Introduction
Endometrial carcinoma is the most common genital
malignancy of women in developed countries. Globally, >280,000
new cases are diagnosed each year (1). The prognosis of endometrial carcinoma is
generally thought to be favorable, predominantly due to detection
and diagnosis in the early stages of disease. However, there are
subtypes among endometrial carcinomas with poor prognosis and
outcome after therapy (2).
Several types of tumors may be classified as
high-risk. This includes serous adenocarcinoma, a highly malignant
histological type that was first described by Hendrickson et
al (3) more than three decades
ago. Clear cell carcinomas, similarly to renal carcinomas, also
belong to a high-risk group and have a mutation profile more like
that of serous carcinomas than common endometrioid carcinomas
(4). During recent years,
carcinosarcomas (mixed malignant mesodermal tumors) have been
reclassified and moved from the uterine sarcoma group to the
endometrial adenocarcinomas of high-risk type group (5). The incidence of recurrences,
predominantly at distant sites (65%), is more frequent for these
high-risk types of non-endometrioid carcinomas than for
endometrioid carcinomas (6,7).
In certain studies, a number of early-stage serous
carcinoma patients appear to have a good prognosis; however,
reliable prognostic indicators are lacking (8). Investigation of predictive and
prognostic factors (9) and definition
of clinically relevant risk groups (10) are important steps to improve
diagnosis, classification and treatment planning. To design
clinical trials and to select optimal treatment, establishing
reliable and reproducible risk groups is mandatory.
At present, no consensus exists regarding which
predictive or prognostic factors should be used and how to combine
them in the definition of various risk groups. Although numerous
randomized phase III trials (11–16) have
been presented in the literature during the last two decades, the
results are difficult to compare and final conclusions remain
uncertain and under debate. The low statistical power of certain
studies dealing with predictive and prognostic factors has also
prevented definitive conclusions regarding prognosis and optimal
therapy. Analysis of data from large registry studies may allow
circumvention of some of these problems (9,10);
however, scanty clinical data and selection bias may be
shortcomings in these types of study.
Since 1980, a number of prospective randomized
studies have been conducted to elucidate the value of external beam
radiotherapy following surgery in early-stage endometrial carcinoma
(11–15). The risk groups have differed between
these trials, including low-risk, low to medium-risk, medium to
high risk and high-risk groups. The type of primary surgery and
staging have also varied, from no staging (11,17) to
staging with lymph node sampling or complete lymphadenectomy
(pelvic and/or paraaortic) (12,13).
Subgroup analyses performed in these studies have suffered from low
power (e.g., for pure high-risk cases), and no level-one data are
generated. Vaginal brachytherapy, external beam pelvic radiation
and adjuvant chemotherapy have been addressed in these studies
(15,16,18).
In the present retrospective study, a series of
high-risk endometrial carcinomas was selected from a large
consecutive, non-selected population of >4,500 endometrial
carcinomas of International Federation of Gynecology and Obstetrics
(FIGO) stages I–IV. Three types of high-risk tumors (serous
carcinoma, clear cell carcinoma and carcinosarcoma) were selected
among non-endometrioid carcinomas for further studies of predictive
and prognostic factors, with special emphasis on the impact of DNA
ploidy measured by flow cytometry.
Patients and methods
Patients
The Department of Gynecological Oncology, Örebro
University Hospital recruited patients with all stages (FIGO I–IV)
of endometrial carcinomas into this retrospective, observational
study. The period of recruitment was between January 1975 and
December 2009. In total, 4,543 patients were included.
Postoperative external pelvic irradiation and/or vaginal
brachytherapy were administered to the majority of the patients.
For high-risk tumors, adjuvant platinum-based chemotherapy was also
commonly administered.
From this consecutive series of endometrial
carcinomas, tumors with high-risk pathology (serous carcinomas,
clear cell carcinomas and carcinosarcomas) were selected for
further analyses with regard to predictive and prognostic factors,
with special focus on the importance of DNA ploidy. A total of 373
tumors fulfilled these high-risk criteria, of which 94 cases were
serous carcinomas, 48 were clear cell carcinomas and 231 were
carcinosarcomas. Further characteristics of the tumors are
presented in Table I. Tumor size and
lymphovascular space invasion were not included in the present
study, as data regarding these variables were not regularly
available in the pathology reports. Other types of high-risk tumors
(endometrioid type) were not included in this review. All pathology
reports were reviewed by one experienced pathologist from the
Department of Pathology, Örebro University Hospital. The mean age
of this subgroup of patients was 67.6 years (range, 32–94
years).
 | Table I.Characteristics of the series of
high-risk tumors. |
Table I.
Characteristics of the series of
high-risk tumors.
| Variable | n | % total | % evaluable |
|---|
| Histology |
|
|
|
| Serous
carcinoma | 94 | 25.2 |
|
| Clear
cell carcinoma | 48 | 12.9 |
|
|
Carcinosarcoma | 231 | 61.9 |
|
| Tumor stage
(surgical) |
|
|
|
| I | 148 | 39.7 | 55.9 |
| II | 30 | 8.0 | 11.3 |
| III | 47 | 12.6 | 17.7 |
| IV | 40 | 10.7 | 15.1 |
|
Unknown | 108 | 29.0 |
|
| Myometrial
infiltration |
|
|
|
| <50% | 83 | 22.3 | 54.3 |
| ≥50% | 70 | 18.8 | 45.7 |
|
Unknown | 220 | 59.0 |
|
| DNA
ploidya |
|
|
|
|
Diploid | 34 | 9.1 | 32.1 |
|
Non-diploid | 72 | 19.3 | 67.9 |
|
Unknown | 267 | 71.6 |
|
| p53 status |
|
|
|
|
Negative | 11 |
2.9 | 24.4 |
|
Positive | 34 |
9.1 | 75.6 |
|
Unknown | 328 | 87.9 |
|
DNA ploidy status
DNA ploidy analysis was conducted by flow cytometry
(FCM) as part of the routine histopathological evaluation of the
endometrial carcinomas. In the majority of the cases, the S-phase
fraction was not reported, and this variable was therefore not
included in this study. The outcome was presented as diploid or
non-diploid (including multiple aneuploid and tetraploid cases). A
DNA diploid histogram was defined as containing a single major peak
with a DNA index (DI) ranging from 0.90 to 1.10; one or more major
peaks outside this range defined non-diploid histograms. A
tetraploid histogram displayed a peak with a DI in the range of
1.80–2.20 (>15% of all cells measured). Among the endometrioid
tumors, 21% were non-diploid (not further presented in this study),
whilst serous carcinomas comprised 79% non-diploid cases, clear
cell carcinomas comprised 50%, and carcinosarcomas included 65%
non-diploid cases. Tetraploid tumors were few, accounting for 47 of
1,601 evaluable cases (2.9%). In clear cell carcinomas, no
tetraploid cases were recorded.
Primary surgery
The primary surgery included a total abdominal
hysterectomy, bilateral salpingo-oophorectomy, appendectomy, node
sampling of enlarged lymph nodes and peritoneal washing with
cytology. Lymphadenectomy was not routinely performed at the
centers referring patients to the regional clinic. Surgeries were
performed at the following institutions: Department of Gynecology
and Obstetrics, Örebro University Hospital; Department of
Gynecology and Obstetrics, Central Hospital, Karlstad; Department
of Gynecology and Obstetrics, Eskilstuna; Department of Gynecology
and Obstetrics, County Hospital, Karlskoga; Department of
Gynecology and Obstetrics, County Hospital, Nyköping. All patients
were subsequently referred to the gynecological oncology center for
postoperative evaluation and treatment. The time interval between
surgery and postoperative pelvic irradiation was 4–8 weeks. All
patients were planned for a 10-year follow-up. The median follow-up
period at the time of analysis was 64 months (range, 2–191 months)
for surviving patients. During all visits, symptoms and signs
associated with the therapy were recorded. However, the
treatment-related side effects are not further presented in the
current study. No patients were lost to follow-up.
Brachytherapy
For the brachytherapy treatments, micro-Selectron
HDR machines (Elekta Instruments AB, Stockholm, Sweden) with an
iridium source (Ir-192) were used. Plastic vaginal cylinders with a
diameter of 20, 25 or 30 mm were used as standard. The length of
the vagina was measured from the vault to the level of introitus.
The proximal two-thirds of the vaginal length were defined as the
target volume. The dose per fraction was specified at a depth of 5
mm from the surface of the vaginal cylinder. Library dose plans
that covered different vaginal lengths in steps of 10 mm and the
different diameters of the cylinders were used. The dose
calculations were made on the Nucletron Planning System (version
10; Elekta Instruments AB) and the PLATO Brachytherapy Planning
System (version 14; Elekta Instruments AB). Six fractions were
administered during an 8-day period. The dose per fraction was
assigned as 2.5–3.0 Gy. Thus, the total doses delivered were
15.0–18.0 Gy. Recalculated to 2-Gy-equivalent doses, the total
doses were 15.6–19.5 Gy at a depth of 5 mm (α/β=10.0; α, the linear
term; β, the quadratic terms of the linear quadratic equation to
describe the cell killing effect in radiotherapy).
External beam radiotherapy
External beam therapy was administered to patients
with high-risk tumors. The target volume was the previous site of
the uterus and adnexa, the parametrial tissues, the proximal
two-thirds of the vagina, and the lymphatic drainage regions along
the iliac vessels up to the promontory. The total median dose
delivered to this volume was 46.0 Gy (range, 6.0–50.0 Gy) and daily
fractions were 1.8–2.0 Gy.
Chemotherapy regimens
Following the completion of radiotherapy,
platinum-based chemotherapy regimens [cisplatin (50 mg/m2) +
doxorubicin (50 mg/m2); cisplatin (75 g/m2) + cyclophosphamide (750
mg/m2); or carboplatin (AUC 5) + paclitaxel (175mg/m2)] were
administered (4 cycles every 3 weeks for a duration of 9 weeks) to
patients with high-risk tumors, including grade 3 endometrioid
tumors with deep myometrial invasion.
Data management
All data were collected in a computerized database
registry at the Regional Gynecological Oncology Center (Örebro,
Sweden). The study was a retrospective, observational registry
study. All analyzed data were retrieved from this clinical database
and no further data were added from the patient records or from
other sources.
Statistical analyses
For statistical analyses, survival curves were
generated using the Kaplan-Meier method, and differences were
tested with the log-rank test. The Pearson χ2 test was used for
comparison of proportions, and an independent samples t-test
or analysis of variance (ANOVA) for comparing the means of two or
more groups. Multivariate analysis of prognostic factors was
performed using the Cox proportional hazards model for survival
data, and logistic regression analysis for binary outcome data
(tumor recurrences). Best subset analysis was performed with a
multivariate technique to find the most important prognostic
factors and to find the most powerful and convenient combination of
these factors. All P-values were based on two-sided tests, with
P<0.05 considered to indicate a statistically significant
difference. The Statistica software package (StatSoft, Inc., Tulsa,
OK, USA; version 12; 2013) was used for the statistical
analyses.
Results
Patient series
The mean age of the complete series of patients was
67.6 years (range, 32–94 years). Patients with carcinosarcomas
(mean age, 65.4 years) were significantly younger than patients
with serous (mean age, 70.8 years) or clear cell (mean age, 71.7
years) carcinomas (ANOVA; P<0.001). Stage distribution
demonstrated a significant difference, with more carcinosarcomas in
stages I and IV [serous carcinomas 29/75 (38.7%), clear cell
carcinomas 18/38 (47.4%) and carcinosarcomas 101/152 (66.5%)], and
more serous carcinomas and clear cell carcinomas in stages II and
III [serous carcinomas 35/75 (46.7%), clear cell carcinomas 15/38
(39.5%) and carcinosarcomas 27/152 (17.8%)] (Pearson χ2; P=0.004).
In the complete series with available data on myometrial
infiltration, significantly more serous carcinomas and clear cell
carcinomas exhibited deep myometrial infiltration (≥50% of
myometrial thickness) than carcinosarcomas (Pearson χ2; P=0.049).
p53 positivity was detected in 94.7% of evaluable serous
carcinomas, 60.0% of clear cell carcinomas, and 62.5% of
carcinosarcomas; there was a statistically significant difference
in the rate of p53-positive staining between the serous and clear
cell carcinomas vs. the carcinosarcomas (Pearson χ2; P=0.037).
Endometrioid carcinomas, not further analyzed in this study, were
p53-positive in 46.8%.
Primary cure rate
In the complete series, primary cure (complete
remission) was achieved in 292 out of 373 high-risk cases (78.3%).
The primary cure rates were 77.7% in serous carcinomas, 85.4% in
clear cell carcinomas and 77.1% carcinosarcomas. Thus, there were
no statistically significant differences between the three
histopathological subtypes (Pearson χ2; P=0.435).
Recurrence rate
The overall recurrence rates of the complete series
of high-risk endometrial carcinomas was 108 out of 373 cases
(29.0%), and 108 out of the 292 cases (37.0%) that had achieved
primary complete remission. Of these, 25 cases (6.7%) had
locoregional recurrence and 83 cases (22.3%) had distant
metastases. There were 8 single vaginal recurrences (2.1%) and 17
pelvic recurrences outside the vagina (4.6%). Among distant
metastases, lung (46 cases; 12.3%), abdomen (21 cases; 5.6%), liver
(5 cases; 1.3%), bone (9 cases; 2.4%), paraaortic nodes (3 cases;
0.8%) and central nervous system (2 cases; 0.5%) were the most
frequent sites. Between the three histopathological high-risk types
(serous carcinomas, clear cell carcinomas and carcinosarcomas), no
significant differences were observed in the overall recurrence
rate (21.3%, 33.3% and 31.2%, respectively; Pearson χ2; P=0.158) or
in the sites of recurrences (Pearson χ2; P=0.602), with the
exception of lung metastases; lung metastases were significantly
more frequent (19.1%) in carcinosarcomas than in serous (5.5%) and
clear cell carcinomas (12.2%) (Pearson χ2; P=0.019). The median
time to relapse was 24.8 months (range, 2–132 months). Out of 108
recurrences, 84 (77.8%) were diagnosed within 3 years, and 97
(89.8%) within 5 years. The mean age of patients with recurrences
(68.1 years) was similar to the mean age of patients without
recurrences (67.4 years).
Predictive factors for primary cure
rate
DNA ploidy (diploid vs. non-diploid) was a
statistically significant predictive factor for primary complete
remission following therapy [logistic regression analysis; odds
ratio, 2.90; 95% confidence interval (CI), 2.36–3.43; P<0.050].
The primary cure rates were 85.3% among diploid tumors and 66.7%
among non-diploid tumors. Similar differences (diploid vs.
non-diploid) in primary cure rate were observed for serous
carcinomas (80 vs. 68%), clear cell carcinomas (92 vs. 75%) and
carcinosarcomas (83 vs. 59%). FIGO stage was a strong and highly
significant predictive factor for primary cure rate (Pearson χ2;
P<0.001). The cure rate varied from 95% in stage I to 23% in
stage IV. Depth of myometrial invasion was also a significant
predictive factor for primary cure rate (92% for superficial
invasion and 77% for deep myometrial invasion; Pearson χ2;
P=0.013). In a multivariate logistic regression analysis FIGO stage
was the only significant (P=0.003) and independent predictive
factor. Tumor expression of p53 (>30% staining of tumor cells)
and age of the patients were not significant predictive factors for
the primary cure rate of the tumor.
Predictive factors for tumor
recurrences
In univariate and multivariate logistic regression
analyses, tumor infiltration (superficial vs. deep) was a
significant and independent predictive factor (P=0.016) in serous
and clear cell carcinomas with regard to overall recurrence rate,
following correction for age, tumor stage (FIGO) and DNA ploidy
status. Age (P=0.463), tumor stage (P=0.718) and DNA ploidy
(P=0.895) were non-significant factors in this analysis as well as
in univariate analyses. A best subset multivariate analysis
confirmed that FIGO stage and depth of myometrial infiltration were
the two most important predictive factors in a model with four
included factors. Age and ploidy status had minor influence on the
likelihood score of the model. When carcinosarcomas were included
in the analysis, depth of tumor infiltration remained a significant
predictive factor in univariate analysis (P=0.009) but not in
multivariate analysis (P=0.131) (Table
II). For distant recurrences, tumor infiltration was a
significant (P=0.003) predictive factor in serous and clear cell
carcinomas; however, this was not the case when carcinosarcomas
were included in the analysis (P=0.076) (Table III). No significant predictive
factors for distant recurrences and lung metastases could be
identified in carcinosarcomas. DNA ploidy was a stronger predictive
factor in carcinosarcomas than in clear cell carcinomas and serous
carcinomas in univariate analyses with regard to overall recurrence
rate. FIGO grade (P<0.001) and nuclear grade (P=0.005), highly
significant and independent predictive factors in endometrioid
carcinomas, were not applicable for the non-endometrioid carcinomas
analyzed in the current study.
 | Table II.Logistic regression analyses.
Predictive factors vs. overall recurrence rate. |
Table II.
Logistic regression analyses.
Predictive factors vs. overall recurrence rate.
| Predictive
factor | OR | 95% CI | P-value |
|---|
| Univariate
analyses |
|
|
|
| Age
(per year) | 1.016 | 0.996–1.036 | 0.129 |
| FIGO
stage (I–II vs. III–IV) | 0.567 | 0.251–0.884 | 0.079 |
| DNA
ploidya | 0.562 | 0.092–1.032 | 0.230 |
|
Infiltrationb | 0.384 | 0.024–0.743 | 0.009 |
| Multivariate
analysis |
|
|
|
| Age
(per year) | 1.024 | 0.960–1.089 | 0.463 |
| FIGO
stage (I–II vs. III–IV) | 0.758 | 0.070–1.587 | 0.744 |
| DNA
ploidya | 0.576 | 0.060–1.212 | 0.395 |
|
Infiltrationb | 0.355 | 0.316–1.027 | 0.131 |
 | Table III.Logistic regression analyses.
Predictive factors vs. distant recurrence rate. |
Table III.
Logistic regression analyses.
Predictive factors vs. distant recurrence rate.
| Predictive
factor | OR | 95% CI | P-value |
|---|
| Univariate
analyses |
|
|
|
| Age
(per year) | 1.008 | 0.987–1.029 | 0.462 |
| FIGO
stage (I–II vs. III–IV) | 0.722 | 0.394–1.050 | 0.330 |
| DNA
ploidya | 0.630 | 0.143–1.117 | 0.352 |
|
Infiltrationb | 0.429 | 0.068–0.789 | 0.021 |
| Multivariate
analysis |
|
|
|
| Age
(per year) | 0.981 | 0.914–1.048 | 0.573 |
| FIGO
stage (I–II vs. III–IV) | 1.006 | 0.255–1.757 | 0.994 |
| DNA
ploidya | 0.557 | 0.070–1.185 | 0.361 |
|
Infiltrationb | 0.302 | 0.360–0.963 | 0.076 |
Survival analyses
The overall 5-year survival rate of the complete
series of patients was 39.2% (95% CI, 33.8–44.6%), whilst the
cancer-specific survival rate was 46.9% (95% CI, 41.1–52.7%), and
the recurrence-free survival rate was 37.2% (95% CI, 31.8–42.6%).
No significant differences were identified between the three
histopathological subtypes with regard to overall (χ2; P=0.929) or
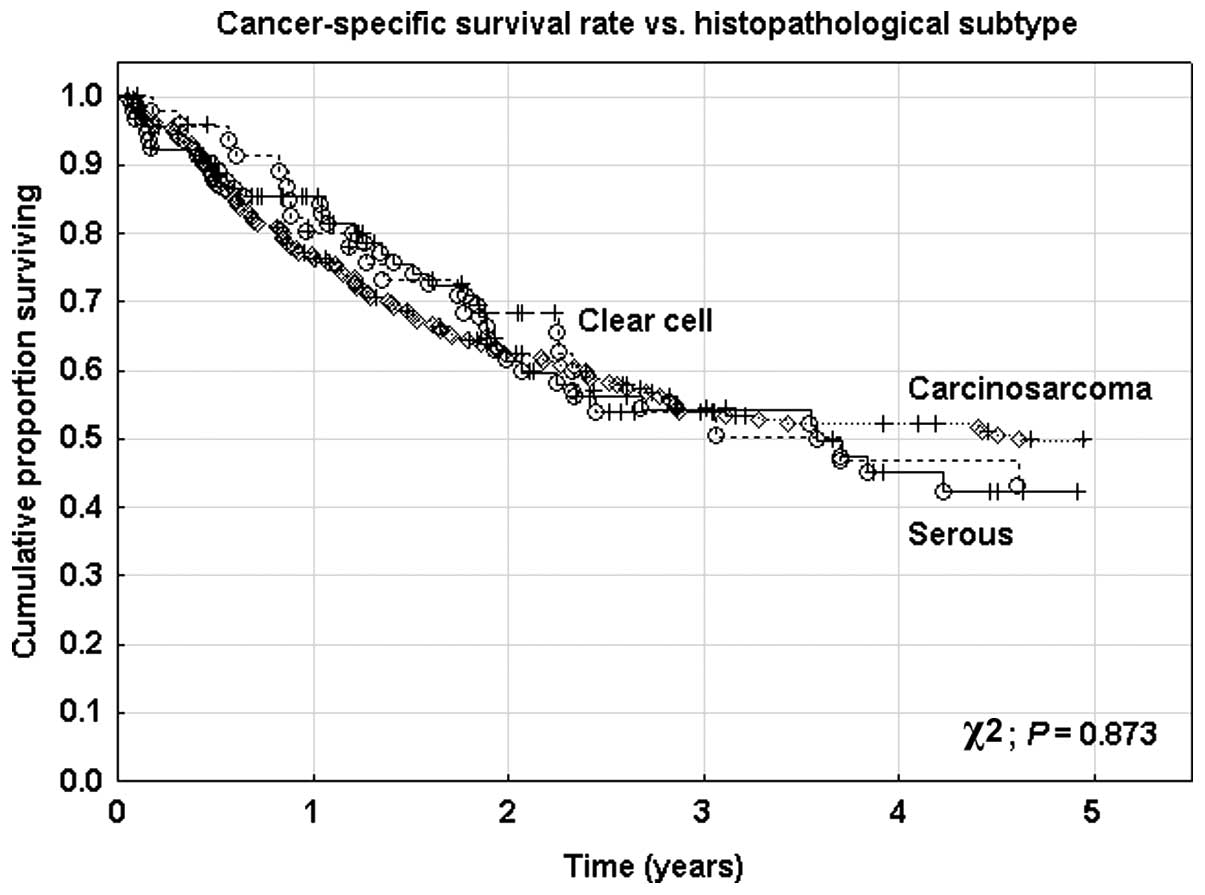
cancer-specific (χ2; P=0.873) survival rates (Fig. 1). The 5-year overall survival rate
following any type and site of recurrence was only 3.3% in this
series of high-risk carcinomas.
Prognostic factors for survival
Four prognostic factors (age, FIGO stage, tumor
infiltration and DNA ploidy status) were analyzed with a Cox
proportional hazard regression analysis (Table IV). In univariate analyses, all four
factors were statistically significant with regard to overall,
cancer-specific and recurrence-free survival rates. In multivariate
analyses with all factors included, age alone was a significant and
independent prognostic factor with regard to overall,
cancer-specific and recurrence-free survival rates. DNA ploidy was
a significant and independent factor when corrected for age,
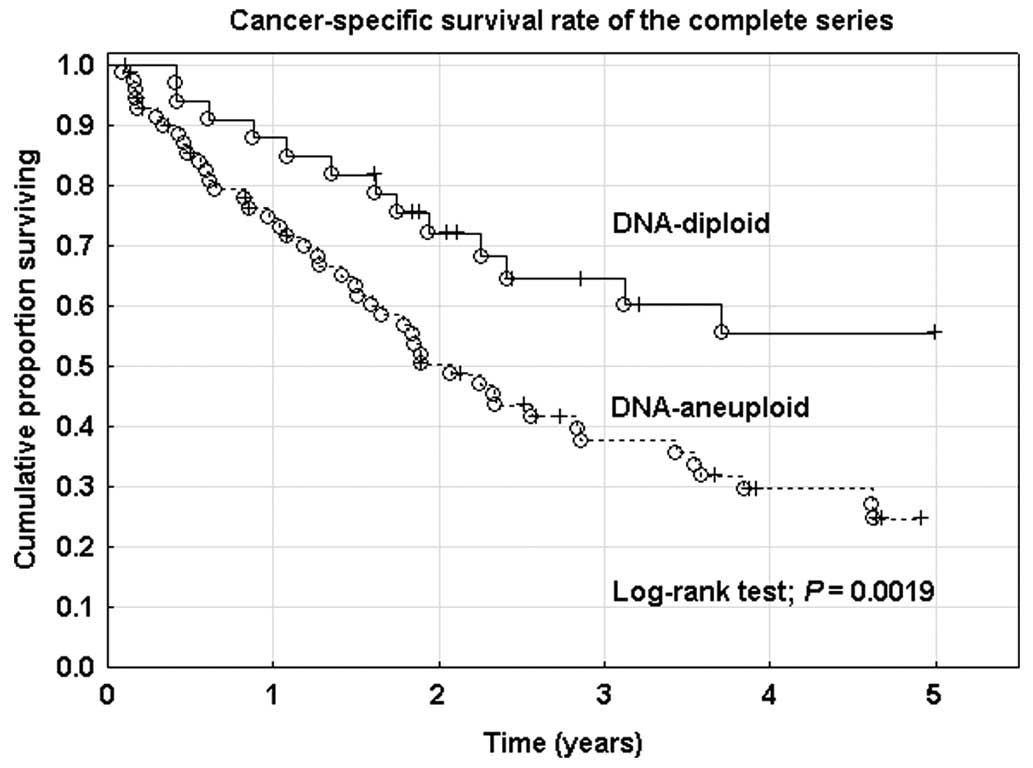
histopathological subtype and myometrial infiltration (Fig. 2), but not after correction for FIGO
stage. DNA ploidy (non-diploid vs. diploid) had a stronger
prognostic impact in carcinosarcomas (HR, 5.976) than in clear cell
carcinomas (HR, 1.874) and serous carcinomas (HR, 1.456) with
regard to cancer-specific survival rate. Tumor expression of p53
was a non-significant predictive and prognostic factor in all
analyses.
 | Table IV.Cox proportional hazard regression
analyses. Prognostic factors vs. cancer-specific survival rate. |
Table IV.
Cox proportional hazard regression
analyses. Prognostic factors vs. cancer-specific survival rate.
| Prognostic
factor | β | SE | HR | 95% CI | P-value |
|---|
| Univariate
analyses |
|
|
|
|
|
| Age
(per year) | 0.034 | 0.007 | 1.035 | 1.021–1.049 | <0.001 |
| FIGO
stage (III–IV vs. I–II) | 0.620 | 0.084 | 3.456 | 2.483–4.811 | <0.001 |
| DNA
ploidy (non-diploid vs. diploid) | 0.441 | 0.152 | 2.414 | 1.331–4.379 |
0.004 |
|
Infiltration (≥50% vs.
<50%)a | 0.429 | 0.114 | 2.360 | 1.512–3.682 | <0.001 |
| Multivariate
analysis |
|
|
|
|
|
| Age
(per year) | 0.038 | 0.018 | 1.039 | 1.003–1.076 |
0.035 |
| FIGO
stage (III–IV vs. I–II) | 0.292 | 0.188 | 1.792 | 0.857–3.746 |
0.121 |
| DNA
ploidy (non-diploid vs. diploid) | 0.252 | 0.190 | 1.655 | 0.786–3.488 |
0.185 |
|
Infiltration (≥50% vs.
<50%)a | 0.098 | 0.198 | 1.217 | 0.560–2.644 |
0.619 |
Discussion
Non-endometrioid endometrial carcinomas have a
significantly poorer prognosis than endometrioid carcinomas
(2). Although the definition of
high-risk carcinomas varies, the non-endometrioid histopathological
subtypes (e.g., serous carcinoma, clear cell carcinoma and
carcinosarcoma) are commonly included in this risk group (19). Poorly differentiated endometrioid
carcinomas with deep myometrial invasion also belong to the group
of high-risk carcinomas of the endometrium. Identification of
predictive and prognostic factors is an important prerequisite for
definitions of risk groups in this disease and is the basis for
design of clinical trials and for evaluation of the optimal
treatment modalities for the individual patient (9). A number of predictive and prognostic
factors in endometrioid carcinomas are described in the literature,
certain of which are effective in the clinical definition of risk
groups (10). However, for
non-endometrioid carcinomas, the situation is different; predictive
and prognostic factors are not so well-known and studied for these
tumor types.
In the present study, 373 high-risk non-endometrioid
tumors were selected from a large consecutive database of >4,500
cases of endometrial carcinomas. In all, 94 serous carcinomas, 48
clear cell carcinomas and 231 carcinosarcomas were identified.
Predictive and prognostic factors, including the age of the
patients, FIGO stage, histopathology, depth of myometrial
infiltration, DNA ploidy and p53 expression, were analyzed with
regard to primary cure rate of the tumor, recurrences rates (local,
regional and distant) and survival rates (overall, cancer-specific
and recurrence-free). Particular emphasis was placed on the
prognostic impact of the DNA ploidy status measured by FCM.
In a prior study of endometrioid carcinomas, DNA
ploidy was found to be a highly significant and independent risk
factor together with the FIGO grade, histological subtype and depth
of myometrial infiltration (9). For
non-endometrioid carcinomas, the importance of these various
predictive and prognostic factors is less clear (20), and this is also true for DNA ploidy
status (21). A reason for this may
be a significantly different distribution of the established
predictive and prognostic factors between the endometrioid and the
non-endometrioid tumors. Another reason may be the rarity of these
tumors and, thus, the few published studies and small sample sizes
of the individual histopathological subtypes. In the present study,
three types of non-endometrioid carcinomas were included. A reason
for this was the apparent similarities in the various outcome
variables for these tumor types. Another reason is the
morphological overlap amongst these groups, with problems often
faced at diagnosis due to the observation of certain pathological
features in all three tumor types (22). At present, carcinosarcomas, or mixed
mesodermal tumors, are considered to be part of the high-risk
endometrial carcinoma group rather than the endometrial sarcoma
group (5). The high-risk group is a
group of carcinomas with an extremely poor prognosis, few
established prognostic factors, and without consensus regarding the
optimal therapy. From our center and another Swedish center, we
have presented data on a series of 322 endometrial carcinosarcomas
and the clinical outcome following an adjuvant radiotherapy
treatment schedule (23). In this
series, 38% recurrences occurred, with 28% at distant sites, and
the overall survival rate was 30% (23). Combined radiotherapy and chemotherapy
was the most efficient postoperative adjuvant therapy for
locoregional tumor control (23).
The primary cure rates (complete remission) in the
present series were similar for the three histopathological
subtypes (77–85%). DNA ploidy was a significant predictive factor
for complete remission in the complete series and for the three
subgroups. Tumor stage and depth of myometrial infiltration were
also significant predictive factors. However, in a multivariate
logistic regression analysis, FIGO stage was the only significant
and independent factor.
The recurrence rate of the complete series was high,
with 37% experiencing recurrence following primary complete
remission, and the majority of the recurrences were of distant
type. The overall recurrence rate was in the same range for the
three tumor types; however, lung metastases were significantly more
frequent (19%) in carcinosarcomas. Uterine sarcomas are known to
spread hematogenously, and the prognosis in advanced stages is
extremely poor (23). In the current
study, 78% of recurrences were diagnosed within the three years
following therapy. DNA ploidy was a significant predictor for
overall recurrence rate in carcinosarcomas, but not in serous and
clear cell carcinomas. However, the most important predictive
factor was myometrial infiltration, significant in univariate and
multivariate analyses. Myometrial infiltration appeared to be a
less important predictive factor in carcinosarcomas than in serous
and clear cell carcinomas. The same result was seen for distant
recurrences, where infiltration was significant in serous and clear
cell carcinomas. For lung metastases, no significant risk factors
could be identified except type of histopathology. FIGO grade and
nuclear grade, known to be highly significant risk factors for
recurrence and metastasis in endometrioid carcinomas, were not
applicable in this group of non-endometrioid, high-risk carcinomas;
according to the definitions, these tumors already have a high
nuclear grade in the majority of the cases (3).
The survival of non-endometrioid carcinomas in this
series was poor, with a 5-year overall survival rate of 39% in the
complete series and with no significant differences between the
histopathological subtypes. This survival rate is in agreement with
a previous study (23). However, for
early stage serous carcinomas, a favorable 5-year overall survival
rate of 80% has been reported in the literature (8), which is not in agreement with the
present results of 53%.
Following tumor recurrence, survival was extremely
poor, with a 3% overall 5-year survival rate. The four prognostic
factors studied (age, stage, myometrial infiltration and DNA
ploidy) were all significant in univariate Cox proportional hazard
regression analysis. DNA ploidy status was also significant and
independent of age, histopathological subtype, and myometrial
infiltration; however, it was not significant following correction
for tumor stage. The two most important, significant and
independent prognostic factors for cancer-specific survival rate
were age and tumor stage. In a recent study from Norway on serous
carcinomas, the only significant prognostic marker in univariate
analyses was the 5c excessive rate (ExR), a ploidy-related
parameter used in the analysis of DNA-deviations, indicating an
abnormal amount of DNA in tumor cells; it has also been
demonstrated to be of prognostic importance in endometrial
carcinoma and other tumor types. The explanation was that 5c ExR
was high in proliferating tetraploid tumors and in aneuploid tumors
with high DNA index (20). In an
earlier study by Strang et al (24), 5c ExR was also a significant
prognostic factor in endometrioid carcinomas. The 5c ExR DNA
pattern has also been found to be a prognostic factor in other
types of carcinomas (25). However, a
different study revealed no association between DNA ploidy and
survival in serous carcinomas (21).
The advantages and disadvantages of FCM compared with image
cytometry (20) have been discussed,
and may be one explanation for different results regarding
predictive and prognostic value of DNA ploidy in the presented
studies. Image analysis seems to detect tetraploid and multiple
aneuploid peaks more efficiently than FCM (20,26,27). In
contrast to the results of the current study, tumor stage and depth
of infiltration did not predict prognosis in serous carcinomas in
the Norwegian study (20), and a
similar finding was reported by Goff et al (28) in a series of 50 serous carcinomas.
Expression of estrogen and progesterone receptors, as well as of
Ki-67 (a proliferation marker), were non-significant prognostic
factors in the aforementioned study (28).
Tumor expression of p53, analyzed by
immunohisto-chemistry, was not a significant predictive or
prognostic factor in any of the analyses performed. Overexpression
of p53 has been reported to be a prognostic factor in endometrioid
carcinomas (29,30), but not in non-endometrioid disease,
including serous carcinomas (31).
For endometrioid carcinomas, it has been shown that
DNA ploidy is an important predictive and prognostic factor and, if
used in combination with the FIGO grade and type of histopathology,
may replace myometrial invasion in the definition of preoperative
high-risk cases requiring more extensive surgery (10). For high-risk non-endometrioid
carcinomas, DNA ploidy is a significant predictive and prognostic
factor on univariate analyses, but does not have the same
importance and prognostic impact as for endometrioid
carcinomas.
Acknowledgements
The author wishes to thank Mr. Peter Jansson, IT
Coordinator at the Department of Oncology, Örebro University
Hospital, for his work with the database and retrieval of the
patient data included in this study. This work was supported by the
research Foundation at the Department of Oncology, and the
Foundation for Research in Gynecological Cancer, Örebro,
Sweden.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Setiawan VW, Yang HP, Pike MC, McCann SE,
Yu H, Xiang YB, Wolk A, Wentzensen N, Weiss NS, Webb PM, et al:
Type I and II endometrial cancers: Have they different risk
factors? J Clin Oncol. 31:2607–2618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hendrickson M, Ross J, Eifel P, Martinez A
and Kempson R: Uterine papillary serous carcinoma: A highly
malignant form of endometrial adenocarcinoma. Am J Surg Pathol.
6:93–108. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoang LN, McConechy MK, Meng B, McIntyre
JB, Ewanowich C, Gilks Blake C, Huntsman DG, Köbel M and Lee CH:
Targeted mutation analysis of endometrial clear cell carcinoma.
Histopathology. 66:664–674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McCluggage WG: Uterine carcinosarcomas
(malignant mixed Mullerian tumors) are metaplastic carcinomas. Int
J Gynecol Cancer. 12:687–690. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sood BM, Jones J, Gupta S, Khabele D, Guha
C, Runowicz C, Goldberg G, Fields A, Anderson P and Vikram B:
Patterns of failure after the multimodality treatment of uterine
papillary serous carcinoma. Int J Radiat Oncol Biol Phys.
57:208–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pradhan M, Abeler VM, Danielsen HE,
Sandstad B, Tropé CG, Kristensen GB and Risberg BÅ: Prognostic
importance of DNA ploidy and DNA index in stage I and II
endometrioid adeno-carcinoma of the endometrium. Ann Oncol.
23:1178–1184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Creasman WT, Odicino F, Maisonneuve P,
Quinn MA, Beller U, Benedet JL, Heintz AP, Ngan HY and Pecorelli S:
Carcinoma of the corpus uteri. FIGO 26th Annual Report on the
Results of Treatment in Gynecological Cancer. Int J Gynaecol
Obstet. 95(Suppl 1): S105–S143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kosary CL: FIGO stage, histology,
histologic grade, age and race as prognostic factors in determining
survival for cancers of the female gynecological system: An
analysis of 1973–87 SEER cases of cancers of the endometrium,
cervix, ovary, vulva and vagina. Semin Surg Oncol. 10:31–46. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sorbe B: Predictive and prognostic factors
in definition of risk groups in endometrial carcinoma. ISRN Obstet
Gynecol. 2012:3257902012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Creutzberg CL, Van Putten WL, Koper PC,
Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, De Winter KA, Lutgens
LC, van den Bergh AC, van de Steen-Banasik E, et al: Surgery and
postoperative radiotherapy versus surgery alone for patients with
stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC
study group. Postoperative radiation therapy in endometrial
carcinoma. Lancet. 355:1404–1411. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keys HM, Roberts JA, Brunetto VL, Zaino
RJ, Spirtos NM, Bloss JD, Pearlman A, Maiman MA and Bell JG:
Gynecologic Oncology Group: A phase III trial of surgery with or
without adjunctive external pelvic radiation therapy in
intermediate risk endometrial adenocarcinoma: A gynecologic
oncology group study. Gynecol Oncol. 92:744–751. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
ASTEC/EN.5 Study Group. Blake P, Swart AM,
Orton J, Kitchener H, Whelan T, Lukka H, Eisenhauer E, Bacon M, Tu
D, et al: Adjuvant external beam radiotherapy in the treatment of
endometrial cancer (MRC ASTEC and NCIC CTG EN. 5 randomised
trials): Pooled trial results, systematic review and meta-analysis.
Lancet. 373:137–146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nout RA, Smit VT, Putter H,
Jürgenliemk-Schulz IM, Jobseb JJ, Lutgens LC, van der Steen-Banasik
EM, Mens JW, Slot A, Kroese MC, et al: Vaginal brachytherapy versus
pelvic external beam radiotherapy for patients with endometrial
cancer of high-intermediate risk (PORTEC-2): An open-label,
non-inferiority, randomised trial. Lancet. 375:816–823. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sorbe B, Horvath G, Andersson H, Boman K,
Lundgren C and Pettersson B: External pelvic and vaginal
irradiation versus vaginal irradiation alone as postoperative
therapy in medium-risk endometrial carcinoma-a prospective
randomized study. Int J Radiat Oncol Biol Phys. 82:1249–1255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sorbe B, Nordström B, Mäenpää J, Kuhelj J,
Kuhelj D, Okkan S, Delaloye JF and Frankendal B: Intravaginal
brachytherapy in FIGO stage I low-risk endometrial cancer: A
controlled randomized study. Int J Gynecol Cancer. 19:873–878.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aalders J, Abeler V, Kolstad P and Onsrud
M: Postoperative external irradiation and prognostic parameters in
stage I endometrial carcinoma. Clinical and histopathologic study
of 540 patients. Obstet Gynecol. 56:419–427. 1980.PubMed/NCBI
|
|
18
|
Högberg T, Signorelli M, de Oliveira CF,
et al: Sequential adjuvant chemotherapy and radiotherapy in
endometrial cancer-results from two randomized studies. Eur J
Cancer. 36:371–378. 2010.
|
|
19
|
Rutgers JK: Update on pathology, staging
and molecular pathology of endometrial (uterine corpus)
adenocarcinoma. Future Oncol. 11:3207–3218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pradhan M, Davidson B, Abeler VM,
Danielsen HE, Tropé CG, Kristensen GB and Risberg BÅ: DNA ploidy
may be a prognostic marker in stage I and II serous adenocarcinoma
of the endometrium. Virchows Arch. 461:291–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kato DT, Ferry JA, Goodman A, Sullinger J,
Scully RE, Goff BA, Fuller AF Jr and Rice LW: Uterine papillary
serous carcinoma (UPSC): A clinicopathologic study of 30 cases.
Gynecol Oncol. 59:384–389. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garg K, Leitao MM Jr, Wynveen CA, Sica GL,
Shia J, Shi W and Soslow RA: p53 overexpression in morphologically
ambiguous endometrial carcinomas correlates with adverse clinical
outcomes. Mod Pathol. 23:80–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sorbe B, Paulsson G, Andersson S and
Steineck G: A population-based series of uterine carcinosarcomas
with long-term follow-up. Acta Oncol. 52:759–766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Strang P, Stenkvist B, Bergström R,
Stendahl U, del Campo Valdes M and Tribukait B: Flow cytometry and
interactive image cytometry in endometrial carcinoma. A comparative
and prognostic study. Anticancer Res. 11:783–788. 1991.PubMed/NCBI
|
|
25
|
Grote HJ, Friedrichs N, Pomjanski N, Guhde
HF, Reich O and Böcking A: Prognostic significance of DNA cytometry
in carcinoma of the uterine cervix FIGO stage IB and II. Anal Cell
Pathol. 23:97–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Danque PO, Chen HB, Patil J, Jagirdar J,
Orsatti G and Paronetto F: Image analysis versus flow cytometry for
DNA ploidy quantitation of solid tumors: A comparison of six
methods of sample preparation. Mod Pathol. 6:270–275.
1993.PubMed/NCBI
|
|
27
|
Huang Q, Yu C, Zhang X and Goyal RK:
Comparison of DNA histograms by standard flow cytometry and image
cytometry on sections in Barrett´s adenocarcinoma. BMC Clin Pathol.
8:52008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goff BA, Kato D, Schmidt RA, Ek M, Ferry
JA, Muntz HG, Cain JM, Tamimi HK, Figge DC and Greer BE: Uterine
papillary serous carcinoma: Patterns of metastatic spread. Gynecol
Oncol. 54:264–268. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Salvesen HB, Iversen OE and Akslen LA:
Prognostic significance of angiogenesis and Ki-67, p53 and p21
expression: A population-based endometrial carcinoma study. J Clin
Oncol. 17:1382–1390. 1999.PubMed/NCBI
|
|
30
|
Alkushi A, Lim P, Quino-Parsons C and
Gilks CB: Markers of proliferative activity are predictors of
patient outcome for low-grade endometrioid adenocarcinoma but not
papillary serous carcinoma of endometrium. Mod Pathol. 15:365–371.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alkushi A, Kobel M, Kalloger SE and Gilks
CB: High-grade endometrial carcinoma: Serous and grade 3
endometrioid carcinomas have different immunophenotypes and
outcomes. Int J Gynecol Pathol. 29:343–350. 2010. View Article : Google Scholar : PubMed/NCBI
|