Introduction
Breast cancer is the most common malignancy in
women, accounting for ~20% of total malignant tumors in females
(1). Numerous studies (2) have shown that breast cancer is a highly
heterogeneous malignancy with significant differences in
histomorphological features, immunophenotype, biological behavior
and response to treatment. Currently, breast cancer is classified
as luminal tumors [hormone receptor expressing tumors, human
epidermal growth factor receptor 2 (HER2)-negative], HER2-negative
(hormone receptor negative) and basal-like tumors (absence of
hormone receptor and HER2 expression) (3).
The aberrant proliferation and migration of tumor
cells are a hallmark of tumor pathology during tumor progression.
Studies conducted on proliferative activity and migration of tumor
cells facilitate the understanding of biological behavior of tumors
and provide clinical evidences for the diagnosis, treatment and
prognosis of tumors (4). Ki-67, a
nuclear antigen associated with cell proliferation, is expressed in
all the phases of the cell cycle except G0. Ki-67 has been
identified as a molecular marker for the effective assessment of
the cell proliferation index (5). It
has been shown that Ki-67 proliferative activity is associated with
the extent of tumor differentiation, invasion and metastasis as
well as prognosis (6,7). In addition, luminal tumors have been
classified into luminal A and luminal B subtypes based on the level
of Ki-67 expression. Furthermore, the involvement of Ki-67
expression in luminal breast cancer tissues has been demonstrated
through large population-based cohort studies (8).
Between August 2009 and November 2014, 62 patients
with luminal type breast cancer and 30 patients with breast
hyperplasia, admitted to the Breast Cancer Center, The Affiliated
Cancer Hospital of Zhengzhou University (Zhengzhou, China) were
included in the present study. The present study aimed to measure
the level of Ki-67 expression in luminal type breast cancer tissues
and investigate the association between Ki-67 and the
clinicopathology of breast cancer. In addition, Ki-67 expression in
the human MCF-7 breast cancer cell line was studied to evaluate the
proliferation and migration of tumor cells to elucidate the
molecular mechanisms underlying the impact of Ki-67 on breast
cancer.
Materials and methods
Clinical data
Breast tumor tissues were collected from 62
patients, who were diagnosed with luminal breast cancer and
underwent surgery at The Affiliated Cancer Hospital of Zhengzhou
University, between August 2009 and November 2014. The patients,
with an age range of 22–57 years and a mean age of 43.15±9.50
years, had no prior history of radio- and chemotherapy. In
addition, 30 patients with breast hyperplasia with an age range of
24–54 years and a mean age of 45.45±11.41 years, served as the
controls.
Reagents
RNA was isolated from tissues and serum using the
TRIzol RNA isolation kit (Life Technologies, Grand Island, NY,
USA). RNA reverse transcription (RT) was performed using the cDNA
reverse transcription system (Promega Corp., Madison, WI, USA). An
RT-polymerase chain reaction (PCR) kit was purchased from Sunshine
Biotechnology Co., Ltd., Nanjing, China.
RT-PCR
Breast cancer tissues were collected from 62
patients during surgery and analyzed. Cancer tissue (200 µg) was
homogenized in 1 ml TRIzol solution and RNA was isolated according
to the manufacturer's instructions. The isolated RNA was dissolved
in 30 µl of diethylpryocarbonate-treated water and quantified using
an ultraviolet spectrophotometer (One Drop, Nanjing, China). RNA
was reverse transcribed into cDNA and stored in a freezer at
−20°C.
The primer sequences used for Ki-67 amplification
were: F: 5′-CGTAGCAGCACAGAAAT-3′ and R:
5′-TGATGGTTGAGGTCGTTCCTTGATG-3′ (9).
U6 was used as an internal control, with the following primers: F:
5′-CTCGCTTCGGCAGCACA-3′ and R: 5′-AACGCTTCACGAATTTGCGT-3′. The PCR
reaction conditions used were: denaturation at 95°C for 20 sec,
followed by 50 cycles of 60°C for 20 sec and 70°C for 1 sec. RT-PCR
data were analyzed on an ABI 7300 RT-PCR system (Applied Biosystems
Life Technologies, Foster City, CA, USA) and the relative
quantitative analysis of gene expression was performed using the
2−ΔΔCt method (10).
Immunohistochemical study and
evaluation criteria
Breast cancer specimens were fixed in 10% formalin
for 48 h, paraffin-embedded and cut into 4 µm thin sections for SP
IHC staining using anti Ki-67 antibody (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China). Phosphate-buffered
saline was used as the negative control. Ki-67 staining was
analyzed under optical microscopy (Olympus, Barrington, NJ,
USA).
A positive Ki-67 expression was detected in the
nuclei of tumor cells identified as brown particles. The IHC
staining result was evaluated based on the extent of staining of
tumor cells and the intensity of staining reaction. Ki-67-positive
tumor cells were counted in 10 randomly selected high-power fields
and evaluated according to the following criteria: <5% was
defined as negative; 5–25% as +; 25–50% as ++; 50–75% as +++.
Cell transfection
Knockdown of Ki-67 was performed in the human MCF-7
breast cancer cell line through transfection of Ki-67 siRNA using
Lipofectamine® 2000 transfection reagent (Invitrogen Life
Technologies, Shanghai, China). Ki-67 siRNA sequences used were:
5′-CCACCAGAGCCAAUAGAUACUUCAG-3′ and
5′-CUGAAGUAUCUAUUGGCUCUGGUGGUGA-3′. The control sequence was
designed as 5′-UCAUAAGUGAUGCUGGAGCTT-3′. Transfection efficiency
was evaluated using RT-PCR.
Cell invasion assay
Transfected MCF-7 cells in the logarithmic phase
were suspended in solution and cell density was adjusted to
3–5×105/ml. Cell suspensions were seeded in the upper
chamber of transwell at a concentration of 0.1 ml/well, and 1 ml
culture medium containing 10% serum was plated in the bottom
chamber. After 24 h culture, transwell chambers were retrieved and
the upper layer of the culture media were aspirated to terminate
the assay. The cells were air-dried at room temperature (20°C),
fixed in ethanol and stained using 0.1% crystal violet for 30 min
at room temperature. Cells that migrated to the bottom chamber were
analyzed under an inverted microscope (Olympus) and the cells in
the bottom chamber were counted in five randomly selected
fields.
Cell proliferation assay
Transfected MCF-7 cells at 0, 24, 48 and 72 h
post-transfection were plated in a mixture of Dulbecco's modified
Eagle's medium (Invitrogen Life Technologies). The cell counting
kit-8 assay (Beyotime, Wuhan, China) was used at a volume ratio of
1:10. The optical density values of samples were measured using a
spectrophotometer at 450 nm to generate the growth curve of
cells.
Statistical analysis
Statistical analysis was performed using SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). Quantitative
data were presented as mean ± standard deviation. Differences
between groups were analyzed using independent samples t-test. The
correlation between Ki-67 and tumor pathology was analyzed using
the Spearman rank correlation test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Increase in Ki-67 levels in the serum
and tissues of breast cancer patients
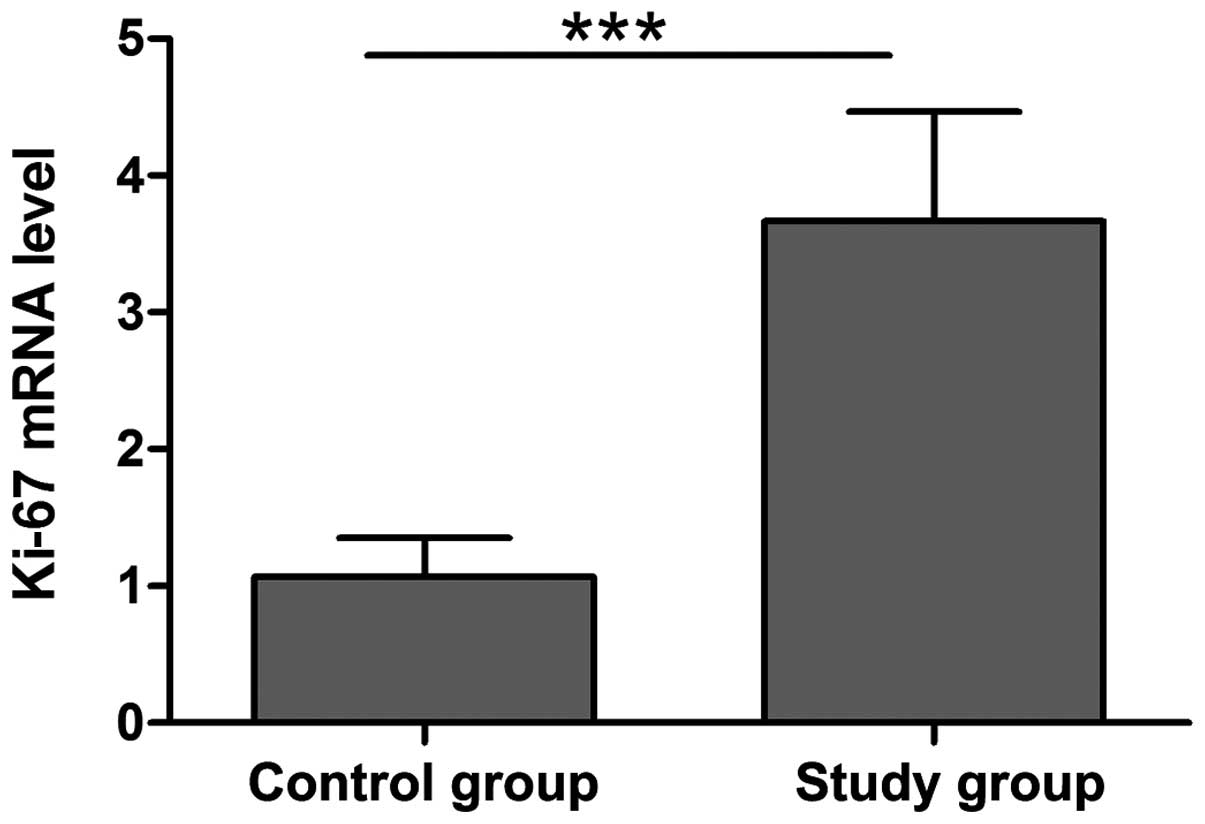
Compared with the control group, Ki-67 mRNA
expression was significantly increased in tissues of breast cancer
patients, indicating an association of Ki-67 in the progression of
breast cancer. The high expression of Ki-67 may be correlated with
the invasion and metastasis of breast cancer, suggesting a poor
prognosis (Fig. 1).
Ki-67 expression in breast cancer
tissues with different histopathological characteristics
According to TNM classification systems in the 7th
edition (2010) of the cancer staging manual of the American Joint
Committee on Cancer, 11 (17.7%) patients were classified as stage
I, 20 (32.3%) as stage II and 31 (50.0%) as stage III. The level of
Ki-67 expression was positively associated with TNM stages
(r=0.589, P<0.01) (Table I). The
Spearman correlation analysis showed that Ki-67 expression levels
in breast cancer tissues were positively associated with tumor size
and the number of metastatic lymph nodes (Table II).
 | Table I.Association between Ki-67 expression
and TNM staging of breast cancer. |
Table I.
Association between Ki-67 expression
and TNM staging of breast cancer.
|
| Ki-67 level, no. |
|
|
|---|
|
|
|
|
|
|---|
| Pathological
stage | + | ++ | +++ | r | P-value |
|---|
| I | 2 | 3 | 6 | 0.589 | 0.004 |
| II | 4 | 6 | 10 |
|
|
| III | 7 | 10 | 14 |
|
|
 | Table II.Correlation of Ki-67 expression in
breast cancer with tumor size and lymphatic metastasis. |
Table II.
Correlation of Ki-67 expression in
breast cancer with tumor size and lymphatic metastasis.
|
| Ki-67 level, no. |
|
|
|---|
|
|
|
|
|
|---|
| Clinical features of
cancer | + | ++ | +++ | r | P-value |
|---|
| Tumor size |
|
|
| 0.452 | 0.016 |
| T1 (T≤2
cm) | 1 | 2 | 6 |
|
|
| T2
(2<T<5 cm) | 5 | 7 | 10 |
|
|
| T3 (T≥5
cm) | 5 | 12 | 14 |
|
|
| Lymphatic
metastasis |
|
|
| 0.331 | 0.021 |
| N1
(1–3) | 2 | 2 | 5 |
|
|
| N2
(4–9) | 5 | 8 | 8 |
|
|
| N3
(>9) | 8 | 8 | 12 |
|
|
Correlation of low expression of Ki-67
with breast cancer staging
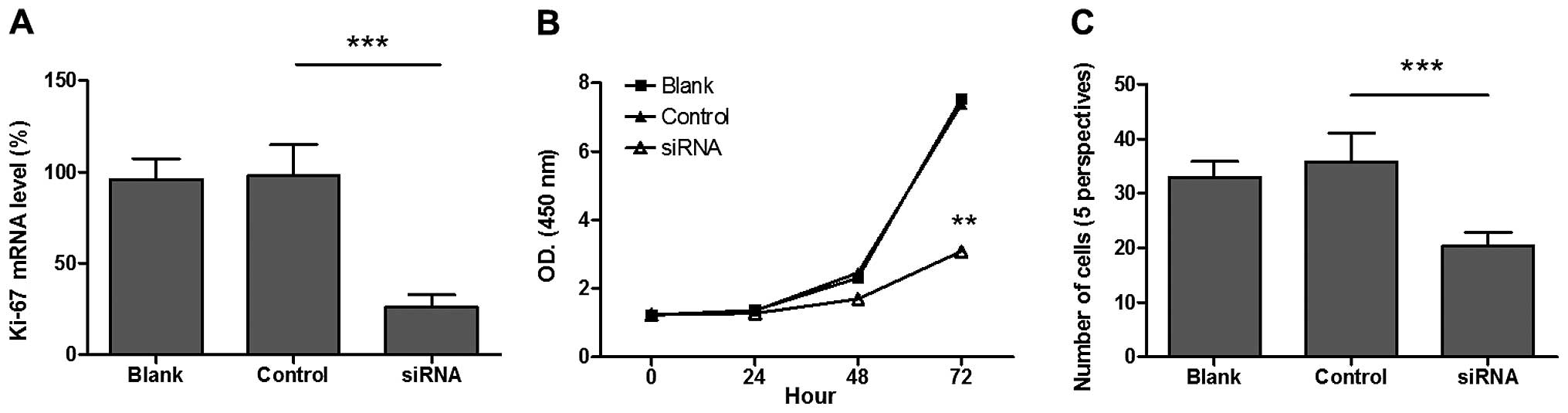
Ki-67 expression was reduced by the knockdown of
Ki-67 in the human MCF-7 breast cancer cell line in an effort to
examine the mechanism by which Ki-67 influences breast cancer. The
results demonstrated that Ki-67 mRNA expression was significantly
reduced in MCF-7 cells 48 h after transfection (Fig. 2A). Compared to the control group, cell
proliferation activity was significantly decreased in breast cancer
cells with low expression of Ki-67 (P<0.05) (Fig. 2B). In addition, cell migration was
significantly reduced in breast cancer cells with low Ki-67
expression compared to that of controls (P<0.05) (Fig. 2C).
Discussion
Breast cancer is one of the most common malignancies
in middle-aged and elderly women globally, representing 1.20
million cases per annum in women worldwide (11). In some regions of China, breast cancer
has become the leading cancer in women, causing serious damage to
their physical and mental health (12). Due to the increase in number of breast
cancer patients and younger age of patients, increasing attention
has been paid to the treatment and prognosis of breast cancer. In
recent years, significant progress has been made in the treatment
of breast cancer as further basic and clinical studies were
conducted, and the study on breast cancer treatment has become one
of the hot topics of cancer research (13).
Invasion and migration is the hallmark of malignant
tumors as well as the major cause for breast cancer death (14,15).
However, the development details and underlying pathological
mechanisms remain to be determined. Ki-67, a gene located on
chromosome 10, can detect a nuclear protein in the nuclear matrix
of proliferating cells and its function has been shown to be
associated with mitosis. In particular, closely associated with
tumor cell proliferation (16–18).
Currently, however, Ki-67 expression and its function in luminal
breast cancer has not been completely elucidated.
Results of the present study have shown that Ki-67
mRNA expression was significantly higher in tissues of breast
cancer than that of breast hyperplasia, indicating that Ki-67 is
closely associated with the pathogenesis and development of breast
cancer. Warth et al (19) have
shown that Ki-67 expression is increased in non-small-cell lung
cancer and suggested that Ki-67 can serve as a predictor for cancer
prognosis (19). Pollack et al
(20) and Khor et al (21) have also shown increased Ki-67
expression in prostate cancer. In addition, Inwald et al
(22) have shown a significantly
increased Ki-67 expression in breast cancer and suggest its role as
a prognostic parameter in breast cancer. Taken together, those
findings corroborate the results of the present study.
Further analysis was performed to investigate the
relationship between Ki-67 expression in breast cancer tissues and
clinicopathological characteristics of the cancer. The results
revealed that the level of Ki-67 expression in breast cancer tissue
was positively correlated with the TNM stages of the cancer. In
addition, the Ki-67 expression level was positively correlated with
tumor size and the number of metastatic lymph nodes. These findings
suggest that a high expression of Ki-67 plays a central role in the
promotion of the pathogenesis and development of breast cancer. In
addition, Ki-67 plays important roles in promoting the genesis and
metastasis of breast cancer.
The mechanism underlying the tumor-promoting
function of Ki-67 in breast cancer was further studied by reducing
Ki-67 expression in the human MCF-7 breast cancer cell line. The
results demonstrate that the proliferation activity and migration
of cancer cells were reduced in breast cancer cells with a low
expression of Ki-67, indicating that Ki-67 may affect the
malignancy of breast cancer cells. Zheng et al (23) have revealed that the knockdown of
Ki-67 in renal carcinoma cells significantly inhibits cancer cell
proliferation and promotes cell apoptosis, thereby indicating that
Ki-67 may be involved in the progression of renal carcinoma by
influencing the growth and apoptosis of the cancer cells.
Furthermore, the knockdown of Ki-67 in bladder cancer cells has
been shown to inhibit tumor growth (24). Taken together, the results of the
present study were corroborated with those findings, which suggest
that interference with Ki-67 expression is involved in the
progression of breast cancer probably by affecting the
proliferation and migration of cancer cells.
In summary, a high expression of Ki-67 is important
in the genesis and development of breast cancer, possibly by
influencing the proliferation and migration of cancer cells.
Detection and interference of Ki-67 have a certain implication for
guiding the treatment and prognosis of breast cancer in clinical
practice.
Acknowledgements
This study was supported by the project of efficacy
of anthracycline and amplification of HER-2, TOP2A and Ki-67 in
breast cancer (grant no. 201401016) from Henan Medical Science and
Technique Foundation, China.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karuturi M, VanderWalde N and Muss H:
Approach and management of breast cancer in the elderly. Clin
Geriatr Med. 32:133–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Savas P, Salgado R, Denkert C, Sotiriou C,
Darcy PK, Smyth MJ and Loi S: Clinical relevance of host immunity
in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. Dec
15–2015.doi: 10.1038. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Z, Shi Y, Shen Y, Cao L, Zhang W and
Guan X: Analysis of different HER-2 mutations in breast cancer
progression and drug resistance. J Cell Mol Med. 19:2691–2701.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim DK, Kim DW, Kim SW, Kim DY, Lee CH and
Rhee CS: Ki67 antigen as a predictive factor for prognosis of
sinonasal mucosal melanoma. Clin Exp Otorhinolaryngol. 1:206–210.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cuzick J, Dowsett M, Pineda S, Wale C,
Salter J, Quinn E, Zabaglo L, Mallon E, Green AR, Ellis IO, et al:
Prognostic value of a combined estrogen receptor, progesterone
receptor, Ki-67, and human epidermal growth factor receptor 2
immunohistochemical score and comparison with the Genomic Health
recurrence score in early breast cancer. J Clin Oncol.
29:4273–4278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krüger K, Stefansson IM, Collett K, Arnes
JB, Aas T and Akslen LA: Microvessel proliferation by co-expression
of endothelial nestin and Ki-67 is associated with a basal-like
phenotype and aggressive features in breast cancer. Breast.
22:282–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Panel members: Strategies for
subtypes - dealing with the diversity of breast cancer: Highlights
of the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou J, Wang P, Lin L, Liu X, Ma F, An H,
Wang Z and Cao X: MicroRNA-146a feedback inhibits RIG-I-dependent
Type I IFN production in macrophages by targeting TRAF6, IRAK1, and
IRAK2. J Immunol. 183:2150–2158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cintra JR, Teixeira MT, Diniz RW,
Gonçalves Junior H, Florentino TM, Freitas GF, Oliveira LR, Neves
MT, Pereira T and Guerra MR: Immunohistochemical profile and
clinical-pathological variables in breast cancer. Rev Assoc Med
Bras. 58:178–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao ZS, Li Y, Guan YL and Li JG: GSTT1
polymorphism and breast cancer risk in the Chinese population: an
updated meta-analysis and review. Int J Clin Exp Med. 8:6650–6657.
2015.PubMed/NCBI
|
|
13
|
Hong W and Dong E: The past, present and
future of breast cancer research in China. Cancer Lett. 51:1–5.
2014. View Article : Google Scholar
|
|
14
|
Arjonen A, Kaukonen R, Mattila E, Rouhi P,
Högnäs G, Sihto H, Miller BW, Morton JP, Bucher E, Taimen P, et al:
Mutant p53-associated myosin-X upregulation promotes breast cancer
invasion and metastasis. J Clin Invest. 124:1069–1082. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Macpherson IR, Rainero E, Mitchell LE, van
den Berghe PV, Speirs C, Dozynkiewicz MA, Chaudhary S, Kalna G,
Edwards J, Timpson P, et al: CLIC3 controls recycling of late
endosomal MT1-MMP and dictates invasion and metastasis in breast
cancer. J Cell Sci. 127:3893–3901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beresford MJ, Wilson GD and Makris A:
Measuring proliferation in breast cancer: Practicalities and
applications. Breast Cancer Res. 8:2162006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller HC, Drymousis P, Flora R, Goldin R,
Spalding D and Frilling A: Role of Ki-67 proliferation index in the
assessment of patients with neuroendocrine neoplasias regarding the
stage of disease. World J Surg. 38:1353–1361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rademakers SE, Hoogsteen IJ, Rijken PF,
Terhaard CH, Doornaert PA, Langendijk JA, van den Ende P, van der
Kogel AJ, Bussink J and Kaanders JH: Prognostic value of the
proliferation marker Ki-67 in laryngeal carcinoma: results of the
accelerated radiotherapy with carbogen breathing and nicotinamide
phase III randomized trial. Head Neck. 37:171–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Warth A, Cortis J, Soltermann A, Meister
M, Budczies J, Stenzinger A, Goeppert B, Thomas M, Herth FJ,
Schirmacher P, et al: Tumour cell proliferation (Ki-67) in
non-small cell lung cancer: A critical reappraisal of its
prognostic role. Br J Cancer. 111:1222–1229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pollack A, DeSilvio M, Khor LY, Li R,
Al-Saleem TI, Hammond ME, Venkatesan V, Lawton CA, Roach M III,
Shipley WU, et al: Ki-67 staining is a strong predictor of distant
metastasis and mortality for men with prostate cancer treated with
radiotherapy plus androgen deprivation: Radiation Therapy Oncology
Group Trial 92-02. J Clin Oncol. 22:2133–2140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khor LY, Bae K, Paulus R, Al-Saleem T,
Hammond ME, Grignon DJ, Che M, Venkatesan V, Byhardt RW, Rotman M,
et al: MDM2 and Ki-67 predict for distant metastasis and mortality
in men treated with radiotherapy and androgen deprivation for
prostate cancer: RTOG 92-02. J Clin Oncol. 27:3177–3184. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inwald EC, Klinkhammer-Schalke M,
Hofstädter F, Zeman F, Koller M, Gerstenhauer M and Ortmann O:
Ki-67 is a prognostic parameter in breast cancer patients: Results
of a large population-based cohort of a cancer registry. Breast
Cancer Res Treat. 139:539–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng Z, Li HZ and Li YQ: Prognostic
factors and associated models for metastatic renal cell carcinoma
treated with targeted therapy. Zhongguo Yi Xue Ke Xue Yuan Xue Bao.
36:450–453. 2014.(In Chinese). PubMed/NCBI
|
|
24
|
Pichu S, Krishnamoorthy S, Shishkov A,
Zhang B, McCue P and Ponnappa BC: Knockdown of Ki-67 by
dicer-substrate small interfering RNA sensitizes bladder cancer
cells to curcumin-induced tumor inhibition. PLoS One. 7:e485672012.
View Article : Google Scholar : PubMed/NCBI
|