Introduction
Mesenchymal tumors are uncommon in the ovary,
occurring more frequently in other regions of the body (1–3). Primary
ovarian leiomyosarcoma (POLMS) and fibroma are mesenchymal tumors.
Fibroma represents 3–5% of all ovarian tumors, and its histogenesis
remains to be elucidated (4). The
majority of patients exhibiting ovarian fibroma are asymptomatic
due to the tumor's small size (4).
However, patients may experience abdominal pain, abdominal
enlargement or urinary symptoms. Furthermore, ascites is a common
associated finding and may be observed in half of all cases with
fibroma measuring >5 cm in diameter (4). When accompanied by ascites and pleural
effusion, ovarian fibroma develops into Meigs' syndrome, which
occurs in 1–3% of cases. To the best of our knowledge, only 72
examples of POLMS (including the present case) have been reported
(1,5–29). When
leiomyosarcoma is encountered in the ovary, it may be difficult to
diagnose (4); its treatment and
etiology remain complicated and controversial. In addition, the
prognosis of POLMS is unfavorable and >50% of patients succumb
to the disease following a mean time of 24 months (1).
To the best of our knowledge, the current report
presents the first case of concurrent POLMS and fibroma in a single
ovary in a premenopausal woman, and highlights the association
between malignant and benign ovarian tumors. A detailed literature
review concerning POLMS and fibroma is followed by the discussion
of several associated histogenetic and clinical issues. Written
informed consent was obtained from the patient's family for the
publication of this study.
Case report
Patient and diagnosis
The patient was a 46-year-old premenopausal woman
(gravida 1, para 1), who experienced regular menstrual cycles and
had no relevant family history. The patient was diagnosed with an
ovarian mass at Dalian Women and Children's Health Hospital
(Dalian, China) in September 2005. The size of the mass at that
time was 3 cm in diameter. As no symptoms associated with the
abdominal mass were present, the patient was not concerned and no
treatment was administered. During the following 5 years, the size
of the mass did not alter and the patient was in good health. On
November 5, 2010, the patient was admitted to Dalian Women and
Children's Health Hospital (Dalian, China) with the chief complaint
of lower abdominal pain, which had persisted for 4 days.
Gynecological examination revealed cervical hypertrophy, and a
uterus that was similar in size to that of a 40-day pregnant woman.
In addition, a palpated, firm, tender mass, with poor activity and
dimensions approximate to that of a duck egg, was observed to be
adherent to the left posterior uterus. Color Doppler ultrasound
(Medison 8000EX; Samsung Medison, Seoul, Korea) revealed two masses
in the left ovary. One hybrid echoic mass was irregular in shape,
and was encapsulated by a discontinuous membrane and filled with an
opaque dark area of fluid, which scattered a number of thick light
spots. Furthermore, color Doppler ultrasound imaging identified an
abundant blood supply and indicated a vascular resistance index
(RI) of 0.77, which suggested that the mass may have been benign
(17). The remaining solid mass was
regular in shape, and was encapsulated by a continuous membrane and
filled with a hypo-echoic area. The RI of this mass was 0.6. These
two masses measured 42×42×40 mm and 49×43×40 mm in size,
respectively. The endometrial thickness was 15 mm, which was
thicker than normal. Fractional curettage revealed no
abnormalities. Two uterine fibroids were detected, one measuring
21×21×19 mm located on the anterior wall of the uterus, and the
other 20×18×15 mm on the posterior wall.
Laboratory test results were all normal and were as
follows: Carcinoembryonic antigen, 1.98 ng/ml (normal range, 0–3.4
ng/ml), α-fetoprotein, <0.5 ng/ml (normal range, 0–5.8 ng/ml);
and cancer antigen 125, 27.73 U/ml (normal range, 0–35 U/ml).
On November 10, 2010, an exploratory laparoscopy was
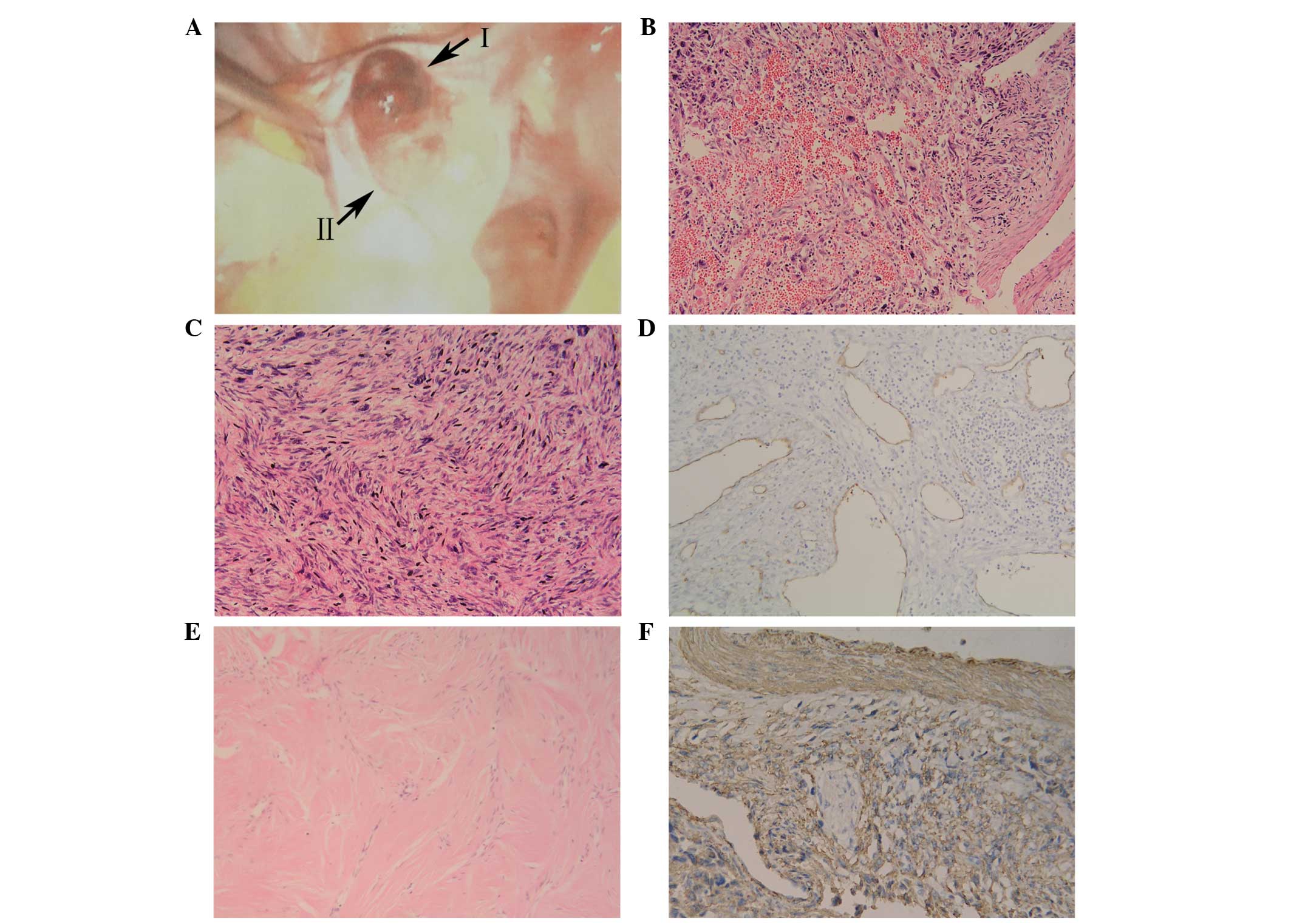
performed. An enlarged left ovary was observed, with a prominent
hemorrhagic smooth mass on the surface (Fig. 1A). The mass measured 5 cm in diameter
and exhibited no adherence to the surrounding tissue. In addition,
the right ovary was atrophied. Gross examination of the excised
left ovarian mass revealed a phyllodes tumor, which consisted of
two regions, measuring up to 75×45×40 mm. The first half of the
tumor (region I) was fragile and pale inside, and exhibited small
hemorrhagic areas and a fine texture. The remaining half (region
II), however, demonstrated a pale yellow interior, and was
resilient and firm. These two regions were partially connected to
each other by an encapsulated membrane. According to frozen
pathology, the phyllodes tumor was composed of spindle cells with
various shapes. Region II with regular shaped cells was confirmed
to be a fibroma (30). Region I
exhibited a number of deformed cells with nuclear fission
concentrated in certain zones, which was suspected to be malignant
transformation of the fibroma. As the patient's family members
refused to allow a staging laparotomy to be performed, a
hysterectomy and bilateral salpingo-oophorectomy were conducted.
The pelvis and greater omentum appeared grossly normal and no
ascites was present. The patient underwent an uneventful
post-operative course.
Pathology
Tumor tissues were sent to the Department of
Pathology, Dalian Women and Children's Health Hospital (Dalian,
China) for sectioning. Microscopic examination of region I of the
tumor revealed a number of infarct-type necrotic and hemorrhagic
areas (Fig. 1B), with pleomorphic and
fusiform cells (Fig. 1C).
Furthermore, the tumor exhibited areas of hypercellular and nuclear
pleomorphism, with up to 15 mitotic figures (MF) per 10 high-power
fields (HPF). In a number of the sections, medium-sized and smaller
blood vessels were encountered with thin muscle coats, and a total
absence of adventitial layers (Fig.
1D). As blood vessel walls exhibit intense immunostaining for
cluster of differentiation (CD)34, anti-CD34 antibody was used for
demonstrating blood vessels absence of adventitial layers.
Microscopic examination of region II of the tumor revealed short
spindle-shaped cells, with narrow or ovoid spindle-shaped nuclei.
The cells formed bundles that were frequently intersected by
hyalinized tissue. Mitotic activity was absent (Fig. 1E).
Tumor tissues were sent to the Department of
Pathology, Dalian Women and Children's Health Hospital (Dalian,
China), for immunohistochemical staining. Immunohistochemical
staining of region II of the tumor revealed smooth muscle actin
negativity, while region I exhibited positivity for vimentin, SMA
(Fig. 1F), desmin, caldesmon and p53,
and negative results for inhibin, Ki-67, progesterone receptor (PR)
and estrogen receptor (ER). The final pathology provided a
diagnosis of concurrent fibroma and stage Ic leiomyosarcoma in a
single ovary (FIGO staging) (31),
which was further confirmed by Peking Union Medical College
Hospital (Beijing, China).
Post-operative positron emission tomography/computed
tomography (PET/CT) observed no additional tumor foci remaining in
the patient's body, indicating that the leiomyosarcoma was POLMS.
The patient underwent a close follow-up subsequent to surgery, and
refused chemotherapy. A total of 13 months after the initial
surgery, PET/CT revealed multiple nodules in the pelvis.
Cytoreductive surgery was performed at Peking Union Medical College
Hospital so that no residual disease remained. The post-operative
pathological diagnosis confirmed recurrent POLMS. Subsequently, 4
cycles of adjuvant chemotherapy were performed. The first cycle was
docetaxel plus gemcitabine (90 mg docetaxel on day 1 and 1 g
gemcitabine on day 2; 21 days; one cycle), and the following 3
cycles consisted of gemcitabine alone due to the patient
demonstrating intolerance to docetaxel (total duration of 4 cycles
was 3 months). A total of 11 months after the second surgery,
PET/CT confirmed a relapse of nodules in the pulmonary organs, a
13-cm mass in the left kidney and a high signal intensity in
segment 4 of the liver. Cytoreductive surgery was conducted for a
second time, with the pulmonary nodules remaining.
Immunohistochemical staining following the surgery revealed total
negativity for CD117, epidermal growth factor receptor and human
epidermal growth factor receptor-2.
Genetic testing was performed at the Cancer
Institute and Hospital, Chinese Academy of Medical Sciences
(Beijing, China) using second-generation sequencing technology. The
result additionally indicated total negativity for vascular
endothelial growth factor (VEGF) receptor, B-raf V600E,
phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit α
codon 545 and echinoderm microtubule-associated protein-like
4-anaplastic lymphoma kinase confusing gene. Thus, no suitable
targeted therapy could be administered, and the patient's disease
gradually progressed. A total of 7 months after the third surgery,
the patient was administered 4 cycles of adjuvant chemotherapy with
epirubicin, ifosfamide and bevacizumab (50 mg epirubicin on days 1
and 2, 2 mg ifosfamide on days 1–5 and 6 mg bevacizumab on days 1
and 15; total duration of 4 cycles was 3 months); however, during
this time the tumor progressed rapidly. Several months later, the
tumor demonstrated extensive invasion. The patient underwent
interstitial brachytherapy and interventional therapy; however,
these treatment attempts proved ineffective. The patient received
supportive care until she succumbed in January 2015.
Discussion
POLMS and fibroma occupy a small number of all
ovarian neoplasms (32). To the best
of our knowledge, concurrent POLMS and fibroma in a single ovary
has not been previously reported. However, a number of cases have
referred to an association between POLMS and fibroma. Seracchioli
et al (5) reported the case of
a 20-year-old woman exhibiting POLMS, who had suffered from nevoid
basal cell carcinoma syndrome at ~1 year old. A total of 4 years
prior to the patient's POLMS excision, a number of fibromas
containing microcalcification were identified in the left ovary. A
total of 3 months after the POLMS excision, multiple small fibromas
were identified in the patient's remnant left ovary. In this case,
POLMS and fibroma were reported to exist in one ovary
asynchronously (5). Lerwill et
al (1) reported a case of
bilateral ovarian tumors with leiomyosarcoma in one ovary and
fibroma in the other. The 82-year-old female patient succumbed to
disease 10 months after the diagnosis. Seinera et al
(33) reported the case of a
25-year-old woman who exhibited multiple nodules in the bilateral
ovaries. Fibrous connective tissues were observable in the larger
tumor. Immunohistochemical analysis confirmed ovarian leiomyoma.
Additionally, Trabelsi et al (18) reported a case of myxoid ovarian
leiomyosarcoma, which was dissociated by fibrohyalinized tissue in
certain areas. Consequently, to the best of our knowledge, the
present case is the first case of concurrent POLMS and fibroma. In
order to investigate the association between these two ovarian
tumors, an overview of the tumors is followed by a detailed
discussion of the histogenesis.
Fibroma represents 3–5% of all ovarian tumors, and
primarily occurs at peri- and postmenopause (4). Fibromas are benign tumors, and the
median age of occurrence is reported to be ~52 years (4). Although certain patients are admitted to
hospital with abdominal pain, abdominal enlargement or urinary
symptoms, ovarian fibroma is typically asymptomatic. When
accompanied by ascites and pleural effusion, ovarian fibroma
develops into Meigs' syndrome (4),
which is observed in 1–3% of all ovarian fibromas. Meigs' syndrome
resolves following resection of the ovarian fibroma. The
histogenesis of ovarian fibroma remains to be elucidated, however,
mesenchymal cells of the ovarian stroma, fibrosed thecoma, Brenner
tumor, ovarian cortex, the walls of the blood and lymphatic vessels
are all potential origins (4,34).
POLMS has been reported in patients aged between 12
and 84 years (1,5–29). Among
the 72 reported cases, 69% of patients were >50 years old.
Although POLMS is typically regarded as being associated with
postmenopausal women (6,8,16), several
of the reported cases occurred during the pediatric period
(6,12). A total of 4 cases occurred in patients
<20 years, 3 of whom had suffered from medulloblastoma at the
age of 1 year. The details of the fourth patient are unknown
(1,5,6,12). The average age of occurrence for all
72 patients was 54.9 years.
Ovarian leiomyosarcoma typically has no significant
symptoms (14). However, a number of
patients were referred to the hospital with abdominal pain or
fullness, a few exhibited constipation and 1 patient exhibited
post-menopausal bleeding (27). Based
on all 72 reported cases of POLMS, it was not possible to conclude
any specific clinical manifestations.
The histogenesis of ovarian leiomyosarcoma remains
to be elucidated, and a number of hypothetical locations have been
proposed, including a totipotent ovarian mesenchyme (35), smooth muscle fibers of ovarian
ligaments (15), the vascular wall
(13,14), and the medulla and hilus (12). Ovarian leiomyosarcoma may additionally
have an origin in ovarian teratoma (35), preexisting serous cystadenoma and
papillary serous cystadenocarcinoma (18,35,36). It is
possible, however, that not all leiomyosarcomas of the ovary
originate in an identical manner or that a single leiomyosarcoma
may possess a number of origins.
Although the etiology of synchronous POLMS and
fibroma remains to be elucidated, the present study proposed that
their occurrence is not coincidental. As ovarian fibroma and
leiomyosarcoma are ovarian non-specific supporting mesenchymal
tissue tumors, their development may involve common carcinogenic
agents. Additionally, in a number of the pathological sections
investigated in the present study, medium-sized and smaller blood
vessels were observed, with thin muscle coats and a total absence
of adventitial layers. This suggested an identical origin for
ovarian fibroma and POLMS from smooth muscle fibers of the vascular
wall. Wellmann (13) published
similar findings. Following the third surgery, the present patient
accepted 3 cycles of bevacizumab, a recombinant humanized
monoclonal antibody that blocks angiogenesis by inhibiting VEGF-A.
However, no improvement was demonstrated. The genetic tests, which
demonstrated VEGF negativity, subsequently explained the
ineffectiveness of bevacizumab treatment.
In addition to the aforementioned discussion points,
compared with the former 71 cases, the present case merited
attention in additional aspects. Clinically, POLMS requires
differentiation with alternative ovarian sarcomas, tumors in other
areas of the body and metastasis to the ovary (1,5,8,37). Under
normal conditions, ultrasonic inspection is the preferred primary
diagnostic method due to its convenience. Based on the theory of
ultrasonics, a high impedance value (RI>0.6) reveals that a mass
is likely to be benign, while a low impedance value (RI<0.4)
reveals that a mass is likely to be malignant (17). However, as the echo texture of solid
pelvic tumors is typically non-specific, these RI criteria are not
completely accurate for the diagnosis of ovarian tumors. In the
present case, prior to surgery, color Doppler sonography identified
a mass with malignant features, while the RI value (0.77) indicated
a benign result. Additionally, Kuscu et al (17) noted that the RI of a previous ovarian
leiomyosarcoma case was 0.54, which was an intermediate value
lacking diagnostic weight. Thus, clinicians should be aware that
the RI value is not necessarily able to differentiate between
benign and malignant ovarian masses. By contrast, pathological
diagnosis has played a critical role in all reported cases of
ovarian leiomyosarcoma. The cell morphology may visually exhibit
the characteristics of malignant tumors. Lerwill et al
(1) additionally proposed that ≥5 MF
per 10 HPF in the presence of significant atypia is a guiding
principle for a diagnosis of leiomyosarcoma, even in the absence of
tumor cell necrosis (1,6). Furthermore, immunohistochemical staining
for ovarian leiomyosarcoma is typically positive for desmin and
muscle-specific actin, and negative for cytokeratins and S100
(1,11,36). Thus,
cell morphology, as well as mitosis criteria and
immunohistochemical staining, together establish the gold standard
for the diagnosis of ovarian leiomyosarcoma.
The effect of hormones on POLMS additionally
requires investigation (1,24,27,35,38–40).
The majority of patients exhibiting POLMS are postmenopausal,
implying that this tumor may be associated with relatively low
hormone levels. However, a number of previous cases have reported
that POLMS occurred in an environment with high levels of hormone.
Walts and Lichtenstein (35) reported
the case of a patient who received Premarin (conjugated estrogens;
Ayerst Laboratories, New York, NY, USA) for 5 years prior to her
POLMS diagnosis. Bouie et al (24) reported the case of a patient who was
undergoing ovulation induction at the time of POLMS diagnosis.
Lerwill et al (1) identified
that several POLMS cases in their study progressed rapidly during
pregnancy. An additional study reported the case of a woman who was
diagnosed with POLMS following admittance to hospital with
post-menopausal bleeding (27). In
the present case, the patient exhibited regular menstrual cycles
when diagnosed with POLMS. The patient demonstrated an apparent
right atrophic ovary and a thickened endometrium. This may indicate
that the patient's left ovary was secreting hormone at an
abnormally high level. In order to identify an appropriate therapy,
additional immunohistochemical tests were conducted, revealing an
almost totally negative outcome for PR and ER, which may suggest
that this POLMS case was hormone-independent, which is different
compared with the aforementioned cases. In conclusion, the effect
of hormones on POLMS requires additional investigation in future
studies.
Cases of POLMS are so rare that no recommended
guidelines on optimal treatment have been established (8). Based on previously reported cases,
surgery is the preferred treatment choice (1,5). In the
present case, although POLMS was removed at an early stage (Ic), as
well as being relatively small in size (3 cm in diameter), and a
series of elaborately designed therapies was conducted, the patient
demonstrated a negative prognosis. It is hypothesized that this
poor prognosis was due to the co-occurrence of POLMS and fibroma,
which increased the uncertainty of treatment and therapeutic
effects. Although no clear causal association has been established
between POLMS and ovarian fibroma, such an observation may lead to
a future research focus investigating synchronous benign and
malignant tumors.
Glossary
Abbreviations
Abbreviations:
|
POLMS
|
primary ovarian leiomyosarcoma
|
|
RI
|
resistance index
|
|
PR
|
progesterone receptor
|
|
ER
|
estrogen receptor
|
|
PET/CT
|
positron emission tomography/computed
tomography
|
References
|
1
|
Lerwill MF, Sung R, Oliva E, Prat J and
Young RH: Smooth muscle tumors of the ovary: A clinicopathologic
study of 54 cases emphasizing prognostic criteria, histologic
variants, and differential diagnosis. Am J Surg Pathol.
28:1436–1451. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamamoto Y, Shomori K, Nosaka K, Haruki T,
Teshima R and Ito H: Prognostic significance of Minichromosome
maintenance protein 7 and Geminin expression in patients with 109
soft tissue sarcomas. Oncol Lett. 1:703–709. 2010.PubMed/NCBI
|
|
3
|
Fukuda T, Sumi T, Nakano Y, Morishita M,
Nobeyama H, Yoshida H, Matsumoto Y, Yasui T, Honda KI and Ishiko O:
Extragastrointestinal stromal tumor originating from the vulva.
Oncol Lett. 2:797–799. 2011.PubMed/NCBI
|
|
4
|
Talerman A and Path FRC: Nonspecific
tumors of the ovary, including mesenchymal tumors and malignant
lymphoma. Blaustein's Pathology of the Female Genital Tract. Kurman
RJ: (3rd). Springer. (New York, NY). 722–741. 1987. View Article : Google Scholar
|
|
5
|
Seracchioli R, Colombo FM, Bagnoli A,
Trengia V and Venturoli S: Primary ovarian leiomyosarcoma as a new
component in the nevoid basal cell carcinoma syndrome: A case
report. Am J Obstet Gynecol. 188:1093–1095. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Monk BJ, Nieberg R and Berek JS: Primary
leiomyosarcoma of the ovary in a perimenarchal female. Gynecol
Oncol. 48:389–393. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mayerhofer K, Lozanov P, Bodner K,
Bodner-Adler B, Mayerhofer-Gallenbacher N, Hudelist G and Czerwenka
K: Immunohistochemical analysis of a primary ovarian
leiomyosarcoma. Case report. Anticancer Res. 23:3433–3436.
2003.PubMed/NCBI
|
|
8
|
Rasmussen CC, Skilling JS, Sorosky JI,
Lager DJ and Buller RE: Stage IIIC ovarian leiomyosarcoma in a
premenopausal woman with multiple recurrences: Prolonged survival
with surgical therapy. Gynecol Oncol. 66:519–525. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anderson AE, Yang X and Young RH:
Epithelioid angiomyolipoma of the ovary: A case report and
literature review. Int J Gynecol Pathol. 21:69–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dixit S, Singhal S, Baboo HA, Vyas RK,
Neema JP, Murthy R and Sooryanaraya U: Leiomyosarcoma of the ovary.
J Postgrad Med. 39:151–153. 1993.PubMed/NCBI
|
|
11
|
Taşkin S, Taşkin EA, Uzüm N, Ataoğlu O and
Ortaç F: Primary ovarian leiomyosarcoma: A review of the clinical
and immunohistochemical features of the rare tumor. Obstet Gynecol
Surv. 62:480–486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Sullivan SG, Das Narla L and Ferraro E:
Primary ovarian leiomyosarcoma in an adolescent following radiation
for medulloblastoma. Pediatr Radiol. 28:468–470. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wellmann KF: Leiomyoma of the ovary:
Report of an unusual case and review of the literature. Can Med
Assoc J. 85:429–432. 1961.PubMed/NCBI
|
|
14
|
Kobayashi Y, Murakami R, Sugizaki K,
Yamamoto K, Sasaki S, Tajima N, Tajima H, Onda M and Kumazaki T:
Primary leiomyoma of the ovary: A case report. Eur Radiol.
8:1444–1446. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nasu M, Inoue J, Matsui M, Minoura S and
Matsubara O: Ovarian leiomyosarcoma: An autopsy case report. Pathol
Int. 50:162–165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bodner K, Bodner-Adler B, Czerwenka K,
Hudelist G, Kimberger O, Leodolter S and Mayerhofer K: Bcl-2
expression in a primary leiomyosarcoma of the ovary: A case report.
Wien Klin Wochenschr. 115:191–195. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuscu E, Erkanli S, Haberal A, Ergin T,
Ozdemir H and Demirhan B: Primary ovarian leiomyosarcoma: A case
report. Eur J Gynaecol Oncol. 26:120–122. 2004.
|
|
18
|
Trabelsi A, Mutijima E, El Hossini Soua A,
Gassoumi M, Bouguizane S, Mokni M, Yacoubi MT and Korbi S: Primary
myxoid leiomyosarcoma of the ovary. A case report with review of
the literature. Tunis Med. 83:288–291. 2005.PubMed/NCBI
|
|
19
|
Dobbs SP, Brown LJ, Hollingworth J and
Ireland D: Surgical treatment of recurrent primary ovarian
leiomyosarcoma. A case report. Eur J Gynaecol Oncol. 20:172–173.
1999.PubMed/NCBI
|
|
20
|
Arslan OS, Sumer C, Cihangiroglu G,
Kanat-Pektas M and Gungor T: A rare tumor of the female genital
tract: Primary ovarian leiomyosarcoma. Arch Gynecol Obstet.
283(Suppl 1): S83–S85. 2011. View Article : Google Scholar
|
|
21
|
Kurian RR, Preethi J and Remadevi A:
Leiomyosarcoma of ovary - a case report. Indian J Pathol Microbiol.
48:19–20. 2005.PubMed/NCBI
|
|
22
|
Piura B, Rabinovich A, Yanai-Inbar I,
Cohen Y and Glezerman M: Primary sarcoma of the ovary: Report of
five cases and review of the literature. Eur J Gynaecol Oncol.
19:257–261. 1998.PubMed/NCBI
|
|
23
|
Inoue J, Gomibuchi H and Minoura S: A case
of a primary ovarian leiomyosarcoma. J Obstet Gynaecol Res.
26:401–407. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bouie SM, Cracchiolo B and Heller D:
Epithelioid leiomyosarcoma of the ovary. Gynecol Oncol. 97:697–699.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Divya Ns and Srinivasamurthy V: Myxoid
leiomyosarcoma of ovary - a rare case report. J Clin Diagn Res.
8:FD05–FD06. 2014.
|
|
26
|
Zygouris D, Androutsopoulos G, Grigoriadis
C, Arnogiannaki N and Terzakis E: Primary ovarian leiomyosarcoma.
Eur J Gynaecol Oncol. 33:331–333. 2012.PubMed/NCBI
|
|
27
|
Kaur J, Mishra M and Goel B: Primary
ovarian leiomyosarcoma: A case report with review. Int J Reprod
Contracept Obstet Gynecol. 3:258–260. 2014. View Article : Google Scholar
|
|
28
|
Khabir A, Boudawara T, Ayadi L, Kharrat M,
Beyrouti I and Jlidi R: Epithelioid bilateral ovarian
leiomyosarcoma: A study. Ann Pathol. 23:47–49. 2003.(In French).
PubMed/NCBI
|
|
29
|
Nogales FF, Ayala A, Ruiz-Avila I and
Sirvent JJ: Myxoid leiomyosarcoma of the ovary: Analysis of three
cases. Hum Pathol. 22:1268–1273. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mackenzie DH: Fibroma: A dangerous
diagnosis. A review of 205 cases of fibrosarcoma of soft tissues.
Br J Surg. 51:607–612. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eluck Petru HJ, Stuart G, Gaffney D,
Millan D and Vergote I: Gynecologic Cancer Intergroup (GCIG)
proposals for changes of the current FIGO staging system. European
Journal of Obstetrics Gynecology & Reproductive Biology.
143:69–74. 2009. View Article : Google Scholar
|
|
32
|
Azoury RS and Woodruff JD: Primary ovarian
sarcomas: Report of 43 cases from the Emil Novak Ovarian Tumor
Registry. Obstet Gynecol. 37:920–941. 1971.PubMed/NCBI
|
|
33
|
Seinera P, Raspollini M, Privitera S,
Farina C and Crana F: Bilateral ovarian leiomyoma. Acta Obstet
Gynecol Scand. 76:488–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sivanesaratnam V, Dutta R and Jayalakshmi
P: Ovarian fibroma - clinical and histopathological
characteristics. Int J Gynaecol Obstet. 33:243–247. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Walts AE and Lichtenstein I: Primary
leiomyosarcoma associated with serous cystadenocarcinoma of the
ovary. Gynecol Oncol. 5:81–86. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lastarria D, Sachdev RK, Babury RA, Yu HM
and Nuovo GJ: Immunohistochemical analysis for desmin in normal and
neoplastic ovarian stromal tissue. Arch Pathol Lab Med.
114:502–505. 1990.PubMed/NCBI
|
|
37
|
Young RH and Scully RE: Sarcomas
metastatic to the ovary: A report of 21 cases. Int J Gynecol
Pathol. 9:231–252. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brinton LA, Lamb EJ, Moghissi KS, Scoccia
B, Althuis MD, Mabie JE and Westhoff CL: Ovarian cancer risk after
the use of ovulation-stimulating drugs. Obstet Gynecol.
103:1194–1203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rossing MA, Daling JR, Weiss NS, Moore DE
and Self SG: Ovarian tumors in a cohort of infertile women. New
Engl J Med. 331:771–776. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Venn A, Jones P, Quinn M and Healy D:
Characteristics of ovarian and uterine cancers in a cohort of in
vitro fertilization patients. Gynecol Oncol. 82:64–68. 2001.
View Article : Google Scholar : PubMed/NCBI
|