Introduction
Cisplatin (DDP) is one of the most frequently
utilized chemotherapeutic agents. The Food and Drug Administration
first approved DDP for the treatment of ovarian and testicular
cancer in 1978 (1). Since then,
therapeutic indications for DDP use (in single-drug and combination
therapies) have been significantly extended. DDP is currently used
for the treatment of lung, head and neck, and ovarian cancers, as
well as germ cell tumors.
DDP has a characteristic toxicity profile that
includes nephrotoxicity, neurotoxicity and ototoxicity (2). It is additionally a highly emetogenic
agent, particularly in doses >50 mg/m2 (2). Complications affecting the
cardiovascular system, whilst typical for various other agents
(including anthracyclines, 5-fluorouracil, taxanes and targeted
drugs including trastuzumab, lapatinib or bevacizumab), are rare
with DDP treatment (3). Advancements
in the understanding of cardio-oncology are important due to the
increased frequency of use of combination therapies, the
cardiotoxicity of novel therapies (including targeted therapies
that interfere with physiological signaling pathways), and the
continually increasing rates of long-term survival of patients, for
whom delayed treatment-associated complications may occur (4).
As cardiotoxicity is not a typical side-effect of
DDP, the present study reports a case of cardiac arrhythmia
occurring in a patient with a metastatic neuroendocrine carcinoma
of the posterior mediastinum, which was treated with etoposide and
DDP (EP regimen) chemotherapy.
Case report
In September 2012, a 58-year-old Caucasian man was
referred from the Pulmonary Hospital Zakopane (Zakopane, Poland) to
the Department of Oncology, University Hospital in Kraków (Kraków,
Poland), due to metastatic neuroendocrine carcinoma, with a primary
lesion in the posterior mediastinum. Histopathological examination
performed prior to referral to University Hospital in Kraków
revealed a large cell neuroendocrine carcinoma, which was positive
for cluster of differentiation 56, chromogranin, synaptophysin and
cytokeratin 7, and had a Ki-67 index of 80%.
The histopathological findings and metastatic stage
of the disease qualified the patient for palliative chemotherapy
with an EP regimen (100 mg/m2 etoposide, days 1–3; 25
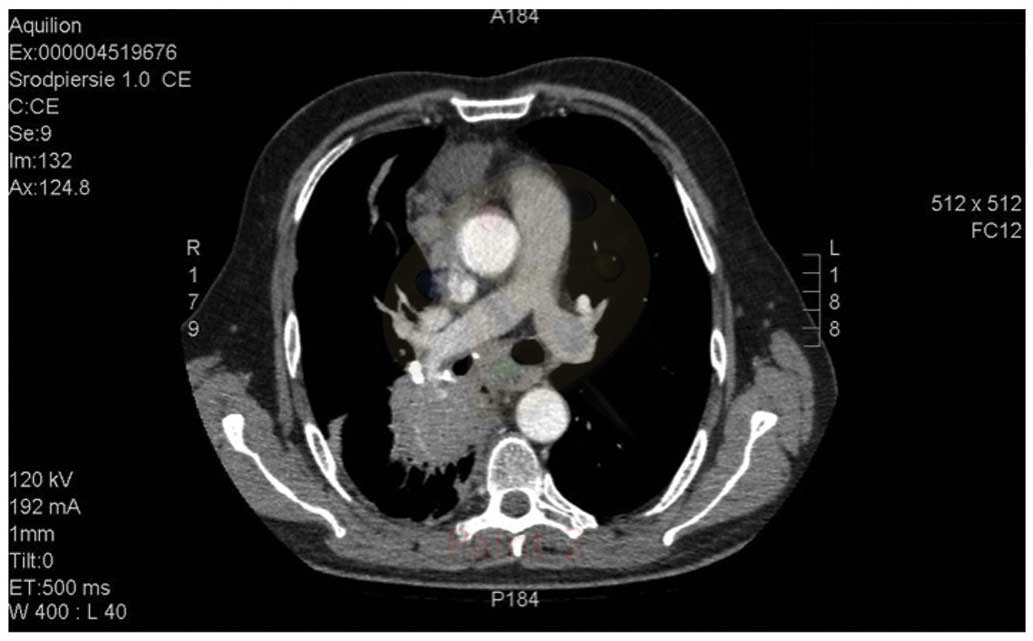
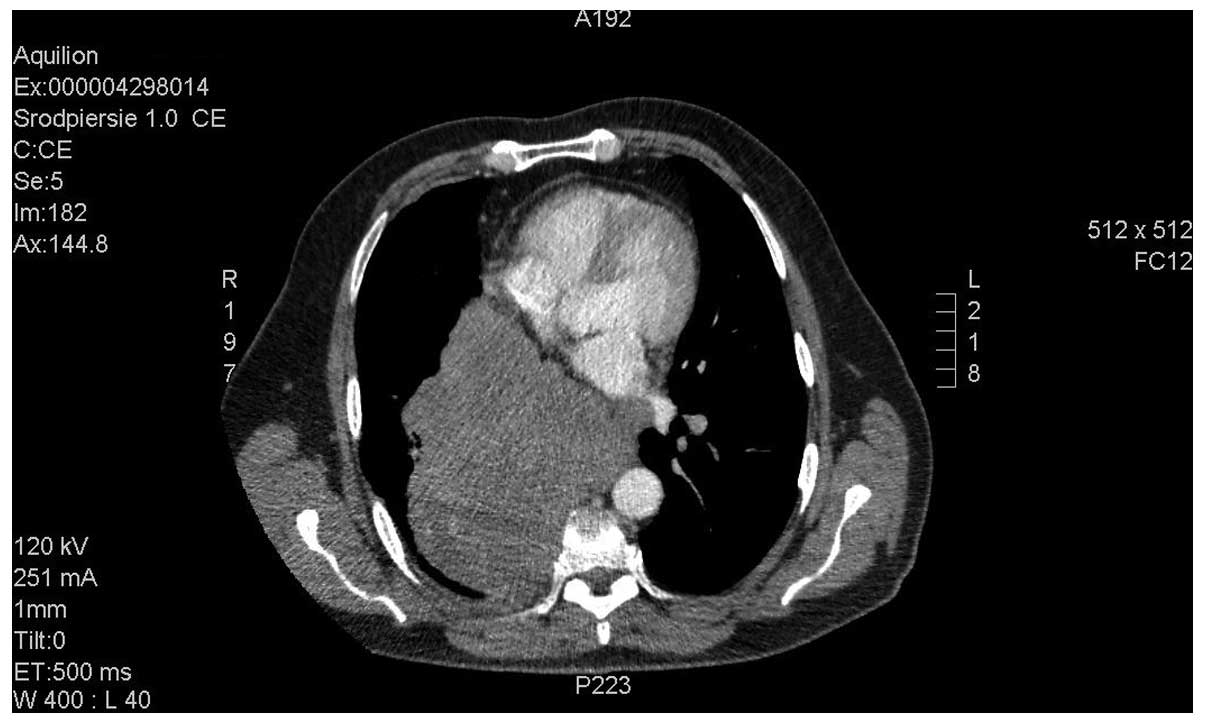
mg/m2 DDP, days 1–3). Computed tomography (CT; Aquilion
64 CT scanner; Toshiba, Tokyo, Japan) imaging of the chest revealed
a posterior mediastinum tumor measuring 16.5×13.0×13.5 cm. The
tumor infiltrated the aorta and the branch of the right pulmonary
artery, with subsequent obstruction of the lower right pulmonary
vein, compression of the atria and constriction of the right middle
and inferior bronchus. Numerous metastatic lesions were identified
in the lungs (Figs. 1 and 2).
Upon admission, the patient complained of heart
palpitations and mild resting dyspnea. The patient's Eastern
Cooperative Oncology Group performance score was 1 (5), and physical examination revealed
tachycardia (~110 bpm; normal range, 60–100 bpm). Laboratory
analysis did not reveal any significant deviations from normal
values. An electrocardiogram (ECG; FX 2000; Emtel, Zabrze, Poland)
revealed sinus tachycardia of 110 bpm, a left axis deviation, a
left anterior hemiblock (LAHB) and a corrected QT (QTc) interval of
410 msec. The decision was made to administer 25 mg of
immediate-release metoprolol, twice per day over two days. This
reduced the patient's heart rate to 80 bpm. The patient was
discharged from hospital in a stable condition with beta-blockers.
The first cycle of EP was initiated on the following day.
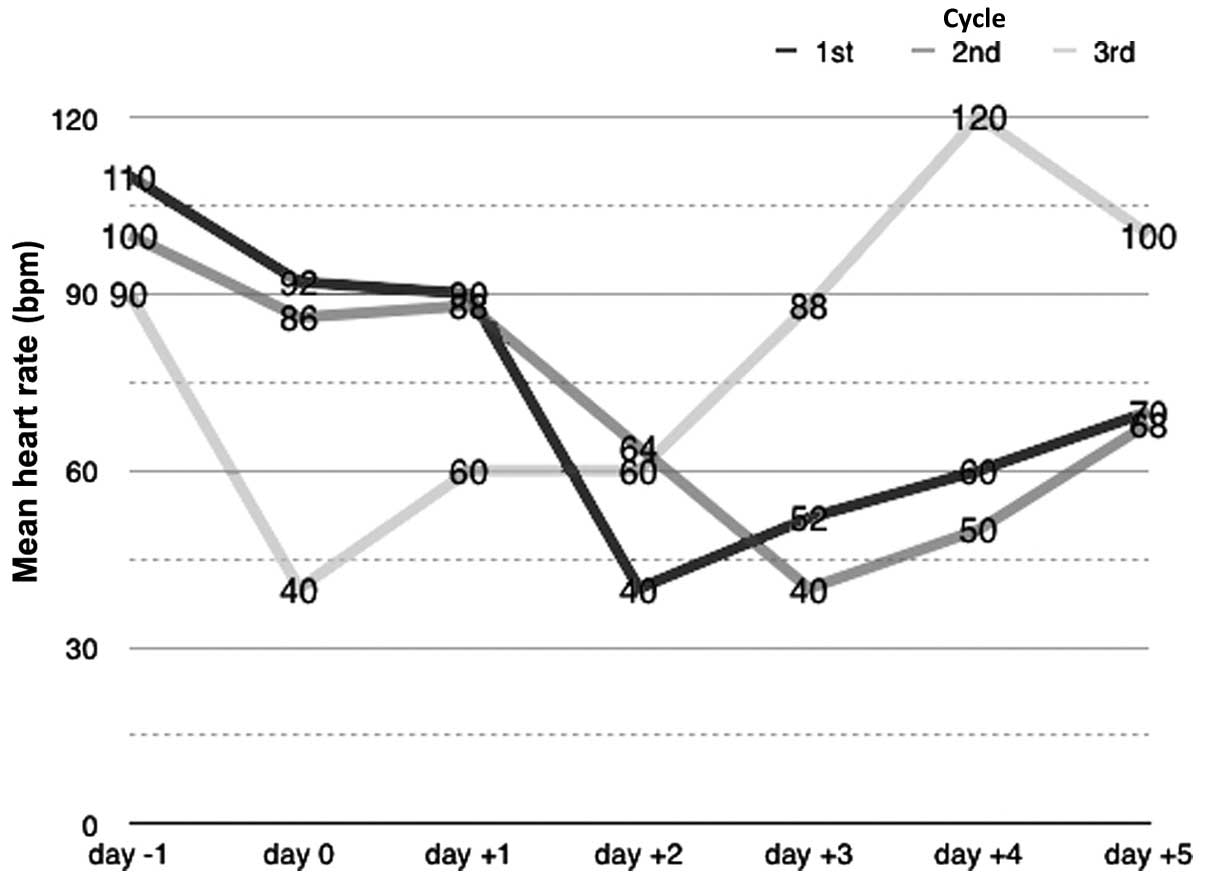
Following the initial 48 h of chemotherapy, the
patient exhibited resting dyspnea, chest discomfort, a heart rate
reduction to 40 bpm (normal range, 60–100 bpm) and blood pressure
of 130/80 mmHg (normal range, 90/60–140/90 mmHg). ECG revealed
sinus bradycardia of 40 bpm, left axis deviation, LAHB and a QTc
interval of 424 msec (normal range, <440 msec), with none of the
typical changes for acute coronary syndrome. Laboratory tests were
negative for markers of cardiac necrosis; potassium, calcium and
magnesium levels were all within normal ranges. The patient was
administered intravenous atropine (0.50 mg every 6 h), which raised
the heart rate to ~70 bpm, and metoprolol treatment was
discontinued. Due to suspected infiltration of the tumor into the
electrical conduction system of the heart, an echocardiogram was
performed, which revealed that the ejection fraction of the left
ventricle was 61% (normal range, 55–70%), and the right ventricle
systolic pressure was 43 mmHg (normal range, 15–30 mmHg). There was
an insignificant amount of fluid in the pericardium, and a
constriction of the inferior vena cava to 6 mm at its outlet into
the atrium, with no clear infiltration into the heart
structures.
During the subsequent three days, the patient was
administered oral atropine (0.25 mg every 8 h), which led to a
gradual improvement of his clinical state and raised his heart
rate. On day 4 following the initial occurrence of bradycardia,
atropine treatment was ceased and the patient's heart rate was ~110
bpm. The patient was seen by a consultant in internal medicine, and
the suggestion to re-administer the beta-blocker drug was made
(single 25 mg dose of extended-release metoprolol per day). As a
consequence of this therapy, the patient's heart rate was reduced
to 80–90 bpm. The patient was discharged from hospital in a stable
and relatively good condition. Subsequently, the patient was
admitted for the next course of chemotherapy. Due to the potential
chemosensitivity of the tumor and a lack of alternative treatment
options, the decision was made to continue chemotherapy despite the
previous complications. Episodes of bradycardia (~40 bpm), were
again observed during the second and third treatment cycles. These
episodes occurred on the third and second days of chemotherapy,
respectively.
Identical cardiac treatments to those given during
the first cycle were administered. A CT scan of the chest,
performed after the third treatment cycle, revealed disease
progression according to the Response Evaluation Criteria in Solid
Tumors 1.1. guidelines (6). The
ineffectiveness and the patient's low tolerance of the treatment
resulted in termination of the chemotherapy. The patient was
referred to a local palliative care center and lost to
follow-up.
Discussion
Cardiotoxicity may be an early or late complication
in the systemic treatment of cancer. Early complications are
frequently associated with pathophysiological mechanisms, including
coronary artery contraction, effects on the autonomic nervous
system or electrolyte disturbances. Late complications are
primarily associated with the development of cardiomyopathy
(7).
Damage to the cardiovascular system, including
cardiac ischemia, diastolic disturbances, hypertension and
microalbuminuria, has been reported in association with DDP-based
therapy (7). These symptoms have been
described for patients who underwent testicular cancer therapy with
DDP-based regimens (7). Tassinari
et al (8) described three
cases of patients experiencing sudden atrial fibrillation following
DDP infusion. In the available literature, to the best of our
knowledge, there have been only two descriptions of DDP-induced
bradycardia (9,10). Schlaeffer et al (9) described a patient with nasopharyngeal
squamous cell carcinoma, whose pulse dropped each time combination
chemotherapy with cisplatin was administered, and the symptoms of
the patient became worse; no other possible causes of bradycardia
were observed. Altundağ et al (10) presented the case of a patient with
relapsed Hodgkin's lymphoma that was treated with cisplatin-based
salvage chemotherapy. Asymptomatic bradycardia was observed in the
patient on days 3–5 following the first cycle of treatment and on
days 2–5 following subsequent treatment cycles. The patient did not
require any treatment for bradycardia, and a dose reduction or
chemotherapy discontinuation was not required. The mechanism of
DDP-associated cardiac arrhythmia remains to be elucidated. It may
be induced by various factors, including intracellular and
extracellular fluctuations of K+ and Mg2+
concentrations caused by DDP (11),
or by the accumulation of DDP in the electrical conduction system
of the heart (for example, in the cells of the sinoatrial node),
which may lead to electrical transmission disturbances (7).
Additional pathological mechanisms may include
infiltration involving the cardiac muscle or the electrical
conduction system of the heart, infiltration affecting the vagus
nerve and, in neuroendocrine tumors, the influence of tumor
excretions, which may affect the cardiovascular system directly or
indirectly and should be taken into account in patients with
malignancies located within the mediastinum (12). The toxic influence of DDP on the
cardiac muscle appears to be the most likely cause of arrhythmia in
the present case. This is supported by the disturbances recorded by
the ECG, and by the temporal association observed between the
administration of DDP and the onset of bradycardia (Fig. 3). In summary, the present case
suggests that medical oncologists should be aware of the
cardiotoxicity of DDP, as it may occur in patients with no
pre-existing cardiac disease. Particular attention should be paid
to patients with a history of arrhythmias (in particular
bradycardia) and those being administered beta-adrenolytics or
non-dihydropyridine calcium channel blockers, as such patients are
prone to bradycardia.
References
|
1
|
Prestayko AW, D'Aoust JC, Issell BF and
Crooke ST: Cisplatin (cis-diamminedichloroplatinum II). Cancer
Treat Rev. 6:17–39. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loehrer PJ and Einhorn LH: Drugs five
years later. Cisplatin. Ann Intern Med. 100:704–713. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bovelli D, Plataniotis G and Roila F: ESMO
Guidelines Working Group: Cardiotoxicity of chemotherapeutic agents
and radiotherapy-related heart disease: ESMO Clinical Practice
Guidelines. Ann Oncol. 21(Suppl 5): v277–v282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yeh ET, Tong AT, Lenihan DJ, Yusuf SW,
Swafford J, Champion C, Durand JB, Gibbs H, Zafarmand AA and Ewer
MS: Cardiovascular complications of cancer therapy: Diagnosis,
pathogenesis, and management. Circulation. 109:3122–3131. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meinardi MT, Gietema JA, van der Graaf WT,
van Veldhuisen DJ, Runne MA, Sluiter WJ, de Vries EG, Willemse PB,
Mulder NH, van den Berg MP, et al: Cardiovascular morbidity in
long-term survivors of metastatic testicular cancer. J Clin Oncol.
18:1725–1732. 2000.PubMed/NCBI
|
|
8
|
Tassinari D, Sartori S, Drudi G, Panzini
I, Gianni L, Pasquini E, Abbisciano V, Ravaioli A and Iorio D:
Cardiac arrhythmias after cisplatin infusion: Three case reports
and a review of the literature. Ann Oncol. 8:1263–1267. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schlaeffer F, Tovi F and Leiberman A:
Cisplatin-induced bradycardia. Drug Intell Clin Pharm. 17:899–901.
1983.PubMed/NCBI
|
|
10
|
Altundağ O, Celik I and Kars A: Recurrent
asymptomatic bradycardia episodes after cisplatin infusion. Ann
Pharmacother. 35:641–642. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blachley JD and Hill JB: Renal and
electrolyte disturbances associated with cisplatin. Ann Intern Med.
95:628–632. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maier HC and Sommers SC: Neuroendocrine
carcinoma of lung associated with bradycardia and episodic cardiac
asystole. Ann Thorac Surg. 41:560–562. 1986. View Article : Google Scholar : PubMed/NCBI
|