Introduction
Breast cancer is the most common cancer and the
leading cause of cancer-associated mortality in women worldwide
(1). Breast cancer mortality rates
have decreased in North America and several European countries over
the past 25 years, principally due to earlier detection and
improved treatments (2,3). However, in numerous African and Asian
countries, the incidence and mortality rates have increased
(4). Therefore, mortality from breast
cancer remains a significant health issue globally. In general, the
primary risk factors for breast cancer in women are old age (>50
years) and circulating estrogen (5).
There are two strategies for enhancing the survival of breast
cancer patients: Early detection and appropriate treatment. Primary
treatments include surgery, radiotherapy, chemotherapy, endocrine
therapy and targeted therapy. Studies have shown that the
integrated use of a variety of treatments is advantageous to the
prognosis of breast cancer patients (6). At present, formulation of a treatment
plan, including surgery and adjuvant therapy, is primarily based on
clinical stage and the presence of several limited biomarkers,
including estrogen receptor, progesterone receptor and human
epidermal growth factor receptor-2 (6). However, the tumor-node-metastasis
staging system and these biomarkers do not meet the requirements
for personalized therapy and/or precise therapy. Identifying
biomarkers for precisely predicting prognosis or for future
targeted therapy is one of the major challenges in this
disease.
Previous studies have demonstrated that a large
proportion of human microRNAs (miRNAs) are located in
cancer-associated regions of the genome (7), which implies that miRNAs are important
in the pathogenesis of various human cancers (8–14). miRNA
expression is usually dysregulated in various cancer types
(15,16), including breast cancer (17). miRNAs specifically bind to the
3′-untranslated region of target mRNAs, which results in the
degradation of the mRNA or inhibition of its translation (18). In addition, miRNAs may be capable of
affecting the transcription of target genes through promoter
activation (19) or transcriptional
silencing (20). Therefore, miRNAs
are critical in cancer diagnosis, prognosis and individualized
therapy (21).
In the present study, miR-711 expression was
detected using reverse transcription (RT)-quantitative polymerase
chain reaction (qPCR) in 30 pairs of formalin-fixed
paraffin-embedded (FFPE) breast cancer and non-cancerous matched
tissue samples. miR-711 is located on chromosome 3. The majority of
previous research concerning miR-711 has focused on organ injury
(22–24), including myocardial infarction. The
findings indicated that miR-711 was overexpressed in breast cancer.
Therefore, the present study revealed a functional role of miR-711
in human cancer, particularly in breast cancer. In addition,
analysis of 161 FFPE breast cancer samples demonstrated that
increased levels of miR-711 expression were associated with poor
overall survival time of patients. Overall, the present in
vitro findings suggest that miR-711 has an oncogenic role in
breast cancer cells.
Materials and methods
Patients and tissue samples
For the current study, 30 paired FFPE breast cancer
and non-cancerous samples were obtained from patients who underwent
surgical treatment for breast cancer between January 2004 and
December 2005 at Sun Yat-sen University Cancer Center (Guangzhou,
Guangdong, China). In addition, 161 FFPE breast cancer samples were
obtained from patients who underwent radical mastectomy between
June 2002 and December 2006 at the First Affiliated Hospital of
Guangzhou Medical University (Guangzhou, Guangdong, China). All
breast cancer samples were pathologically diagnosed as invasive
ductal carcinoma by two experienced pathologists. None of the 161
patients had distant metastasis at diagnosis. The percentage of
tumor cells in the FFPE breast cancer tissues was >70%. The 161
patients had not received radiotherapy or chemotherapy prior to
mastectomy. Clinical staging was based on the American Joint
Committee on Cancer staging manual (25). The clinical characteristics of the 161
patients are summarized in Table I.
The median follow-up time for patients from the First Affiliated
Hospital of Guangzhou Medical University was 75.2 months (range,
6.7–115.8). Overall survival (OS) time was calculated between the
date of surgery and the date of mortality due to any cause or of
last follow-up, and the disease-free survival (DFS) time was
calculated between the date of surgery and the date of the first
distant metastasis, relapse or mortality due to any cause. The
study was reviewed and approved by the Ethics Committees of Sun
Yat-sen University Cancer Center and the First Affiliated Hospital
of Guangzhou Medical University.
 | Table I.Association between miR-711 expression
level and clinical characteristics. |
Table I.
Association between miR-711 expression
level and clinical characteristics.
|
| miR-711
expressiona |
|
|---|
|
|
|
|
|---|
| Clinical
characteristics | High, n (%) | Low, n (%) | P-value |
|---|
| All patients | 80
(100) | 81
(100) |
|
| Age, years |
|
| 0.88 |
|
>45 | 43 (54) | 45 (56) |
|
|
≤45 | 37 (46) | 36 (44) |
|
| Menopause |
|
| 0.82 |
| No | 42 (52) | 44 (54) |
|
|
Yes | 38 (48) | 37 (46) |
|
| Pathological
grade |
|
| 0.52 |
| I | 2 (3) | 5 (6) |
|
| II | 68 (85) | 66 (82) |
|
|
III | 10 (13) | 10 (12) |
|
| Estrogen
receptor |
|
| 0.21 |
|
Positive | 55 (69) | 48 (59) |
|
|
Negative | 25 (31) | 33 (41) |
|
| Progesterone
receptor |
|
| 0.58 |
|
Positive | 48 (60) | 52 (64) |
|
|
Negative | 32 (40) | 29 (36) |
|
| HER2 |
|
| 0.69 |
|
Positive | 30 (37) | 28 (34) |
|
|
Negative | 50 (63) | 53 (66) |
|
| Tumor stage |
|
| 0.23 |
| T1 | 17 (21) | 19 (24) |
|
| T2 | 47 (59) | 55 (68) |
|
| T3 | 14 (18) | 6 (7) |
|
| T4 | 2 (3) | 1 (1) |
|
| Node stage |
|
| 0.10 |
| N0 | 36 (45) | 47 (58) |
|
| N1 | 23 (29) | 25 (31) |
|
| N2 | 15 (19) | 6 (7) |
|
| N3 | 6 (8) | 3 (4) |
|
| TNM stage |
|
| 0.17 |
| I | 11 (14) | 15 (19) |
|
| II | 43 (54) | 50 (62) |
|
|
III | 26 (33) | 16 (20) |
|
| Radiotherapy |
|
| 0.35 |
| No | 16 (20) | 22 (27) |
|
|
Yes | 64 (80) | 59 (73) |
|
| Chemotherapy |
|
| 0.86 |
| No | 24 (30) | 23 (28) |
|
|
Yes | 56 (70) | 58 (72) |
|
Total RNA extraction
The breast cancer and non-cancerous tissues were cut
into 8–10 µm sections, deparaffinized and rehydrated. Total RNA was
extracted from the tissue sections using a modified
phenol/chloroform extraction method, as previously described
(26). Total RNA from cell lines was
extracted using Invitrogen TRIzol reagent (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. A NanoDrop® ND-1000 spectrophotometer
(Thermo Fisher Scientific, Inc.) was used to estimate the
concentration and quality of total RNA.
RT-qPCR
Reverse transcription was performed on the 30 paired
tissue samples using 10 ng of total RNA, 1X miRNA-specific reverse
transcription primers (Thermo Fisher Scientific, Inc.), 100 µM
nucleoside triphosphates (Thermo Fisher Scientific, Inc.), 3.33
U/µl MultiScribe™ Reverse Transcriptase (Thermo Fisher Scientific,
Inc.), 1X Reverse Transcription Buffer (Thermo Fisher Scientific,
Inc.) and 1.33 U/µl RNase inhibitor (Thermo Fisher Scientific,
Inc.) in a final volume of 15 µl. The reaction was conducted at
16°C for 30 min, followed by 30 min at 42°C and 5 min at 85°C. The
qPCR reaction was performed using a 20 µl volume containing 1.33 µl
reverse transcription products, 1X TaqMan® Small RNA Assay solution
(including specific primers and probes; Applied Biosystems; Thermo
Fisher Scientific, Inc.) and 1X Universal PCR Master Mix II (no
UNG; Thermo Fisher Scientific, Inc.). The RT-qPCR was performed in
triplicate for each sample using an Applied Biosystems PRISM 7900HT
System (Thermo Fisher Scientific, Inc.) with the following
conditions: 50°C for 2 min; 95°C for 10 min; and 45 cycles of 95°C
for 15 sec and 60°C for 60 sec. The primer sequences used were as
follows: MiR-711 forward, 5′-ACACTCCAGCTGGGGGGACCCAGGGAGAGA-3′; and
reverse, 5′-TGGTGTCGTGGAGTCG-3′. Small nuclear RNA U6 was used as a
normalization control. The 2−ΔΔCq equation was used to
represent the relative expression of miRNA (27) and Student's t-test was used to
analyze the results.
Cell culture and transfection
MCF-7, MDA-MB-231 and ZR-75-30 human breast cancer
cell lines were cultured in Gibco Dulbecco's modified Eagle's
medium (DMEM; Thermo Fisher Scientific, Inc.) supplemented with 10%
Gibco fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) at
37°C with an atmosphere of 5% CO2. The miR-711 mimic,
inhibitor and miRNA negative control (NC) were designed and
synthesized by Shanghai GenePharma, Ltd. (Shanghai, China). When
the cells reached 60–70% confluence, Invitrogen Lipofectamine® 2000
RNAiMAX reagent (Thermo Fisher Scientific, Inc.) was used to
perform the transfection of cells with 100 nM miR-711 mimic or
inhibitor, or NC, according to the manufacturer's protocol.
Cell growth
Following transfection for 36 h, the cells were
cultured in 96-well plates (1,000 cells/well) for an additional 7
days. The growth of the cells was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. At a specific time every day for 7 days, 20 µl MTT
(dilution, 0.5 mg/ml; Weijia Biology Science and Technology Co.,
Ltd, Guangzhou, China) was added, followed by additional incubation
for 4 h at 37°C. Following the removal of the supernatant, 150 µl
dimethyl sulfoxide (Weijia Biology Science and Technology Co., Ltd)
was added to each well. The cells in the 96-well plate were gently
agitated for 10 min and the absorbance was measured at 490 nm
(SpectraMax® M5 Multi-Mode Microplate Reader; Molecular Devices
LLC, Sunnyvale, CA, USA) to calculate the cell growth rate.
Colony formation assay
At 36 h subsequent to transfection, MCF-7 cells were
seeded on a 6-well plate (1,000 cells/well) and incubated at 37°C
in an atmosphere of 5% CO2 for 2 weeks. The surviving
colonies (>50 cells/colony) were counted using crystal violet
staining.
Apoptosis assay
Following transfection with miR-711 inhibitors for
48 h, the cells were washed twice with ice-cold phosphate-buffered
saline and resuspended in 400 µl 1X Binding Buffer (Bestbio Co.,
Shanghai, China). The cells were stained with Annexin V-fluorescein
isothiocyanate (Bestbio Co.) for 15 min at 4°C. Subsequently,
propidium iodide was added to the cells for 5 min at 4°C. The
percentage of apoptotic cells was analyzed using flow cytometry (FC
500; Beckman Coulter, Inc., Brea, CA, USA).
In vitro migration and invasion
assays
A Boyden chamber Transwell assay (24-well Transwell
plate; BD Biosciences, Franklin Lakes, NJ, USA) was used to measure
cell migration and invasion, and was performed according to the
manufacturer's protocol. For Transwell migration, 5×104
MDA-MB-231 cells were plated into the top chamber of the Transwell
plate in DMEM without FBS. The bottom chamber of the Transwell
plate was filled with DMEM supplemented with 10% FBS to stimulate
migration. Following an incubation period of 16 h, the cells in the
membrane of the bottom chamber were fixed with 100% methanol,
stained with 0.1% crystal violet (Weijia Biology Science and
Technology Co., Ltd) and counted under a microscope (BX53; Olympus
Corp., Tokyo, Japan). For Transwell invasion, the membrane of the
top chamber of the Transwell plate was coated with Matrigel (BD
Biosciences) and 2×105 cells were plated into the top
chamber. Following an incubation period of 24 h, the fixed and
stained cells in the bottom chamber were counted.
Statistical analysis
The Kaplan-Meier method and the log rank test were
used to analyze OS time and DFS using GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA). Univariate and
multivariate Cox regression analysis were performed to identify the
independent prognostic factors for survival. The association
between miR-711 and clinical characteristics of the patients was
analyzed using Student's t-test, χ2 test or
Fisher's exact test. SPSS version 17.0 software (SPSS, Inc.,
Chicago, IL, USA) was used for all analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-711 is upregulated in breast
cancer
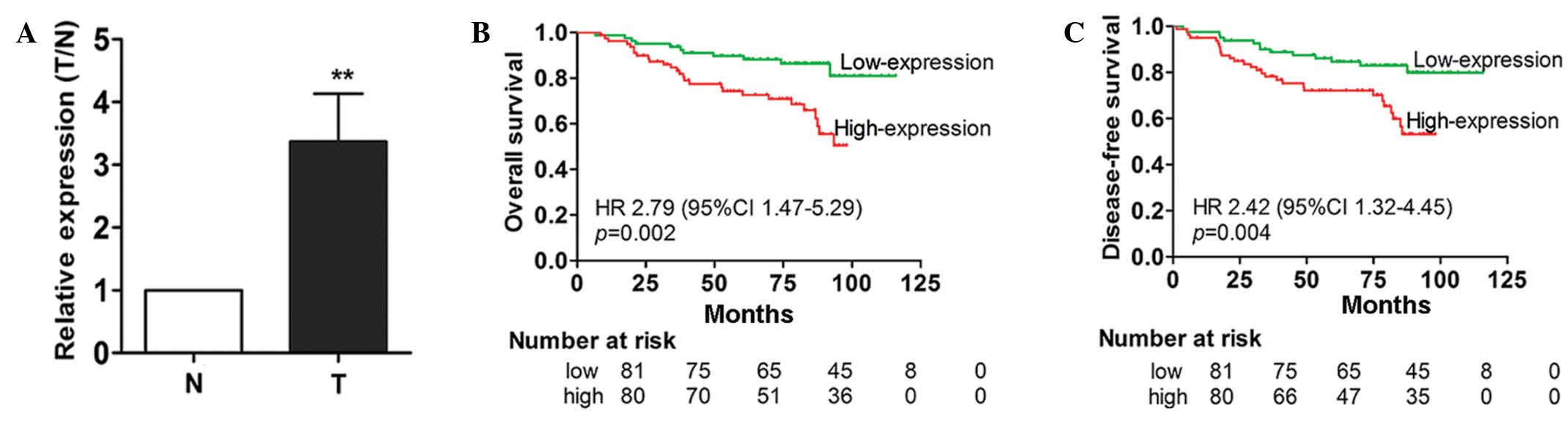
To investigate whether miR-711 is dysregulated in
breast cancer, 30 paired FFPE breast cancer and non-cancer tissue
samples were collected, and miR-711 expression was assessed in the
samples using TaqMan RT-qPCR. The findings demonstrated that the
expression level of miR-711 in breast cancer tissues was
significantly increased compared with the paired non-cancerous
breast tissues (Fig. 1A). This
suggests that miR-711 may be a potential oncogene in breast
cancer.
miR-711 expression is associated with
survival but not clinical characteristics of breast cancer
patients
To additionally investigate the clinical
significance of overexpressed miR-711, RT-qPCR analysis was
conducted in 161 FFPE breast cancer samples. Based on the miR-711
expression levels measured by RT-qPCR, the 161 patients were
divided into a high or low miR-711 expression group using the
median expression level as the cut-off point (0.57; range, 0.23 to
1.21). The associations between miR-711 expression level and
clinical characteristics were evaluated; however, no significant
associations were observed (Table I).
By contrast, a Kaplan-Meier survival analysis indicated that
patients in the high miR-711 expression group demonstrated
significantly poorer OS and DFS times compared with patients in the
low-expression group (P=0.002 and P=0.004, respectively; Fig. 1B and C).
miR-711 is an independent prognostic
factor for breast cancer
Based on the findings that miR-711 expression is
significantly associated with survival time of patients with breast
cancer, the present study investigated whether miR-711 is an
independent prognostic factor for breast cancer. The effects of
miR-711 expression and various clinical characteristics on the
survival time of 161 patients was analyzed using univariate and
multivariate Cox regression models. The results demonstrated that
miR-711 expression was an independent prognostic factor for OS time
[hazard ratio (HR), 2.549; 95% CI, 1.303–4.988; P=0.006] and DFS
time (HR, 2.873; 95% CI, 1.392–5.929; P=0.004) in patients with
breast cancer (Table II).
Tumor-node-metastasis stage was also found to be an independent
prognostic factor for OS (HR, 1.928; 95% CI, 1.162–3.198; P=0.011)
and DFS (HR, 2.068; 95% CI, 1.209–3.536; P=0.008). Furthermore,
miR-711 was not associated with other clinical characteristics,
suggesting that this miRNA independently affects the survival time
of patients with breast cancer. Overall, the results indicate that
miR-711 is an independent prognostic factor in breast cancer.
 | Table II.Univariate and multivariate Cox
regression analysis for the identification of prognostic factors
for OS and DFS time in patients with breast cancer. |
Table II.
Univariate and multivariate Cox
regression analysis for the identification of prognostic factors
for OS and DFS time in patients with breast cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinical
feature | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Analysis for OS
time |
|
|
|
|
|
Expression of miR-711 (high
vs. low) | 1.985
(1.159–3.398) | 0.007 | 2.549
(1.303–4.988) | 0.006 |
| Age,
years (>45 vs. ≤45) | 1.015
(0.944–1.137) | 0.654 | – | – |
|
Menopause (yes vs. no) | 1.187
(0.555–2.539) | 0.659 | – | – |
|
Pathological grade (III vs.
I–II) | 1.426
(0.626–3.251) | 0.398 | – | – |
| HER2
(positive vs. negative) | 1.693
(1.082–2.260) | 0.080 | – | – |
| PR
(positive vs. negative) | 1.045
(0.782–1.398) | 0.764 | – | – |
| ER
(positive vs. negative) | 0.981
(0.737–1.307) | 0.897 | – | – |
| TNM
stage (III vs. II vs. I) | 1.985
(1.159–3.398) | 0.012 | 1.928
(1.162–3.198) | 0.011 |
| Analysis for DFS
time |
|
|
|
|
|
Expression of miR-711 (high
vs. low) | 2.927
(1.406–6.092) | 0.004 | 2.873
(1.392–5.929) | 0.004 |
| Age,
years (>45 vs. ≤45) | 1.021
(0.979–1.107) | 0.785 | – | – |
|
Menopause (yes vs. no) | 1.377
(0.610–3.105) | 0.441 | – | – |
|
Pathological grade (III vs.
I–II) | 1.095
(0.421–2.848) | 0.852 | – | – |
| HER2
(positive vs. negative) | 1.310
(1.082–1.704) | 0.092 | – | – |
| PR
(positive vs. negative) | 1.129
(0.826–1.543) | 0.448 | – | – |
| ER
(positive vs. negative) | 0.923
(0.681–1.251) | 0.606 | – | – |
| TNM
stage (III vs. II vs. I) | 2.235
(1.252–3.989) | 0.007 | 2.068
(1.209–3.536) | 0.008 |
miR-711 overexpression promotes
oncogenic growth of breast cancer cells
In order to understand how miR-711 may affect the
survival time of breast cancer patients, the present study
investigated the mechanisms by which miR-711 promotes the
development and progression of breast cancer. MCF-7, MDA-MB-231 and
ZR-75-30 human breast cancer cell lines were transiently
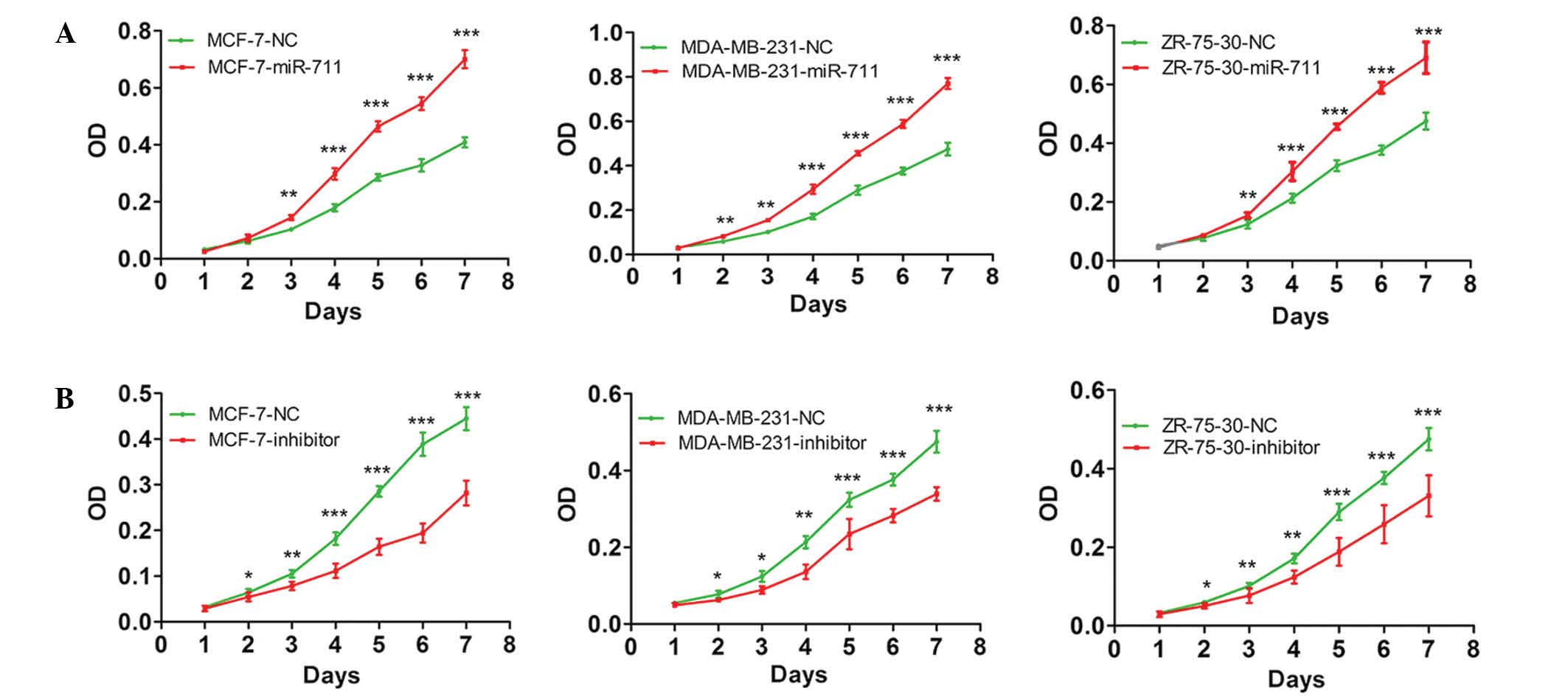
transfected with a miR-711 mimic and the cell proliferation was
observed. MTT assay demonstrated that the growth rate of the cells
transfected with miR-711 mimic was significantly increased compared
with cells transfected with the NC miRNA (P=0.002; Fig. 2A). Subsequently, cells transfected
with a miR-711 inhibitor were analyzed. The findings demonstrated
that the proliferation of the cells transfected with the miR-711
inhibitor were markedly decreased compared with cells transfected
with NC (P=0.001; Fig. 2B). A colony
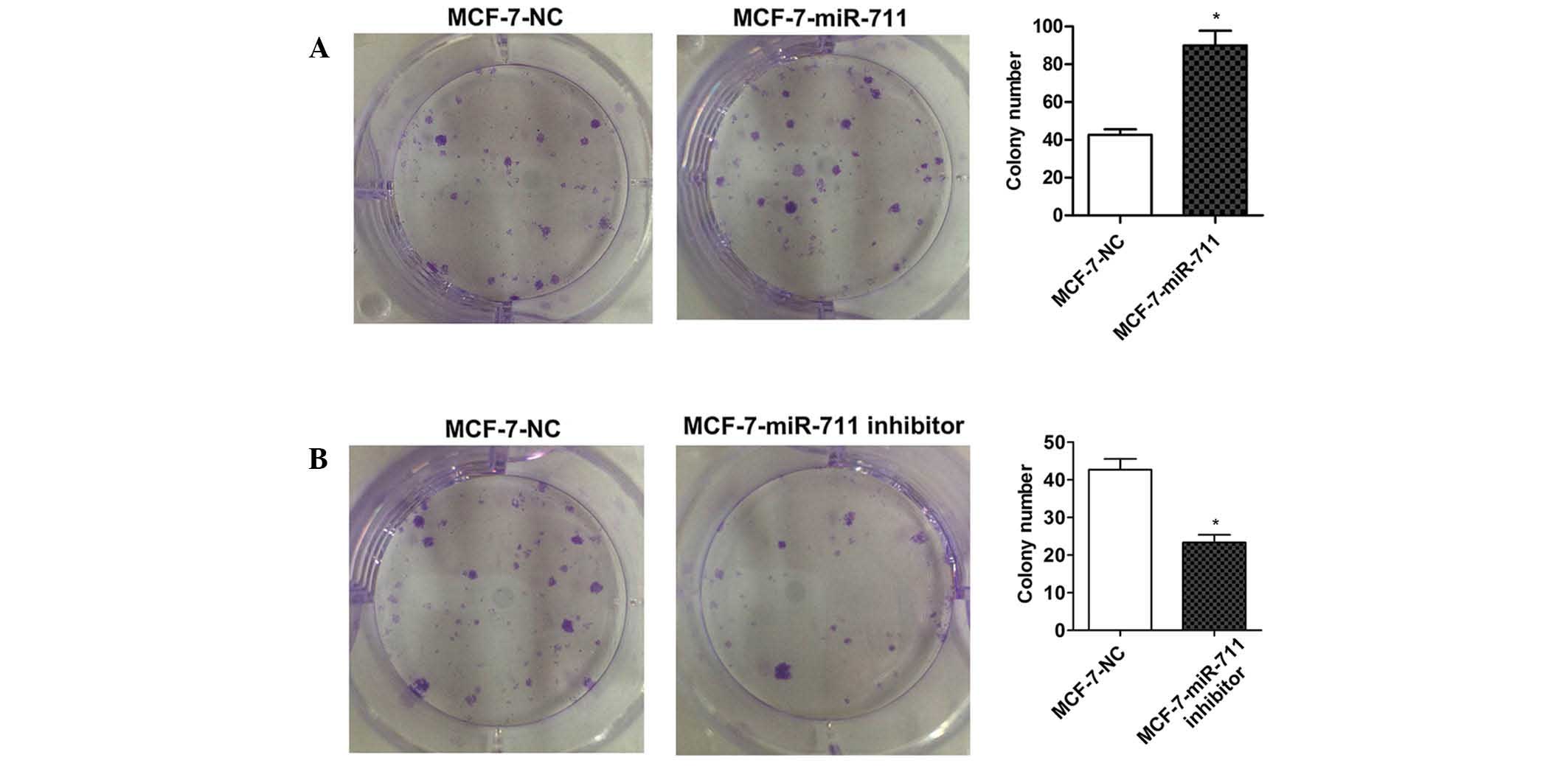
formation assay was performed on MCF-7 cells transfected with a
miR-711 mimic or inhibitor. As expected, the colony numbers of
MCF-7 cells transiently transfected with miR-711 mimics was
significantly increased compared with MCF-7 cells transfected with
NC (P=0.007; Fig. 3A), and the colony
numbers were significantly decreased in cells transfected with a
miR-711 inhibitor compared with cells transfected with NC (P=0.008;
Fig. 3B). These findings indicate
that miR-711 promotes the oncogenic growth of breast cancer
cells.
Knockdown of miR-711 induces apoptosis
in breast cancer cells
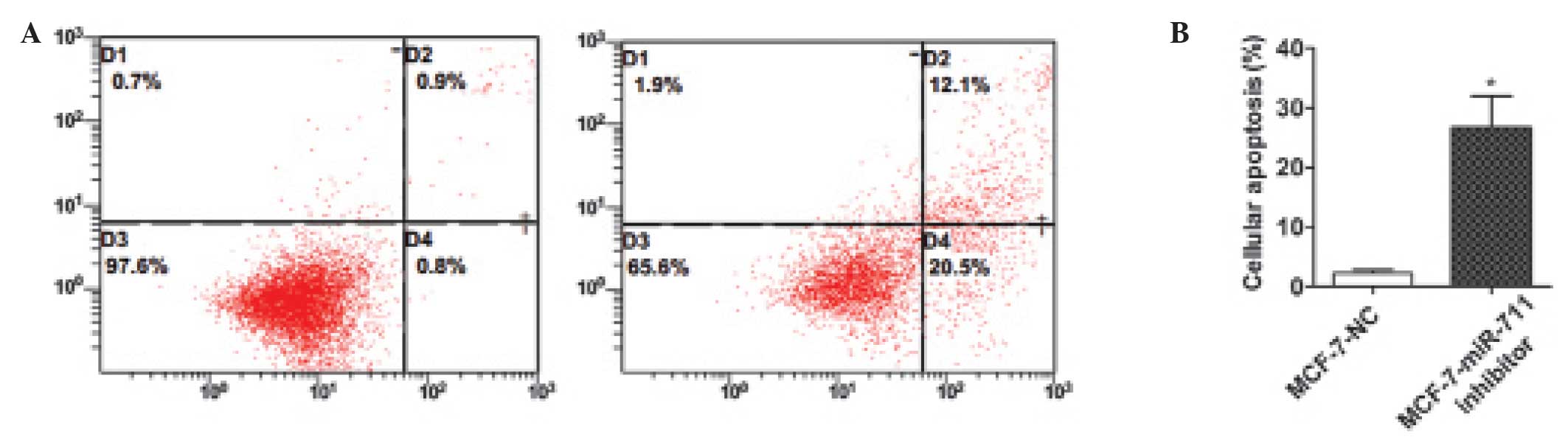
The present study aimed to identify whether
apoptosis affects the growth of breast cancer cells induced by
miR-711. In the apoptosis assay, MCF-7 cells were transiently
transfected with a miR-711 inhibitor or NC, and flow cytometry was
used to evaluate the results. The findings demonstrated that the
apoptotic rate of MCF-7 cells transfected with the miR-711
inhibitor was markedly increased compared with cells transfected
with NC (Fig. 4). This suggests that
downregulation of miR-711 may induce apoptosis in breast cancer
cells. Therefore, miR-711 may inhibit cell apoptosis, leading to
the proliferation of breast cancer cells.
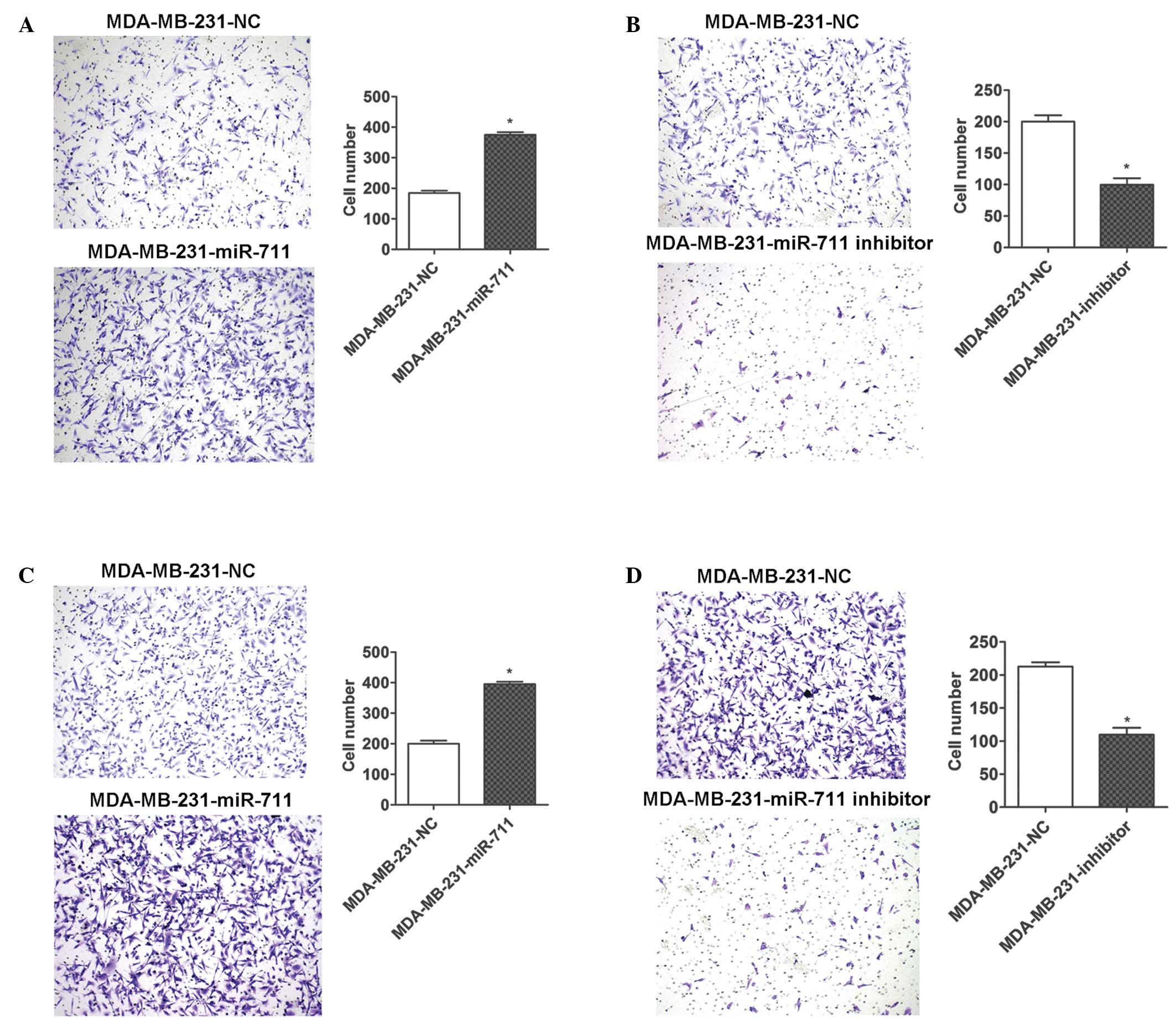
miR-711 affects the migration and
invasion of breast cancer cells
To additionally investigate miR-711 in the
progression of breast cancer, migration and invasion assays were
conducted using Transwell plates. MDA-MB-231 cells were transiently
transfected with miR-711 mimic, inhibitor or NC and were seeded in
the wells of the Transwell plate. The ectopic expression of miR-711
significantly promoted the migration and invasion of MDA-MB-231
cells (Fig. 5A and C), and the
downregulation of miR-711 using a miR-711 inhibitor resulted in
marked inhibition of migration and invasion of the cells (Fig. 5B and D). Therefore, the present
results indicate that miR-711 serves a functional role in tumor
progression and metastasis, which is consistent with the poor
survival time observed in patients with breast cancer who have an
increased expression of miR-711.
Discussion
In recent years, mortality rates of breast cancer
have continued to decrease due to advances in early diagnosis and
treatment (2,3). However, thousands of women succumb to
this disease each year worldwide. In clinical practice, positive
outcomes and increased survival time have been proven to be
associated with early diagnosis and precise therapy in breast
cancer (1). Therefore, identification
of effective prognostic biomarkers is urgently required to improve
the survival time of patients with breast cancer. The dysregulation
of miRNAs in breast cancer has been extensively investigated. In
2008, Gregory et al (28)
revealed that the expression of the miR-200 family was abnormal in
regions of metaplastic breast cancer tissues lacking E-cadherin. Wu
et al (29) reported that
miR-205 expression was significantly reduced in breast cancer
tissues compared with matched normal breast tissues by using miRNA
TaqMan PCR. Furthermore, it was observed that certain breast cancer
cell lines, including MCF-7 and MDA-MB-231, expressed a decreased
level of miR-205 compared with the non-malignant breast MCF-10A
cell line (29). In addition, it was
observed that ectopic expression of miR-205 significantly inhibited
cell proliferation and growth, as well as cell invasion, and in
animal models miR-205 suppressed lung metastasis (29). In 2012, Rivas et al (30) reported that miR-16 functioned as a
tumor suppressor in breast cancer. It was demonstrated that miR-16
was involved in progestin-induced tumor growth, and overexpression
of miR-16 repressed progestin-induced breast tumor growth in
vitro and in vivo (30).
Furthermore, it was demonstrated that miR-16 was significantly
downregulated by hormonotherapy in an in vivo setting
(30). These results suggested a
novel mechanism of progestin-induced breast cancer growth that may
have the potential to modulate a wide array of genes (30). Notably, it also demonstrated the
involvement of miR-16 in HRG-induced breast cancer cell
proliferation, confirming the ability of miR-16 to act as a tumor
suppressor during breast cancer cell proliferation (30). To the best of our knowledge, there
have been no studies that have investigated the role of miR-711 in
human cancer. However, previous studies have investigated the role
of miR-711 in organ injury and have reported miR-711 upregulation
leading to various effects, including inhibition of collagen-1
expression in myocardial infarction and induction of neuronal cell
death following traumatic brain injury (31).
The present study reports that miR-711 is aberrantly
overexpressed in breast cancer tissues; this is similar to results
observed in cutaneous T-cell lymphoma, in which miR-711 is
overexpressed (32). Notably, the
present study revealed that miR-711 overexpression is associated
with poor OS and DFS times and is an independent prognostic factor
in patients with breast cancer. Furthermore, the present in
vitro experiments demonstrated that overexpression of miR-711
promotes proliferation, colony formation, migration and invasion of
breast cancer cells, and that a knockdown of miR-711 significantly
increases the percentage of apoptotic breast cancer cells. This
indicates that miR-711 promotes the oncogenic proliferation of
breast cancer cells by inhibiting the apoptosis of cells.
Therefore, additional studies are required to investigate the
target genes of miR-711 and the mechanism by which it functions in
breast cancer cells.
In summary, the present study demonstrates for the
first time, to the best of our knowledge, that miR-711 is an
independent prognostic factor and plays an important oncogenic role
in patients with breast cancer; however, the exact nature of this
role remains to be determined. Thus, miR-711 may potentially be
used as a biomarker for prognosis, and may be a future targeted
therapy in patients with breast cancer.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China/Joint Research Fund
for Oversea Scholar (grant no. 81228104) and the Research Fund of
State Key Laboratory of Oncology in South China.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Autier P, Boniol M, La Vecchia C, Vatten
L, Gavin A, Héry C and Heanue M: Disparities in breast cancer
mortality trends between 30 European countries: Retrospective trend
analysis of WHO mortality database. BMJ. 341:(aug11 1): c36202010.
View Article : Google Scholar
|
|
4
|
Doll R, Payne P and Waterhouse JAH: Cancer
Incidence in Five Continents. I:Geneva: Union Internationale Contre
le Cancer. 1966. View Article : Google Scholar
|
|
5
|
Lacroix M: Significance, detection and
markers of disseminated breast cancer cells. Endocr Relat Cancer.
13:1033–1067. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saini KS, Taylor C, Ramirez AJ, Palmieri
C, Gunnarsson U, Schmoll HJ, Dolci SM, Ghenne C, Metzger-Filho O,
Skrzypski M, et al: Role of the multidisciplinary team in breast
cancer management: Results from a large international survey
involving 39 countries. Ann Oncol. 23:853–859. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu W, Sun M, Zou GM and Chen J: MicroRNA
and cancer: Current status and prospective. Int J Cancer.
120:953–960. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calin GA, Ferracin M, Cimmino A, Di Leva
G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et
al: A MicroRNA signature associated with prognosis and progression
in chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ueda T, Volinia S, Okumura H, Shimizu M,
Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, et al:
Relation between microRNA expression and progression and prognosis
of gastric cancer: A microRNA expression analysis. Lancet Oncol.
11:136–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu SL, Chen HY, Chang GC, Chen CY, Chen
HW, Singh S, Cheng CL, Yu CJ, Lee YC, Chen HS, et al: MicroRNA
signature predicts survival and relapse in lung cancer. Cancer
Cell. 13:48–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yousef M, Showe L and Showe M: A study of
microRNAs in silico and in vivo: Bioinformatics
approaches to microRNA discovery and target identification. FEBS J.
2762150:21562009.Liu CG, Calin GA, Volinia S and Croce CM: MicroRNA
expression profiling using microarrays. Nat Protoc 3: 563-578,
2008.
|
|
15
|
Liu CG, Calin GA, Volinia S and Croce CM:
MicroRNA expression profiling using microarrays. Nat Protoc.
3:563–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dvinge H, Git A, Gräf S, Salmon-Divon M,
Curtis C, Sottoriva A, Zhao Y, Hirst M, Armisen J, Miska EA, et al:
The shaping and functional consequences of the microRNA landscape
in breast cancer. Nature. 497:378–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li LC, Okino ST, Zhao H, Pookot D, Place
RF, Urakami S, Enokida H and Dahiya R: Small dsRNAs induce
transcriptional activation in human cells. Proc Natl Acad Sci USA.
103:17337–17342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morris KV, Chan SW, Jacobsen SE and Looney
DJ: Small interfering RNA-induced transcriptional gene silencing in
human cells. Science. 305:1289–1292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho WC: MicroRNAs: Potential biomarkers
for cancer diagnosis, prognosis and targets for therapy. Int J
Biochem Cell Biol. 42:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Redell JB, Liu Y and Dash PK: Traumatic
brain injury alters expression of hippocampal microRNAs: potential
regulators of multiple pathophysiological processes. J Neurosci
Res. 87:1435–1448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang K, Zhang S, Marzolf B, Troisch P,
Brightman A, Hu Z, Hood LE and Galas DJ: Circulating microRNAs,
potential biomarkers for drug-induced liver injury. Proc Natl Acad
Sci USA. 106:4402–4407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao N, Yu H, Sun M, Zhang Y, Xu M and Gao
W: MiRNA-711-SP1-collagen-I pathway is involved in the
anti-fibrotic effect of pioglitazone in myocardial infarction. Sci
China Life Sci. 56:431–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
American Joint Committee on Cancer: Cancer
Staging Manual. Greene FL, Page DL, Fleming ID and Fritz AG: (6th).
(New York, NY). Springer Science+Business Media. 221–240. 2002.
|
|
26
|
Körbler T, Grsković M, Dominis M and
Antica M: A simple method for RNA isolation from formalin-fixed and
paraffin-embedded lymphatic tissues. Exp Mol Pathol. 74:336–340.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu H, Zhu S and Mo YY: Suppression of cell
growth and invasion by miR-205 in breast cancer. Cell Res.
19:439–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rivas MA, Venturutti L, Huang YW,
Schillaci R, Huang TH and Elizalde PV: Downregulation of the
tumor-suppressor miR-16 via progestin-mediated oncogenic signaling
contributes to breast cancer development. Breast Cancer Res.
14:R772012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sabirzhanov B, Stoica BA, Zhao Z, Loane
DJ, Wu J, Dorsey SG and Faden AI: miR-711 upregulation induces
neuronal cell death after traumatic brain injury. Cell Death
Differ. Oct 16–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ralfkiaer U, Hagedorn PH, Bangsgaard N,
Løvendorf MB, Ahler CB, Svensson L, Kopp KL, Vennegaard MT,
Lauenborg B, Zibert JR, et al: Diagnostic microRNA profiling in
cutaneous T-cell lymphoma (CTCL). Blood. 118:5891–5900. 2011.
View Article : Google Scholar : PubMed/NCBI
|