Ul16 binding
protein expression has been identified as an independent factor for
recurrent hepatocellular carcinoma (27). NK cell cytotoxicity is mediated
through the NKG2D-major histocompatability complex (MHC) class
I-related chain A/B interaction (28). In the present study, the expression of
NKG2D increased significantly following incubation with IL-2 and
the expression of NKB1, an inhibitory receptor, was elevated
moderately. The expression of cell markers for activated NK cells
(granzyme and perforin) increased significantly. Overall, the data
indicate that NK cells exert inhibitory effects on tumor cell
proliferation, migration and invasion.
Killer immunoglobulin receptors (KIRs) are NK cell
receptors that are associated with liver cancer incidence. KIRs are
involved in the regulation of NK cell activation through
recognition and binding with specific human leukocyte antigen (HLA)
class I allotypes. Thus, the type of HLAs expressed on tumor cells
may determine the antitumor efficacy of the NK cells (29). NKB1 (KIR3DL1) is a member of the KIR
family. The interaction of NKB1 with specific HLA-B antigens on a
target cell inhibits cell-mediated cytotoxicity, possibly by
delivering a negative signal that prevents NK cell activation.
Therefore, the interactions between MHC I and KIRs may regulate NK
responses to antigenic challenge (30).
The mechanisms underlying the antitumor and
anti-metastatic activity of NK cells have not been well
characterized. Few studies have indicated that NK cells may mediate
the removal of micro-metastatic cells from circulation and attack
metastatic cells present inside organs. Therefore, methods to
further improve the antitumor functions of NK cells are of
considerable interest. The role of signaling pathways and gene
expression in the development of NK cells has been previously
investigated. One study demonstrated that activation of the Notch
signaling pathway supports the development of cytokine-producing NK
cells that are hyporesponsive and have low receptor and poor
effector functions (31).
Furthermore, microRNA-30c-1 has been identified to strengthen NK
cell cytotoxicity against hepatocellular carcinoma cells by
regulating the expression of the transcription factor homeobox
containing 1 (32). When combined,
these studies indicate that the NK cell antitumor activity may be
improved by regulating the signal transduction pathways or the gene
expression of NK cells.
In conclusion, in the present study, it was
demonstrated that NK cells inhibited the proliferation, migration
and invasion of the MHCC97-H cells in vitro, and that the NK
cells survived in the livers of the nude mice. Furthermore, the NK
cells inhibited lung metastasis of hepatocellular carcinoma in
vivo. However, the NK cells did not inhibit tumor growth in the
liver of the nude mice. The data also indicated that IL-2 may
enhance NK cell-mediated metastasis inhibition by inducing the
expression of immunologically relevant molecules. However, further
investigation is required to identify the mechanisms that enable NK
cells to inhibit cancer metastasis from the liver to the lungs.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81171976) and the Fujian
University of Traditional Chinese Medicine (no. XB2015039).
References
|
1
|
But DY, Lai CL and Yuen MF: Natural
history of hepatitis-related hepatocellular carcinoma. World J
Gastroenterol. 14:1652–1656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hao K, Luk JM, Lee NP, Mao M, Zhang C,
Ferguson MD, Lamb J, Dai H, Ng IO, Sham PC and Poon RT: Predicting
prognosis in hepatocellular carcinoma after curative surgery with
common clinicopathologic parameters. BMC Cancer. 9:3892009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sangiovanni A and Colombo M: Treatment of
hepatocellular carcinoma: Beyond international guidelines. Liver
Int. 36(Suppl 1): 124–129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Invernizzi F, Viganò M, Grossi G and
Lampertico P: The prognosis and management of inactive HBV
carriers. Liver Int. 36(Suppl 1): 100–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seki S, Nakashima H, Nakashima M and
Kinoshita M: Antitumor immunity produced by the liver Kupffer
cells, NK cells, NKT cells, and CD8 CD122 T cells. Clin Dev
Immunol. 2011:8683452011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geissler M, Mohr L and Blum HE:
Immunotherapy of hepatocellular carcinoma. Dtsch Med Wochenschr.
126:1464–1466. 2001.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gersuk GM, Westermark B, Mohabeer AJ,
Challita PM, Pattamakom S and Pattengale PK: Inhibition of human
natural killer cell activity by platelet-derived growth factor
(PDGF). III. Membrane binding studies and differential biological
effect of recombinant PDGF isoforms. Scand J Immunol. 33:521–532.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barkholt L, Alici E, Conrad R, Sutlu T,
Gilljam M, Stellan B, Christensson B, Guven H, Björkström NK,
Söderdahl G, et al: Safety analysis of ex vivo-expanded NK and
NK-like T cells administered to cancer patients: A phase I clinical
study. Immunotherapy. 1:753–764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doskali M, Tanaka Y, Ohira M, Ishiyama K,
Tashiro H, Chayama K and Ohdan H: Possibility of adoptive
immunotherapy with peripheral blood-derived
CD3−CD56+ and CD3+CD56+
cells for inducing antihepatocellular carcinoma and antihepatitis C
virus activity. J Immunother. 34:129–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang X, Liang L, Zhang XF, Jia HL, Qin Y,
Zhu XC, Gao XM, Qiao P, Zheng Y, Sheng YY, et al: MicroRNA-26a
suppresses tumor growth and metastasis of human hepatocellular
carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology.
58:158–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ciaglia E, Pisanti S, Picardi P, Laezza C,
Sosa S, Tubaro A, Vitale M, Gazzerro P, Malfitano AM and Bifulco M:
N6-isopentenyladenosine affects cytotoxic activity and cytokines
production by IL-2 activated NK cells and exerts topical
anti-inflammatory activity in mice. Pharmacol Res. 89:1–10. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu LY, Zhou J, Liu YZ and Pan WD:
Prognostic significance of natural killer cell infiltration in
hepatocellular carcinoma. Ai Zheng. 28:1198–1202. 2009.(In
Chinese). PubMed/NCBI
|
|
13
|
Matsuoka S, Maeda N, Izumiya C, Yamashita
C, Nishimori Y and Fukaya T: Expression of inhibitory-motif killer
immunoglobulin-like receptor, KIR2DL1, is increased in natural
killer cells from women with pelvic endometriosis. Am J Reprod
Immunol. 53:249–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li T, Yang Y, Hua X, Wang G, Liu W, Jia C,
Tai Y, Zhang Q and Chen G: Hepatocellular carcinoma-associated
fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer
Lett. 318:154–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao
D, Liu Y, Zhu F, Zhang L, Sun W, et al: T cell immunoglobulin- and
mucin-domain-containing molecule-3 (Tim-3) mediates natural killer
cell suppression in chronic hepatitis B. J Hepatol. 52:322–329.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohira M, Nishida S, Tryphonopoulos P,
Tekin A, Selvaggi G, Moon J, Levi D, Ricordi C, Ishiyama K, Tanaka
Y, et al: Clinical-scale isolation of interleukin-2-stimulated
liver natural killer cells for treatment of liver transplantation
with hepatocellular carcinoma. Cell Transplant. 21:1397–1406. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsunematsu H, Tatsumi T, Kohga K, Yamamoto
M, Aketa H, Miyagi T, Hosui A, Hiramatsu N, Kanto T, Hayashi N and
Takehara T: Fibroblast growth factor-2 enhances NK sensitivity of
hepatocellular carcinoma cells. Int J Cancer. 130:356–364. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mikuriya Y, Tashiro H, Kuroda S, Nambu J,
Kobayashi T, Amano H, Tanaka Y and Ohdan H: Fatty liver creates a
pro-metastatic microenvironment for hepatocellular carcinoma
through activation of hepatic stellate cells. Int J Cancer.
136:E3–E13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Faridi RM, Das V, Tripthi G, Talwar S,
Parveen F and Agrawal S: Influence of activating and inhibitory
killer immunoglobulin-like receptors on predisposition to recurrent
miscarriages. Hum Reprod. 24:1758–1764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maat W, van der Slik AR, Verhoeven DH,
Alizadeh BZ, Ly LV, Verduijn W, Luyten GP, Mulder A, van Hall T,
Koning F, et al: Evidence for natural killer cell-mediated
protection from metastasis formation in uveal melanoma patients.
Invest Ophthalmol Vis Sci. 50:2888–2895. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uemura A, Takehara T, Miyagi T, Suzuki T,
Tatsumi T, Ohkawa K, Kanto T, Hiramatsu N and Hayashi N: Natural
killer cell is a major producer of interferon gamma that is
critical for the IL-12-induced anti-tumor effect in mice. Cancer
Immunol Immunother. 59:453–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM,
Wang D, Li XF and Zheng L: Monocyte/macrophage-elicited natural
killer cell dysfunction in hepatocellular carcinoma is mediated by
CD48/2B4 interactions. Hepatology. 57:1107–1116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoon JC, Lim JB, Park JH and Lee JM:
Cell-to-cell contact with hepatitis C virus-infected cells reduces
functional capacity of natural killer cells. J Virol.
85:12557–12569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamagiwa S, Kamimura H and Ichida T:
Natural killer cell receptors and their ligands in liver diseases.
Med Mol Morphol. 42:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoechst B, Voigtlaender T, Ormandy L,
Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten
TF and Korangy F: Myeloid derived suppressor cells inhibit natural
killer cells in patients with hepatocellular carcinoma via the
NKp30 receptor. Hepatology. 50:799–807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Konjević G, Mirjacić Martinović K, Vuletić
A, Jović V, Jurisić V, Babović N and Spuzić I: Low expression of
CD161 and NKG2D activating NK receptor is associated with impaired
NK cell cytotoxicity in metastatic melanoma patients. Clin Exp
Metastasis. 24:1–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kamimura H, Yamagiwa S, Tsuchiya A,
Takamura M, Matsuda Y, Ohkoshi S, Inoue M, Wakai T, Shirai Y,
Nomoto M and Aoyagi Y: Reduced NKG2D ligand expression in
hepatocellular carcinoma correlates with early recurrence. J
Hepatol. 56:381–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morisaki T, Onishi H, Koya N, Kiyota A,
Tanaka H, Umebayashi M, Ogino T, Nagamatsu I and Katano M:
Combinatorial cytotoxicity of gemcitabine and cytokine-activated
killer cells in hepatocellular carcinoma via the NKG2D-MICA/B
system. Anticancer Res. 31:2505–2510. 2011.PubMed/NCBI
|
|
29
|
Pan N, Jiang W, Sun H, Miao F, Qiu J, Jin
H, Xu J, Shi Q, Xie W and Zhang J: KIR and HLA loci are associated
with hepatocellular carcinoma development in patients with
hepatitis B virus infection: A case-control study. PLoS One.
6:e256822011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Townsley E, O'Connor G, Cosgrove C, Woda
M, Co M, Thomas SJ, Kalayanarooj S, Yoon IK, Nisalak A,
Srikiatkhachorn A, et al: Interaction of a dengue virus NS1-derived
peptide with the inhibitory receptor KIR3DL1 on natural killer
cells. Clin Exp Immunol. Oct 6–2015.(Epub ahead of print).
PubMed/NCBI
|
|
31
|
Bachanova V, McCullar V, Lenvik T, Wangen
R, Peterson KA, Ankarlo DE, Panoskaltsis-Mortari A, Wagner JE and
Miller JS: Activated notch supports development of cytokine
producing NK cells which are hyporesponsive and fail to acquire NK
cell effector functions. Biol Blood Marrow Transplant. 15:183–194.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gong J, Liu R, Zhuang R, Zhang Y, Fang L,
Xu Z, Jin L, Wang T, Song C, Yang K, et al: miR-30c-1* promotes
natural killer cell cytotoxicity against human hepatoma cells by
targeting the transcription factor HMBOX1. Cancer Sci. 103:645–652.
2012. View Article : Google Scholar : PubMed/NCBI
|