Introduction
Small-cell lung cancer (SCLC) accounts for 15–20% of
all types of lung cancers (1). SCLC
is characterized by a high incidence of metastatic disease when
patients initially present with SCLC, and a high treatment response
rate. Limited stage-SCLC (LS-SCLC) is diagnosed in 30–40% of
patients with SCLC.
The first-line treatment for patients with LS-SCLC
is cisplatin-based chemotherapy combined with irradiation of the
chest region (2,3), which yields a complete response (CR)
rate of 50–85%, a median duration of survival of 12–20 months, and
a two-year disease-free survival rate of 15–40%. In addition, the
risk of thoracic recurrence decreases with combined treatment;
however, brain metastasis becomes one of the main types of relapse
(2). The cumulative incidence of
brain metastasis two years post-treatment is 45–58% in patients
that receive combined treatment (4,5).
Prophylactic cranial irradiation (PCI) has been
demonstrated to reduce the risk of brain metastases from 58.6 to
33.3% 3 years subsequent to combined therapy, and in addition,
meta-analyses have revealed that PCI results in a 16% reduction in
mortality rate, which modestly increases the 3-year survival rate
between 15.3 and 20.7%. (6). However,
in recent years, there has been a more effective systemic
chemotherapy treatment (etoposide regimen and cisplatin-combination
regimens) that has been combined with more advanced thoracic
radiotherapy, including three-dimensional conformal and
intensity-modulated radiation therapy (7,8);
therefore, the magnitude of benefit from PCI should be
re-evalauated.
The benefit of PCI has been determined, but the
optimal timing of PCI intervention has not been examined (6). Auperin et al demonstrated by
meta-analysis that PCI had a significantly greater effect on the
incidence of brain metastasis in patients that received PCI within
6 months following induction therapy compared with patients that
received PCI after 6 months (P=0.01) (6). However, the results from Auperin et
al were from a subgroup analysis and should be interpreted with
caution. Two prospective randomized studies comparing the optimal
timing of PCI revealed conflicting conclusions; an early randomized
study revealed no difference in the frequency of brain metastases
between PCI performed at the start of induction treatment and PCI
delivered 6 weeks later, whereas a later study demonstrated a
statistically significant decrease in intracranial recurrence when
PCI was performed during chemoradiotherapy as opposed to following
chemoradiotherapy (9,10). Therefore, the optimal timing of PCI
delivery should be established. The aim of the present study was to
re-evaluate the benefits of PCI and investigate whether a delay in
delivering PCI following the start of the first chemotherapy cycle
leads to a detrimental outcome of patients.
Materials and methods
Patients
Histological or cytological evidence of the presence
of SCLC was required. The selected patients were diagnosed with
LS-SCLC and had achieved CR or near CR subsequent to primary
chemotherapy or chemoradiotherapy. Patients underwent a staging
evaluation prior to the initiation of chemotherapy. The staging
evaluation consisted of a computed tomography (CT) scan of the
chest, ultrasonography of the neck and upper abdomen, magnetic
resonance imaging (MRI) or CT of the head, a radionuclide bone
scan, and a CT scan of the upper abdomen. Radiographs of the
regions of increased radionuclide uptake were confirmed by CT or
MRI. A bone marrow biopsy was not used for staging. Lymph nodes
that were suspected of being enlarged were observed using
ultrasonography and confirmed by cytology using a needle
aspirate.
LS-SCLC was defined as cancer limited to one
hemithorax, the mediastinum and supraclavicular nodes, provided
that all volumes were combined in the same radiotherapy field as
the primary tumor.
Patients were excluded from analysis for the
following reasons: Cytological evidence of a malignant pleural
effusion; the presence of a second malignancy; ≤2 cycles of primary
chemotherapy undertaken; the presence of squamous cell carcinoma or
adenocarcinoma; and the presence of progressive disease during
chemo- or radiotherapy.
The present study was approved by the Medical Ethics
Committee of Zhejiang Cancer Hospital (Hangzhou, China; approval
no., IRB-2016-10).
Treatment strategy
In total, ≥3 cycles of cisplatin-based treatment
regimens were administered to patients. Chemotherapy regimens
mainly consisted of etoposide and cisplatin therapy (cisplatin, 25
mg/m2 on days 1–3 or carboplatin, area under the
curve-based dosing of 5 on day 1, and etoposide, 80–100
mg/m2 on days 1–3). Thoracic radiotherapy (TRT) was
performed concurrently, sequentially or alternatively with
chemotherapy. TRT was administered with 3-dimensional conformal
radiotherapy (CRT) or intensity-modulated radiotherapy technique,
and the prescription dose was 40–66 Gy in 20–33 fractions. The
target volume consisted of all gross disease, the ipsilateral hilum
and the entire mediastinum. The supraclavicular fossa was not
irradiated routinely if there were no enlarged lymph nodes in those
sites.
The patients received whole-brain PCI, in which 4–6
MV photons were delivered with opposed lateral portals, with normal
tissue sparing of the lens. The irradiation fields included a ≥1 cm
margin on the calvarium. PCI was performed within 1 month following
primary chemotherapy or chemoradiotherapy.
To investigate the impact of the timing of PCI on
prognosis, early and late PCI groups were established from the
median time between the start of primary chemotherapy and the
initiation of PCI.
Response assessment and follow-up
The response assessment at the end of primary
chemotherapy or chemo- and radiotherapy was based on the results of
a CT scan of the chest and upper abdomen, an MRI of the head and a
radionuclide bone scan. Fiberoptic bronchoscopy was not performed.
CR was defined as the disappearance of all target lesions. Partial
response (PR) was defined as a decrease of ≥50% in the greatest
dimensions of target tumors, using the sum at baseline as a
reference. Near CR was defined as a continuum between CR and
PR.
CT of the chest and ultrasonography of the neck and
upper abdomen were repeated every 3 months for 2 years following
the end of treatment, and every 6 months thereafter. An MRI of the
head was used if the patients presented with a headache or
neurological symptoms suggestive of brain metastases.
Endpoints
The primary endpoint was the cumulative incidence of
brain metastases. Overall survival (OS) time was the secondary
endpoint and was defined as the duration between the date of the
first chemotherapy cycle and the date of mortality from any cause
or the last known date that the patient was alive.
Statistical analysis
All data were calculated using SPSS software version
13.0 (SPSS Inc., Chicago, IL, USA). The differences within each
categorical variable were assessed using χ2 tests. The
Kaplan-Meier estimate was used to calculate brain metastasis rates.
Survival time was calculated as the time elapsed between the date
of primary chemotherapy and the date of mortality. The patients
that remained alive were censored at the date they were last known
to be alive. The Kaplan-Meier estimate was used to calculate
survival time curves and the Mantel-Cox version of the log rank
test was used to determine associations between OS time and
clinical factors, including the age at diagnosis, gender, smoking
status, Eastern Cooperative Oncology Group performance score (ECOG
PS), weight loss, thoracic radiotherapy and PCI. Cox's proportional
hazards model, with a backward-forward, stepwise method, was used
to identify significant variables. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
Between March 2005 and December 2010, 479 patients
with LS-SCLC were treated at Zhejiang Cancer Hospital (Hangzhou,
Zhejiang, China). In total, 399 patients were eligible for the
present study, and 80 patients were excluded from the analysis as
follows: 22 patients underwent ≤2 cycles of the first chemotherapy
regimen; 2 patients possessed mixed carcinoma; 46 patients did not
achieve CR or near CR following first-line treatment; and 10
patients demonstrated progressive disease during chemo- or
radiotherapy, of whom 9 patients developed brain metastases, with 7
developing metastases during thoracic radiotherapy and 2 developing
metastases during chemotherapy, and 1 patient developed liver
metastases during chemotherapy. The median follow-up time was 26.5
months (range, 3.1–77.4 months).
Of the 399 patients whose records were examined, the
median age was 55 years (range, 25–79 years) and 81% of the
patients were men. In total, 98% of the patients possessed an ECOG
PS of 0 or 1 at baseline, and 25 patients underwent thoracic
surgery and 344 patients received thoracic radiotherapy. The mean
dose of thoracic radiotherapy was 54.10±0.43 Gy.
PCI was administered to 185 patients. The mean PCI
dose was 28.22±0.30 Gy in 10 fractions. In 98% of patients, PCI was
delivered subsequent to the end of primary chemotherapy or
chemoradiotherapy. The median interval between the start of primary
chemotherapy and the start of PCI was 6 months. In total, 92
patients received early PCI and 93 patients received late PCI. The
median interval between the start of primary chemotherapy and the
initiation of PCI was 5.0 months (range, 1.5–6.0 months) in the
early PCI group and 7.2 months (range, 6.1–16.9 months) in the late
PCI group. In total, 214 patients did not receive PCI due to the
individual preference of the physician or the patient.
The baseline characteristics of the patients are
presented in Table I. There were no
differences in the distribution of the majority of characteristics
between the PCI group and the group that did not receive PCI, with
the exception of age and the number of patients that underwent
thoracic radiotherapy. An increased number of patients received
thoracic radiotherapy in the PCI group compared to the group that
did not receive PCI (P<0.001). Patients >65 years of age were
significantly less likely to receive PCI compared with patients
aged <65 years (P=0.045). The baseline characteristics were
similar between the early and late PCI groups.
 | Table I.Characteristics of 399 patients with
limited stage-small cell lung cancer. |
Table I.
Characteristics of 399 patients with
limited stage-small cell lung cancer.
|
| Group, n (%) |
|
|---|
|
|
|
|
|---|
| Characteristic | No PCI | Early PCI | Late PCI | P-value |
|---|
| Total | 214
(100.0) | 92
(100.0) | 93
(100.0) |
|
| Age |
|
|
|
|
| <65
years | 176 (82.2) | 83 (90.2) | 85 (91.4) | 0.045 |
| ≥65
years | 38
(17.8) | 9 (9.8) | 8 (8.6) |
| Gender |
|
|
|
|
| Male | 178 (83.2) | 72 (78.3) | 72 (77.4) | 0.399 |
|
Female | 36
(16.8) | 20 (21.7) | 21 (22.6) |
| ECOG PS |
|
|
|
|
| 0–1 | 208 (97.2) | 90 (97.8) | 91 (97.8) | 0.920 |
| 2 | 6
(2.8) | 2 (2.2) | 2 (2.2) |
| Weight loss |
|
|
|
|
|
<5 | 202 (94.4) | 87 (94.6) | 88 (94.6) | 0.996 |
| ≥5 | 12 (5.6) | 5 (5.4) | 5 (5.4) |
| Smoking status |
|
|
|
|
| Yes | 164 (76.6) | 64 (70.0) | 66 (71.0) | 0.346 |
| No | 50
(23.4) | 28 (30.0) | 27 (29.0) |
| TRT |
|
|
|
|
| Yes | 167 (78.0) |
85(92.4) | 92 (98.9) | 0.000 |
| No | 47
(22.0) | 7 (7.6) | 1 (1.1) |
| CT cycles |
|
|
|
|
| 3 | 12 (5.6) | 4 (4.4) | 3 (3.2) | 0.128 |
|
4–6 | 198 (92.5) | 88 (95.6) | 85 (91.4) |
|
7–8 | 4
(1.9) | 0 (0.0) | 5 (5.4) |
Cumulative incidence of brain
metastases
Symptomatic brain metastases were identified in 75
of the 215 patients in the group that did not receive PCI (34.9%)
and in 21 of the 185 patients in the PCI group (11.4%; P<0.001).
The median time between the start of primary chemotherapy and the
incidence of brain metastases was 10.6 months (range, 2.5–46.0
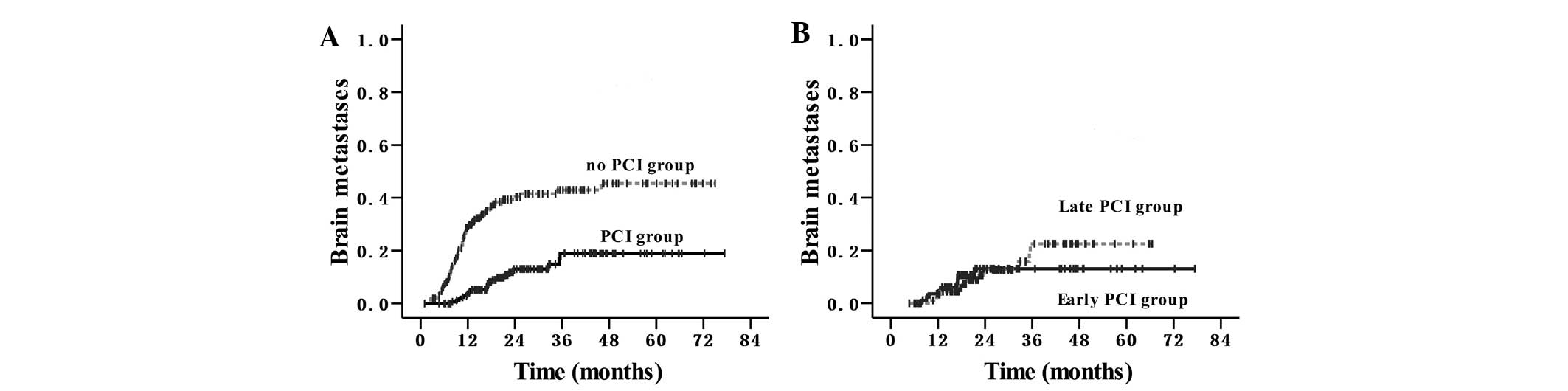
months). The cumulative incidence curves are revealed in Fig. 1A. The cumulative risks of symptomatic
brain metastases at 6, 12 and 24 months were 7, 29 and 42% in the
group that did not receive PCI, and 0, 3 and 13% in the PCI group.
The hazards ratio for the PCI group was 0.24 [95% confidence
interval (CI), 0.15–0.39]. There was no significant difference
between the early PCI and late PCI groups (P=0.875); however, there
was an increasing trend in the incidence of brain metastases in the
late PCI group 3 years subsequent to receiving PCI (Fig. 1B).
Overall survival (OS) rate and
time
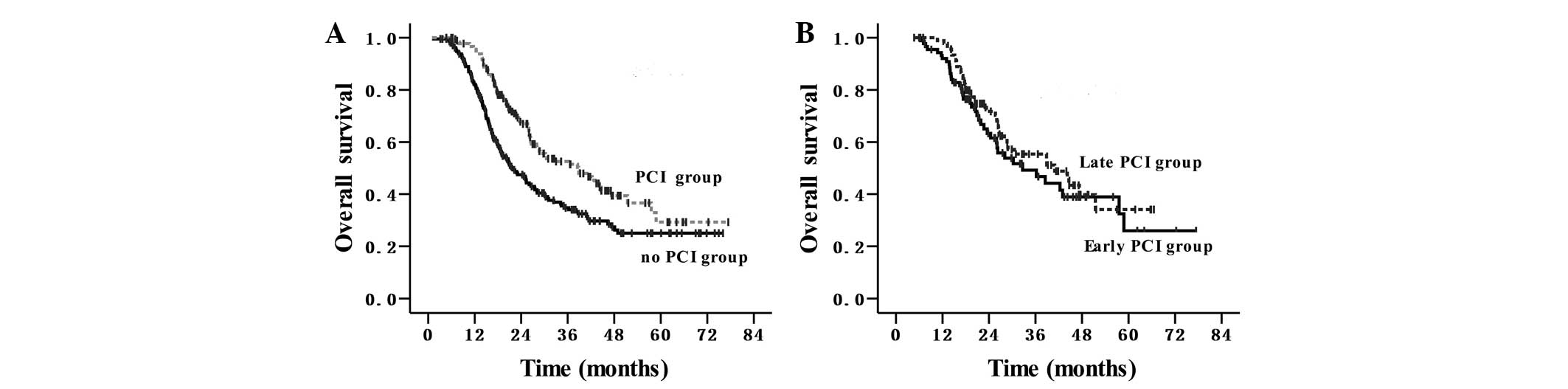
The median survival time was 27.5 months and the OS
rates at 1 and 3-years were 89 and 43%, respectively, for the
entire cohort of patients. The survival outcomes are shown in
Fig. 2A and B. In the group that did
not receive PCI, the median survival time was 21.5 months, and the
1 and 3-year OS rates were 82 and 35%, respectively. In the PCI
group, the median survival time was 38.8 months, and the 1 and
3-year OS rates were 96 and 53%, respectively, (P<0.001; HR,
0.60; 95% CI, 0.45–0.79). In the early PCI group, the median
survival time was 32.6 months, and the 1 and 3-year OS rates were
92 and 50%, respectively. In the late PCI group, the median
survival time was 40.9 months, and the 1 and 3-year OS rates were
99 and 55%, respectively (P=0.361).
Patient and clinical characteristics were evaluated
to determine their prognostic value in terms of OS rate in Table II. In total, 9 characteristics (age,
gender, ECOG PS, weight loss, smoking status, thoracic
radiotherapy, PCI, the number of chemotherapy cycles and the timing
of PCI) were analyzed. Univariate analysis demonstrated that age,
gender, ECOG PS, weight loss, the number of chemotherapy cycles and
the timing of PCI were not significantly associated with the
survival rate; however, smoking status, PCI and thoracic
radiotherapy were significant prognostic factors for the survival
time. As revealed in Table III,
multivariate analysis demonstrated that PCI (P=0.004) and thoracic
radiotherapy (P=0.023) were the only 2 independent favorable
prognostic factors of OS rate. Smoking status demonstrated a
marginally significant effect on the OS rate (P=0.051).
 | Table II.Univariate analysis of the prognostic
factors for survival rate in patients with limited-stage small-cell
lung cancer. |
Table II.
Univariate analysis of the prognostic
factors for survival rate in patients with limited-stage small-cell
lung cancer.
|
|
| OS rate, % |
|
|---|
|
|
|
|
|
|---|
| Characteristic | MST, months | 1-Year | 3-Year | P-value |
|---|
| Age |
|
|
|
|
| <65
years | 28.1 | 88 | 44 | 0.111 |
| ≥65
years | 22.4 | 90 | 32 |
| Gender |
|
|
|
|
|
Male | 26.4 | 88 | 41 | 0.087 |
|
Female | 38.5 | 92 | 52 |
| ECOG PS |
|
|
|
|
|
0–1 | 28.1 | 89 | 43 | 0.427 |
| 2 | 22.4 | 80 | 33 |
| Weight loss |
|
|
|
|
|
<5 | 27.4 | 89 | 43 | 0.948 |
| ≥5 | 34.4 | 73 | 44 |
| Smoking status |
|
|
|
|
|
Yes | 26.1 | 87 | 40 | 0.027 |
| No | 38.5 | 92 | 51 |
| TRT |
|
|
|
|
|
Yes | 30.2 | 90 | 46 | 0.001 |
| No | 19.1 | 78 | 25 |
| CT cycles |
|
|
|
|
|
<4 | 25.1 | 79 | 48 | 0.601 |
| ≥4 | 27.5 | 89 | 43 |
| PCI |
|
|
|
|
|
Yes | 38.8 | 96 | 53 | 0.000 |
| No | 21.5 | 82 | 35 |
| Timing of PCI |
|
|
|
|
| Early
PCI | 32.6 | 92 | 50 | 0.361 |
| Late
PCI | 40.9 | 99 | 55 |
 | Table III.Multivariate analysis of the
prognostic factors for survival rate in patients with limited-stage
small-cell lung cancer. |
Table III.
Multivariate analysis of the
prognostic factors for survival rate in patients with limited-stage
small-cell lung cancer.
| Characteristic | B | HR | 95% CI | χ2 | P-value |
|---|
| Age, years (≥65 vs.
<65) |
0.097 | 1.101 | 0.908–1.336 | 0.956 | 0.328 |
| Gender (female vs.
male) | −0.096 | 0.908 | 0.542–1.522 | 0.133 | 0.715 |
| ECOG PS (2 vs.
0–1) |
0.300 | 1.349 | 0.587–3.100 | 0.498 | 0.480 |
| Weight loss (≥5 vs.
<5%) | −0.066 | 0.936 | 0.512–1.709 | 0.047 | 0.829 |
| Smoking status (yes
vs. no) |
0.329 | 1.390 | 0.999–1.934 | 3.816 | 0.051 |
| TRT (yes vs.
no) | −0.413 | 0.661 | 0.463–0.945 | 5.163 | 0.023 |
| CT cycles (≥4 vs.
<4) | −0.056 | 0.945 | 0.507–1.763 | 0.031 | 0.860 |
| PCI (yes vs.
no) | −0.423 | 0.655 | 0.491–0.873 | 8.337 | 0.004 |
Discussion
Following an individual patient data-based
meta-analysis, published in 1999, PCI became the standard treatment
in patients with LS-SCLC that achieved complete remission
subsequent to chemotherapy and radiotherapy (6). However, in the present retrospective
study, >50% of patients with LS-SCLC did not receive PCI. There
is a significant discrepancy between the recommended evidence-based
optimum rate of radiotherapy in lung cancer and the actual rate
used in patients, since PCI is recommended for all patients
(6), but not all patients receive it,
which suggests that recommending PCI remains controversial among
doctors and patients (11). In
addition, in the present study, elderly patients were less likely
to receive PCI compared with younger patients, indicating that
doctors and patients were more cautious in delivering PCI to
elderly patients, due to PCI potentially causing chronic
neurotoxicity (12–14). Consequently, studies are required to
demonstrate whether PCI is safe and effective in elderly
individuals.
The present retrospective study evaluated PCI in 399
patients with LS-SCLC that achieved CR or near CR following primary
chemo- or radiotherapy and demonstrated that PCI resulted in a
significant decrease in the rate of brain metastases (P<0.001;
HR, 0.24; 95% CI, 0.15–0.39) and a significant improvement in the
OS rate (P<0.001; HR, 0.60; 95% CI, 0.45–0.79). PCI remained a
significant factor following adjustment for age, gender, ECOG PS,
weight loss, smoking status, thoracic radiotherapy and the number
of chemotherapy cycles (HR, 0.655; P=0.004). These favorable
survival rate results were consistent with other studies that
evaluated PCI in patients with LS-SCLC (6,15,16). Notably, in the present study the PCI
group and the group that did not receive PCI possessed a longer
median survival time compared with other studies (17–19). The
magnitude of benefit from PCI in the present study increased the
3-year survival rate between 35 and 53% and increased the median
survival time between 21.5 and 38.8 months, which was greater than
that revealed in other studies (6,12,16). The present study hypothesizes that
this variation is partly due to the improved accuracy of the
diagnostic staging and cisplatin-based chemotherapy combined with
the advanced radiotherapy technology used in the present study,
which resulted in an improved control rate of intrathoracic
diseases (20). Early retrospective
data also suggested that the role of PCI was largely dependent on
the response of the primary tumor to CRT (21). In addition, the current study
demonstrated that 90% of brain metastases appeared within the first
2 years of induction chemotherapy, which was consistent with other
studies (5,11,17).
Despite the disadvantages of a retrospective
analysis, the present study demonstrates the advantage that all
results were acquired from a patient cohort that was treated in a
major hospital over several years. The present retrospective study
demonstrated that the timing of PCI initiation was not
significantly associated with the incidence of brain metastases and
the OS rate or time. The baseline characteristics were similar
between the early and late PCI groups; therefore, this result was
not confounded by other clinical factors.
It is well established that early chest radiotherapy
may improve the survival rate of patients with LS-SCLC (22). As a prophylactic treatment, it is
unknown whether early PCI may be as effective as early chest
radiotherapy. A phase II study revealed that the incidence of brain
metastasis was 7.3% in the early PCI group, when PCI was performed
during chemoradiotherapy, and 20% in the late PCI group, when PCI
was performed following chemoradiotherapy (P=0.00901).
Consequently, the timing of PCI was hypothesized to be an important
factor for decreasing the incidence of brain metastases (10). However, the increased risk of
long-term neurotoxicity should be considered when PCI is used
concurrently with chemotherapy (23–25). In
the present study, 98% of patients received PCI following the end
of primary chemoradiotherapy, and hyperfractionated accelerated
radiotherapy was not used; therefore, the median interval between
the start of primary chemotherapy and the initiation of PCI was as
long as 6 months. This was similar to the interval time in the
meta-analysis by Auperin et al, demonstrating that the
interval has no effect on the risk of mortality of patients
(6).
The present findings are also consistent with two
additional studies (23,24). In a retrospective study, which
assessed 118 patients that received PCI, the risk of developing
cerebral recurrence was not observed to be significantly different
between patients that received PCI 2–5 months subsequent to
diagnosis and those that received it 5–15 months subsequent to
diagnosis (P=0.26) (26). A pooled
analysis on 421 patients with LS-SCLC also revealed that the length
of the interval between the start of chemotherapy and the
initiation of PCI did not affect subsequent survival rates (HR,
1.00; 95% CI, 0.99–1.01; P=0.58) (27). However, the present results should be
interpreted with caution, since 9 patients were excluded from the
primary analysis for developing brain metastases during primary
chemoradiotherapy, with 2 patients developing metastases during
chemotherapy and 7 patients developing metastases during thoracic
radiotherapy, which was administered sequentially with
chemotherapy. The brain was previously considered as a
pharmacological sanctuary where metastases could develop, due to
the protection provided by the blood-brain barrier (BBB). However,
in recent decades, it has become clear that the BBB is disrupted in
metastatic tumor tissue, rendering it permeable to pharmacological
agents, including CPT-11 and cisplatin (28). This is additionally confirmed in the
present observations that intracranial progression is relatively
rare during primary chemotherapy. The present finding that 7
patients developed brain metastases during thoracic radiotherapy
suggests that PCI should be administered as soon as primary
chemotherapy is completed.
The present study has several limitations. First, it
is a single-institution, retrospective review. The present study
attempted to limit the bias inherent in comparing two
non-randomized groups; however, it is likely that there are other
clinical factors that may have contributed to the difference in the
OS time and rate between the groups, including the dose and
different schedules of radiotherapy, chemotherapy combination and
the different treatment options provided during the development of
cancer. In addition, the latest edition of the
tumor-node-metastasis staging system was not used in the present
survival rate and time analysis (29). Second, the follow-up time of the
present study was relatively short. In total, 73 patients were
followed-up for ≤24 months and symptomatic brain metastases may not
have been detected in such a short follow-up period. Third, the
cut-off time of 6 months set in the present study for the early and
late PCI groups may not be optimal.
In conclusion, the present study identified that PCI
significantly decreased the incidence of brain metastases and
improved the overall survival rate in patients with LS-SCLC. Early
PCI administered within 6 months of the start of first-line
chemotherapy was as effective as late PCI, which was PCI that was
administered 6 months later. However, it is recommended that PCI
should be administered to patients as soon as first-line
chemotherapy is completed.
Acknowledgements
The authors are grateful to all the physicians and
patients for their co-operation in the present study.
References
|
1
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing
epidemiology of small-cell lung cancer in the United States over
the last 30 years: analysis of the surveillance, epidemiologic, and
end results database. J Clin Oncol. 24:4539–4544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pignon JP, Arriagada R, Ihde DC, Johnson
DH, Perry MC, Souhami RL, Brodin O, Joss RA, Kies MS, Lebeau B, et
al: A meta-analysis of thoracic radiotherapy for small-cell lung
cancer. N Engl J Med. 327:1618–1624. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Warde P and Payne D: Does thoracic
irradiation improve survival and local control in limited-stage
small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol.
10:890–895. 1992.PubMed/NCBI
|
|
4
|
Komaki R, Cox JD and Whitson W: Risk of
brain metastasis from small cell carcinoma of the lung related to
length of survival and prophylactic irradiation. Cancer Treat Rep.
65:811–814. 1981.PubMed/NCBI
|
|
5
|
Arriagada R, Le Chevalier T, Borie F,
Rivière A, Chomy P, Monnet I, Tardivon A, Viader F, Tarayre M and
Benhamou S: Prophylactic cranial irradiation for patients with
small-cell lung cancer in complete remission. J Natl Cancer Inst.
87:183–190. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aupérin A, Arriagada R, Pignon JP, Le
Péchoux C, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka
H, Wagner H and Aisner J: Prophylactic Cranial Irradiation Overview
Collaborative Group: Prophylactic cranial irradiation for patients
with small-cell lung cancer in complete remission. N Engl J Med.
341:476–484. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sundstrøm S, Bremnes RM, Kaasa S, Aasebø
U, Hatlevoll R, Dahle R, Boye N, Wang M, Vigander T, Vilsvik J, et
al: Norwegian Lung Cancer Study Group: Cisplatin and etoposide
regimen is superior to cyclophosphamide, epirubicin, and
vincristine regimen in small-cell lung cancer: Results from a
randomized phase III trial with 5 years' follow-up. J Clin Oncol.
20:4665–4672. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pujol JL, Carestia L and Daurès JP: Is
there a case for cisplatin in the treatment of small-cell lung
cancer? A meta-analysis of randomized trials of a
cisplatincontaining regimen versus a regimen without this
alkylating agent. Br J Cancer. 83:8–15. 2000.PubMed/NCBI
|
|
9
|
Perez CA, Krauss S, Bartolucci AA, Durant
JR, Lowenbraun S, Salter MM, Storaalsi J, Kellermeyer R and Comas
F: Thoracic and elective brain irradiation with concomitant or
delayed multiagent chemotherapy in the treatment of localized small
cell carcinoma of the lung: A randomized prospective study by the
Southeastern Cancer Study Group. Cancer. 47:2407–2413. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sas-Korczyńska B, Korzeniowski S and
Wójcik E: Comparison of the effectiveness of “late” and “early”
prophylactic cranial irradiation in patients with limited-stage
small cell lung cancer. Strahlenther Onkol. 186:315–319. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Delaney G, Barton M, Jacob S and Jalaludin
B: A model for decision making for the use of radiotherapy in lung
cancer. Lancet Oncol. 4:120–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giuliani M, Sun A, Bezjak A, Ma C, Le LW,
Brade A, Cho J, Leighl NB, Shepherd FA and Hope AJ: Utilization of
prophylactic cranial irradiation in patients with limited stage
small cell lung carcinoma. Cancer. 116:5694–5699. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Glantz MJ, Choy H and Yee L: Prophylactic
cranial irradiation in small cell lung cancer: Rationale, results,
and recommendations. Semin Oncol. 24:477–483. 1997.PubMed/NCBI
|
|
14
|
Wolfson AH, Bae K, Komaki R, Meyers C,
Movsas B, Le Pechoux C, Werner-Wasik M, Videtic GM, Garces YI and
Choy H: Primary analysis of a phase II randomized trial Radiation
Therapy Oncology Group (RTOG) 0212: Impact of different total doses
and schedules of prophylactic cranial irradiation on chronic
neurotoxicity and quality of life for patients with limited-disease
small-cell lung cancer. Int J Radiat Oncol Biol Phys. 81:77–84.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arriagada R, Spielmann M, Koscielny S, Le
Chevalier T, Delozier T, Ducourtieux M, Tursz T and Hill C:
Patterns of failure in a randomized trial of adjuvant chemotherapy
in postmenopausal patients with early breast cancer treated with
tamoxifen. Ann Oncol. 13:1378–1386. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel S, Macdonald OK and Suntharalingam
M: Evaluation of the use of prophylactic cranial irradiation in
small cell lung cancer. Cancer. 115:842–850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takada M, Fukuoka M, Kawahara M, Sugiura
T, Yokoyama A, Yokota S, Nishiwaki Y, Watanabe K, Noda K, Tamura T,
et al: Phase III study of concurrent versus sequential thoracic
radiotherapy in combination with cisplatin and etoposide for
limited-stage small-cell lung cancer: Results of the Japan Clinical
Oncology Group Study 9104. J Clin Oncol. 20:3054–3060. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gregor A, Drings P, Burghouts J, Postmus
PE, Morgan D, Sahmoud T, Kirkpatrick A, Dalesio O and Giaccone G:
Randomized trial of alternating versus sequential
radiotherapy/chemotherapy in limited-disease patients with
small-cell lung cancer: A European Organization for Research and
Treatment of Cancer Lung Cancer Cooperative Group Study. J Clin
Oncol. 15:2840–2849. 1997.PubMed/NCBI
|
|
19
|
Lebeau B, Urban T, Bréchot JM, Paillotin
D, Vincent J, Leclerc P, Meekel P, L'Her P, Lebas FX and Chastang
C: A randomized clinical trial comparing concurrent and alternating
thoracic irradiation for patients with limited small cell lung
carcinoma. “Petites Cellules” Group. Cancer. 86:1480–1487. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen AB, Neville BA, Sher DJ, Chen K and
Schrag D: Survival outcomes after radiation therapy for stage III
non-small-cell lung cancer after adoption of computed
tomography-based simulation. J Clin Oncol. 29:2305–2311. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosen ST, Makuch RW, Lichter AS, Ihde DC,
Matthews MJ, Minna JD, Glatstein E and Bunn PA Jr: Role of
prophylactic cranial irradiation in prevention of central nervous
system metastases in small cell lung cancer. Potential benefit
restricted to patients with complete response. Am J Med.
74:615–624. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Ruysscher D, Pijls-Johannesma M,
Vansteenkiste J, Kester A, Rutten I and Lambin P: Systematic review
and meta-analysis of randomised, controlled trials of the timing of
chest radiotherapy in patients with limited-stage, small-cell lung
cancer. Ann Oncol. 17:543–552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahles TA, Silberfarb PM, Herndon J II,
Maurer LH, Kornblith AB, Aisner J, Perry MC, Eaton WL, Zacharski
LL, Green MR and Holland JC: Psychologic and neuropsychologic
functioning of patients with limited small-cell lung cancer treated
with chemotherapy and radiation therapy with or without warfarin: A
study by the Cancer and Leukemia Group B. J Clin Oncol.
16:1954–1960. 1998.PubMed/NCBI
|
|
24
|
Fonseca R, O'Neill BP, Foote RL, Grill JP,
Sloan JA and Frytak S: Cerebral toxicity in patients treated for
small cell carcinoma of the lung. Mayo Clin Proc. 74:461–465. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ball DL and Matthews JP: Prophylactic
Cranial Irradiation: More Questions Than Answers. Semin Radiat
Oncol. 5:61–68. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramlov A, Tietze A, Khalil AA and Knap MM:
Prophylactic cranial irradiation in patients with small cell lung
cancer. A retrospective study of recurrence, survival and
morbidity. Lung Cancer. 77:561–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schild SE, Foster NR, Meyers JP, Ross HJ,
Stella PJ, Garces YI, Olivier KR, Molina JR, Past LR and Adjei AA:
North Central Cancer Treatment Group: Prophylactic cranial
irradiation in small-cell lung cancer: Findings from a North
Central Cancer Treatment Group Pooled Analysis. Ann Oncol.
23:2919–2924. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van den Bent MJ: The role of chemotherapy
in brain metastases. Eur J Cancer. 39:2114–2120. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|