Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common type of cancer and the third cause of mortality induced by
tumors in the world (1). China has
the highest HCC prevalence in the world, and about 2/3 of all novel
HCC cases occur there (2). Surgical
resection is accepted as the preferred treatment method for early
stage HCC, but a great proportion of patients are in the advanced
stage when diagnosed and therefore miss the opportunity (3). Surgical treatment for these patients is
associated with high recurrence and metastasis rates, so the 5-year
survival rate is <50% (4).
Currently, various anti-tumor agents including Sorafenib,
Doxorubicin, cis-platinum and 5-fluorouracil have been used to
treat HCC, which only increase patient survival by ~2-3 months and
the severe toxic effects greatly affect patient's life quality
(5,6)
HCC's exact mechanisms remain unclear at present. Current knowledge
suggests that HCC development is a multi-step process involving
multiple oncogenes and tumor suppressor genes. Previous studies
have demonstrated that abnormal tumor suppressor gene promoter
methylation could inactivate the corresponding genes and thus
promote tumor development and progression (7,8). The GSTP1
gene is a very important DNA damage repair gene that can inhibit
the effects of cytotoxic agents and carcinogens and is regarded as
a tumor suppressor gene (9). Previous
studies have also demonstrated that hypermethylation of the CpG
islands in the GSTP1 gene promoter is involved in the development
and progression of multiple tumors, and thus could be used as a
promising biomarker to help early screening, diagnosis, and patient
prognosis (10–12).
DNA methylation of tumor suppressor genes is a
reversible process. Reversing abnormal methylation to restore the
normal corresponding gene expression has been accepted as a novel
method to treat tumors (13,14). Methyltransferase inhibitors, including
5-aza-2-deoxycytidine (Aza), may reverse abnormal tumor suppressor
gene methylation to restore gene expression and thus inhibit tumor
growth. A number of clinical studies used 5-Aza to treat leukaemia
and myelodysplastic syndrome (15).
Although several previous studies demonstrated that nucleosides
like 5-Aza could restore HCC-related gene expression and inhibit
solid tumor cell growth, the severe side effects and lack of a safe
and effective dose have restricted investigation in clinical
studies (16). Identifying effective
and less toxic demethylation agents is now a priority. Elemene is a
traditional Chinese medicine (TCM) extracted from Curcuma
zedoaria. As a non-cytotoxic anti-tumor agent, elemene has
minor side effects but may inhibit tumor cell proliferation, induce
apoptosis and differentiation, eliminate tumor cells, reverse
multidrug resistance, and inhibit tumor metastasis, especially in
HCC (17–19). Elemene's anti-tumor effects are very
similar to tumor suppressor genes, but to the best of our
knowledge, no previous study has investigated whether it could
reverse tumor suppressor gene methylation and thus restore gene
activity. In the present study, cultured HCC cell lines QGY7703
were treated with different elemene concentrations and the cell
viability, apoptosis, and cell cycle were measured. GSTP1 gene
methylation was also measured prior to and following treatment to
investigate whether elemene inhibits or reverses the abnormal tumor
suppressor gene methylation and the mechanisms involved in the
anti-tumor effects.
Materials and methods
Materials
The HCC cell line QGY7703 was a gift from the cell
bank of Suzhou University (Suzhou, China). Elemene injection was
purchased from Hualijingang Pharmaceutical Co., LTD (Dalian,
China), 5-Aza and MTT were purchased from Sigma-Aldrich (St. Louis,
MO, USA), Annexin V-FITC apoptosis kits were purchased from
Biyuntian Biotechnology Co., Ltd. (Shanghai, China), genomic DNA
extraction kits were purchased from QIAGEN Biotechnology Co., Ltd.
(Shanghai, China), EZ DNA Methylation-Direct Kit TM was purchased
from ZYMO RESEARCH (USA), primers were purchased from Jierui
Biotechnology Co., Ltd. (Shanghai, China), PCR Mixture 2xMix
(BS-PCR002) was purchased from Bio-serve Company (Shanghai, China),
and 100 bp DNA Marker was purchased from Takara Company
(Japan).
Beckman Coulter Epics XL flow cytometer was from
Beckman Coulter, Inc. (Brea, CA, USA), PCR Thermocycle Instrument
(PTC200) was from MJ Research, Inc. (Waltham, MA, USA), Ultraviolet
Spectrometry Photometer (NanoDrop2000) was from Thermo Fisher
Scientific (Waltham, MA, USA), Gel Imaging System (JS-3000) was
from Peiqing Technology Ltd. (Shanghai, China), and Multi-function
Electrophoresis System (PowerBC-6002S1) was from Shennengbocai
Biotechnology Co., Ltd. (Shanghai, China).
Cell proliferation inhibition
Cell proliferation inhibition induced by elemene was
measured by MTT assay. In brief, QGY7703 cells in logarthmic growth
phase were harvested and suspended, and the cell density was
adjusted to 5×104/ml with plate count method (20). A total of 200 ml suspension was
dispensed into each well of a 96-well plate and cultured for 24 h
to allow adhesion. Then, the culture medium was discarded,
elemene-containing culture medium was added, and the elemene
concentration was adjusted to 20, 40, 80, and 160 µg/ml. For each
concentration, 6 wells of cells were exposed. The final culture
medium volume in each was not >200 µl. After the cells were
cultured for 48 h, 20 µl MTT was added and incubated for 4 h, and
then the supernatant was removed. A total of 150 µl DMSO was then
added into each well, and the plate was oscillated on a shaker at
low speed for 10 min to allow the crystals to dissolve. The
absorbance (A) of each well was measured by ELISA Reader/Microplate
Reader DR-200Bs (Huawen Machinery & Electronics Co., Wuxi,
China). at 570 nm. Cell proliferation inhibition rate (%) was
calculated as: (A in treatment group-A in control group)/(A in
control group-A in blank control)x100.
QGY7703 cell apoptosis induced by
elemene
QGY7703 cells were seeded into a 6-well plate at a
density of 1×105 cells/ml, then complete DMEM was added
until the final volume was 3 ml. Then, elemene-containing culture
medium was added and the final elemene concentration was adjusted
to 10, 20, or 30 µg/ml A blank control containing no elemene was
also used. The cells were harvested, 50,000–100,000 suspended cells
were centrifuged at 1,000 × g for 5 min, the supernatant was
discarded, and 195 µl of Annexin V-FITC binding buffer was added to
resuspend the cells (Annexin V-FITC Apoptosis Detection Kit C1062;
Biyuntian Biotechnology Co., Ltd.). Then, 5 µl of Annexin V-FITC
was added, and the cells were incubated at 20–25°C in the dark for
10 min. The cells were then centrifuged at 1000 × g for 5 min, the
supernatant was discarded, and 190 µl of Annexin V-FITC binding
buffer was added to resuspend the cells. Propidium iodide (PI) (10
µl; Annexin V-FITC Apoptosis Detection Kit C1062; Biyuntian
Biotechnology Co., Ltd.) was added and mixed gently, and the cells
were put on ice for 10 min in darkness. Cell apoptosis was measured
using a Beckman Coulter Epics XL flow cytometer (Beckman Coulter,
Inc., Brea, CA, USA) and FlowJo 7.6.3 software (FlowJo, LLC,
Ashland, OR, USA).
Cell cycle measurement
QGY7703 cells were harvested and 50,000–100,000
suspended cells were centrifuged at 1000 × g for 5 min, the
supernatant was discarded, and 70% ice-cold ethanol was used to fix
the cells overnight (over 12 h) at 4°C. The cells were then
centrifuged at 1000 × g for 5 min to remove the ethanol. After the
cells were washed gently with precooled (4°C) PBS twice, 0.5 ml of
precooled PBS was used to resuspend the cells. Then, 5 µl RNAse A
(10 mg/ml, final concentration: 100 µg/ml; Qiagen19101 RNaseA;
Qiagen GmbH, Hilden, Germany) was added to each well, and the cells
were incubated at 37°C in darkness for 30 min, after which 50 µg/ml
PI was added and incubated with the cells at 4°C in darkness for 30
min. The cell cycle was measured using a Beckman Coulter Epics XL
flow cytometer. Each experiment was performed in triplicate.
GSTP1 gene methylation
measurement
QGY7703 cells in the logarithmic growth phase were
collected, and elemene was added to obtain a final concentration of
0, 40, 80, or 160 µg/m. DNA extracting kits (QIAGEN) were used
according to instruction. Bisulfite conversion of the extracted DNA
was then performed to convert the unmethylated C in CpG into U,
while methylated C was not converted. Then, methylation specific
polymerase chain reaction (MSP) was performed to measure DNA
methylation. All these processes were performed in strict
accordance with kit instructions.
MSP included the PCR of methylated primer sequences
and unmethylated primer sequences. In the present study, primers
were designed to amplify CpG rich regions using online software,
MethPrimer (www.urogene.org/methprimer/). Two primer types,
methylated (M) and unmethylated (U) primers, were used for the
amplification. The primer sequences and PCR conditions are listed
in Table I. The PCR conditions were
as follows: pre-denaturation at 94°C for 3 min, denaturation at
94°C for 30 sec, annealing at 61°C for 30 sec, and extension at
72°C for 30 sec. After 40 cycles were completed, an additional
extension at 72°C for 7 min was performed before the PCR was
completed. A 30 µl reaction volume was used, which included PCR
Mixture 2xMix 15 µl, U or M-Primer F 0.5 µl (10 µM), U or M-Primer
R 0.5 µl (10 µM), Modified DNA 1–5 µl, and ddH2O. No DNA
but ddH2O was added for blank control, while QGY7703
cells treated with 5-Aza were used as a positive control. Agarose
gel electrophoresis (2%; catalog no., 111860; Biowest SAS, Nuaillé,
France) was performed for all amplicons at 120 V for 30 min, and a
gel image analysis system (JC-300; Shanghai Peiqing Science and
Technology Co., Ltd, Shanghai, China) was used to analyze the
results under ultraviolet excitation.
 | Table I.Primers used for GSTP1 gene
methylation-specific polymerase chain reaction. |
Table I.
Primers used for GSTP1 gene
methylation-specific polymerase chain reaction.
| Gene | M/U | Sequences of the
primers (5′-3′) | Annealing
temperature | Product length |
|---|
| GSTP1 | M |
(F)-TTCGGGGTGTAGCGGTCGTC | 61°C | 91 bp |
|
|
|
(R)-GCCCCAATACTAAATCACGACG |
|
|
|
| U |
(F)-GATGTTTGGGGTGTAGTGGTTGTT | 55°C | 91 bp |
|
|
|
(R)-CCACCCCAATACTAAATCACAACA |
|
|
Statistical analysis
SPSS software, version 15.0 (SPSS, Inc., Chicago,
IL, USA) was used for the statistical analysis. Quantitative data
with equal variances were described as means and standard divisions
(SDs). Independent t-test was used to compare means between 2
groups, while one-way analysis of variances (one-way ANOVA) was
used to compare means among 3 or more groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
QGY7703 proliferation inhibition by
elemene
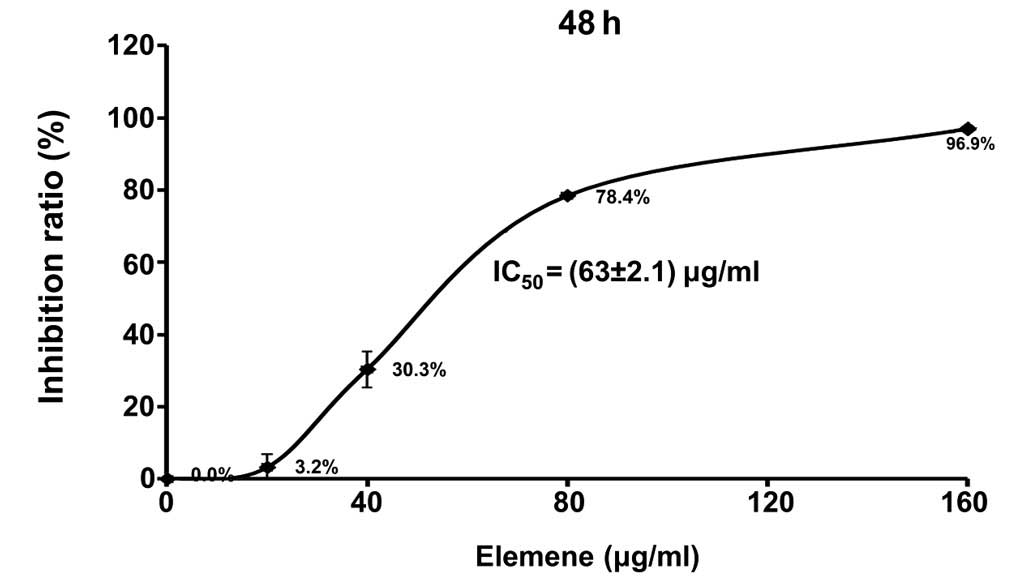
After being treated with elemene for 48 h, an MTT
assay was used to evaluate adhesive QGY7703 cell viability. As
shown in Table II and Fig. 1, elemene significantly inhibited the
proliferation rate of QGY7703 cells, in a dose-dependent manner
(P<0.01). The inhibitive effect increased with elemene
concentration, and the IC50 of elemene was determined as
63±2.1 µg/ml by linear regression.
 | Table II.Elemene's effects on QGY7703 cell
proliferation (n=3, mean ± SD). |
Table II.
Elemene's effects on QGY7703 cell
proliferation (n=3, mean ± SD).
| Group (ug/ml) | A-value | Inhibition rate
(%) |
|---|
| 0 | 0.47±0.038 | 0 |
| 20 | 0.46±0.035 | 3.2 |
| 40 |
0.33±0.030b | 30.3 |
| 80 |
0.10±0.111b | 78.4 |
| 160 |
0.01±0.007b | 96.9 |
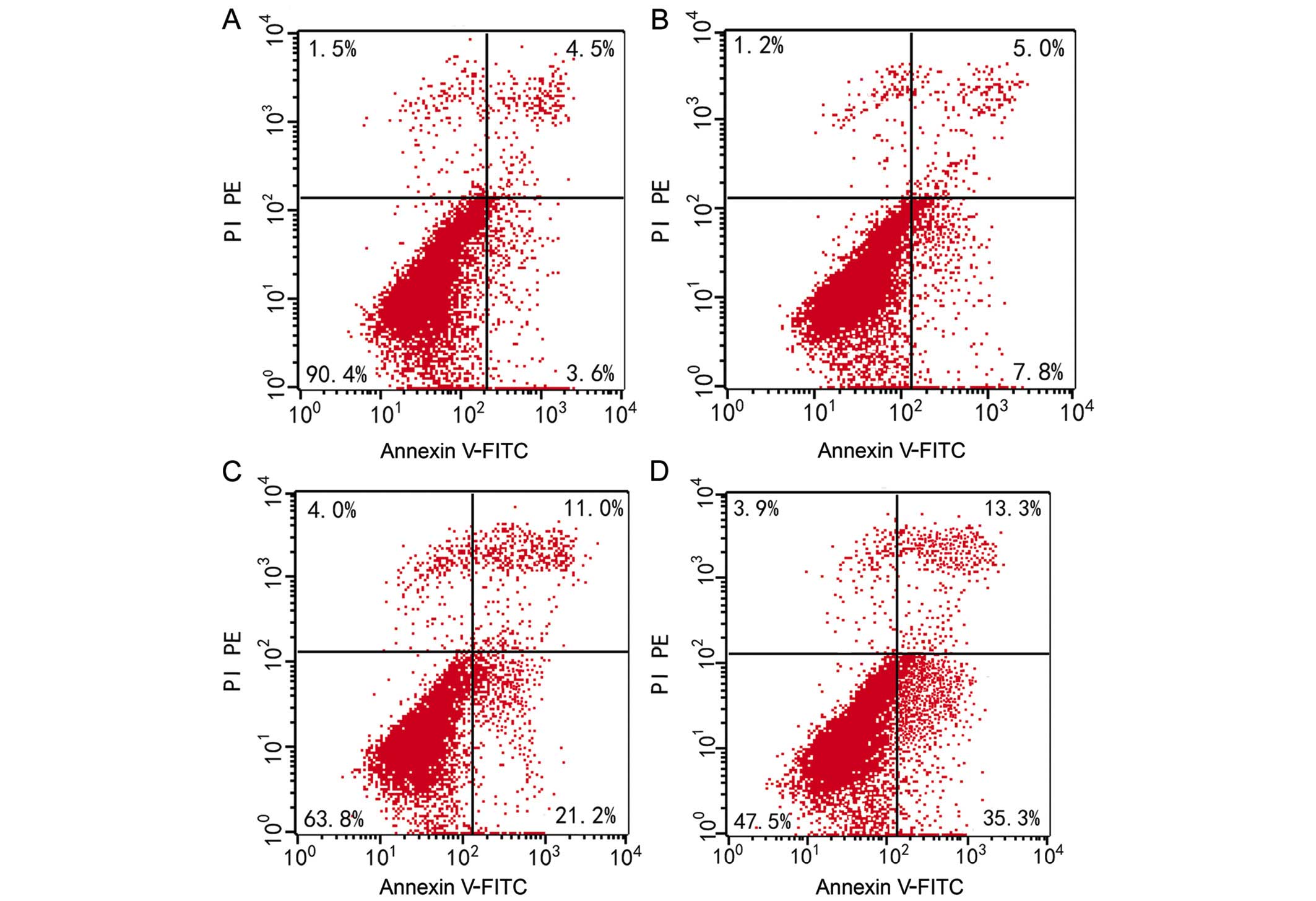
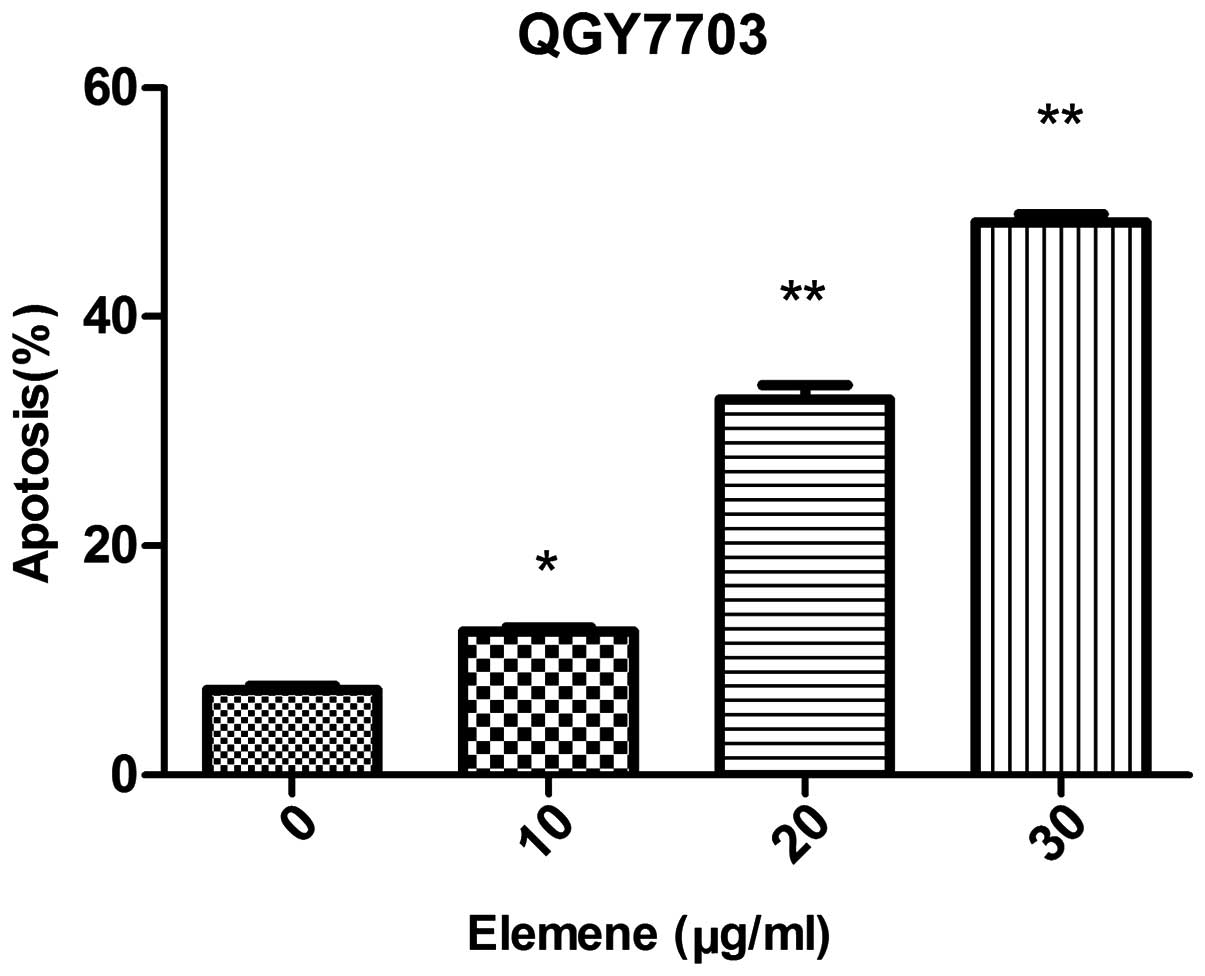
Cell apoptosis
The apoptosis rate was 7.40±0.66% for QGY7703 cells
prior to elemene treatment. Following treatment with different
elemene concentrations, dose-dependent apoptosis rate increases
were found. The apoptosis rate was 12.50±0.61%, 32.77±2.30%, and
48.27±1.23% for cells treated with 10, 20, and 30 µg/ml of elemene,
respectively, and the difference was statistically significant when
compared with the control group (10 µg/ml, P<0.05; 20 and 30
µg/ml, P<0.01) (Table III,
Figs. 2 and 3).
 | Table III.QGY7703 cell apoptosis rates (n=3,
mean ± SD). |
Table III.
QGY7703 cell apoptosis rates (n=3,
mean ± SD).
|
| Different
concentrations of elemene (µg/ml) |
|---|
|
|
|
|---|
| Apoptosis rate
(%) | 0 | 10 | 20 | 30 |
|---|
| Early apoptosis
rate | 3.37±0.17 | 7.37±0.37 | 21.60±1.10 | 36.20±0.79 |
| Necrosis and late
apoptosis rate | 4.03±0.50 | 5.13±0.32 | 11.17±0.96 | 12.07±1.25 |
| Overall apoptosis
rate | 7.40±0.66 |
12.50±0.61a |
32.77±2.30b |
48.27±1.23b |
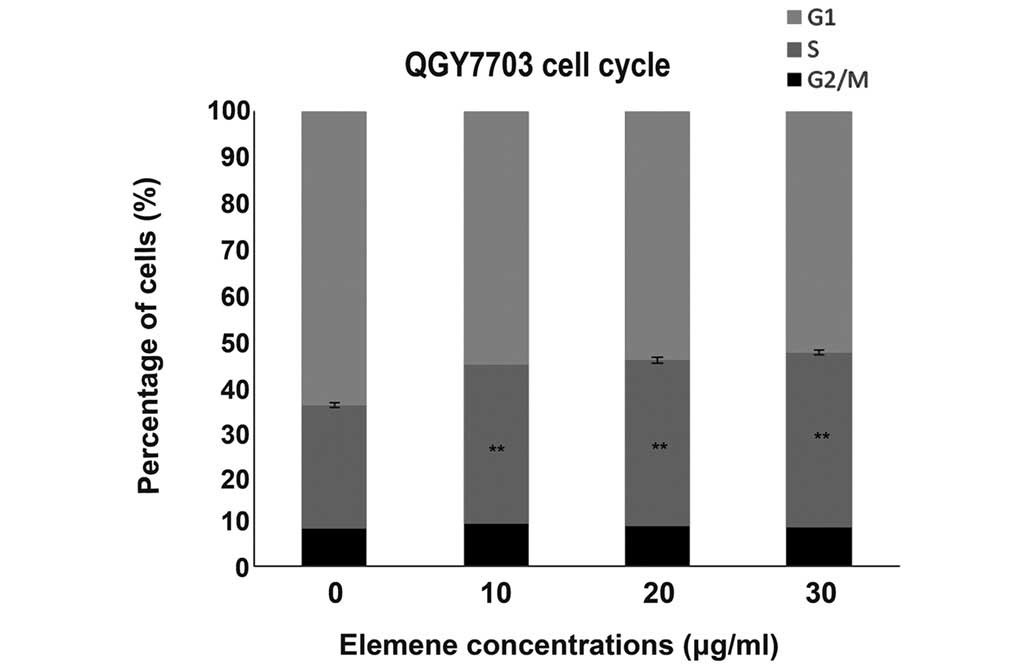
QGY7703 cell cycles after elemene
treatment
After being treated with 10, 20, and 30 µg/ml of
elemene for 48 h (Table IV, Fig. 4), the cells in the G1 phase reduced
gradually with elemene concentration, while cells in the S phase
increased gradually, indicating elemene arrested the cells in S
phase. The differences between the treated groups and control group
were statistically significant (P<0.01).
 | Table IV.QGY7703 cell cycle after being
treated with elemene for 48 h (n=3, mean ± SD). |
Table IV.
QGY7703 cell cycle after being
treated with elemene for 48 h (n=3, mean ± SD).
|
| Different
concentrations of elemene (ug/ml) |
|---|
|
|
|
|---|
| Cell cycle | 0 | 10 | 20 | 30 |
|---|
|
G2/M | 8.39±0.08 | 9.45±0.08 | 8.77±0.73 | 8.57±0.49 |
| S | 27.04±0.45 | 35.05±0.32 | 36.57±0.57 | 38.43±0.60 |
| G1 | 65.56±0.41 | 55.53±0.13 | 54.66±0.33 | 53.00±0.15 |
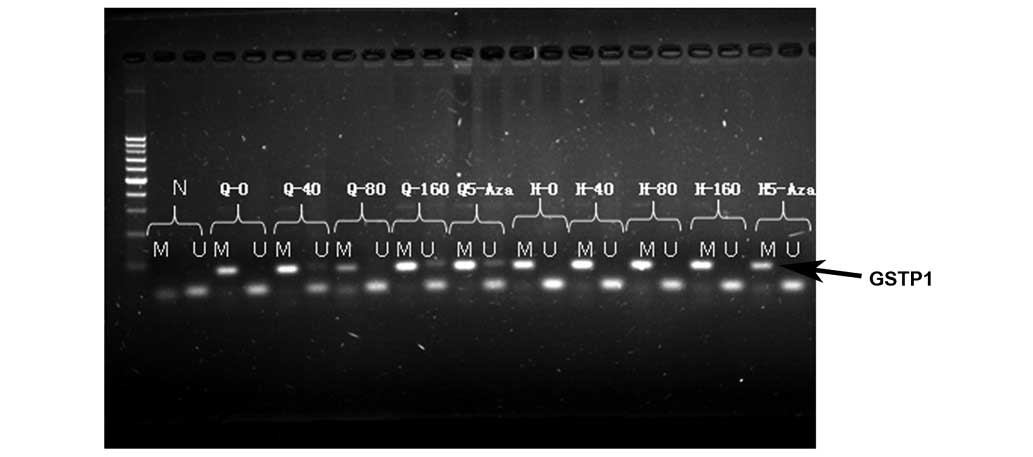
GSTP1 gene methylation in QGY7703
cells
All GSTP1 genes in untreated QGY7703 cells were
found to be methylated, but after being treated with 40 µg/ml or
160 µg/ml of elemene or 10 µmol/l of 5-Aza-dc, unmethylated GSTP1
genes were observed (Fig. 5, black
arrow).
Discussion
Elemene injection with a major β-elemene component
was approved as a second line of anticancer drug for clinical use
in China in 1995 (21). The major
characteristics that distinguish elemene, an effective TCM
extraction, from other anticancer drugs is that its cytotoxic
effects against non-cancerous normal cells are minimal (21). Previous studies have shown that
elemene is effective for liver (22),
ovarian (23), gastric (24), pulmonary (25), and breast (26) cancers and lymphosarcoma, particularly
for patients with malignant pleural effusion and ascites (27). Elemene's major anti-tumor mechanisms
include tumor cell proliferation inhibition, tumor cell
elimination, cell apoptosis and differentiation induction,
multidrug resistance reversion, tumor metastasis inhibition, and
immunity up-regulation (17–19). Recently, several studies investigated
elemene's anti-tumor mechanisms at the molecular and gene levels
(28,29). However, to the best of our knowledge,
no previous study has investigated whether elemene reverses tumor
suppressor gene methylation and thus restores gene activity.
In the present study, an MTT assay was used to
investigate the effects of different elemene concentrations on
QGY7703 cell proliferation. After being treated with 20, 40, 80,
and 160 µg/ml of elemene for 48 h, the results showed that elemene
could significantly inhibit QGY7703 cell proliferation. Further
analysis showed that the inhibition was dose-dependent, and the
differences between each treatment group and the control group were
statistically significant (P<0.05). The IC50 was 63
µg/ml. Flow cytometry was used to investigate elemene's effects on
the cell cycle, and it was determined that elemene could
effectively inhibit cells entering the G2/M phase, in accordance
with the findings of Yang et al (30). In Yang's study, elemene could inhibit
HL-60 and K562 cell lines entering the G2/M phase. The cell cycle
arrest may be associated with intracellular free calcium ion
concentration changes, immunoprophylatic effects, and P53 and Bcl-2
inhibition. In a study by Lee et al (23), the authors found that elemene could
significantly inhibit the A2780 cell line growth and arrest cells
in the G2 phase, which could be associated with the down-regulation
of cyclin-dependent kinases (including CDC2, cyclin A, and cyclin
B1). Another study also showed that elemene could inhibit malignant
glioma cell line entering G1 phase from G0 phase (31). These findings showed that elemene
could inhibit the proliferation of multiple tumor cells. However,
the cells were found arrested in different phases, which could
result from the different mechanisms involved in different tumor
cells.
The present study also investigated elemene's
effects on cell apoptosis in QGY7703 cells, and the results showed
that it could significantly induce and may promote early apoptosis,
and the effects were dose-dependent. Dai et al (32) treated HepG2 cells with
elemene and found that it could significantly inhibit cell
proliferation, promote cell apoptosis, and upregulate Fas/Fasl
protein expression, and thus supposed that the apoptosis induced by
elemene could be associated with Fas/Fasl. Other studies have also
found that elemene could induce tumor cell apoptosis in pulmonary
cancer, laryngeal cancer, leukemia, and glioma (33–36). The
mechanisms involved in the apoptosis induction effects could be as
follows: Influencing the expression of oncogenes and tumor
suppressor genes, influencing MAPK/ERK and PI3K/Akt/mTOR signaling
pathways, activating Caspase cascade, inducing mitochondrial
damages, inducing oxidative damages, inhibiting telomerase
activity, and altering intracellular Ca2+ concentration.
These findings indicated that the pathway involved in elemene's
apoptosis induction effects in different tumor cells could be
different. Elemene may induce cell apoptosis by regulating various
signaling pathways.
The present study further investigated GSTP1 gene
methylation in QGY7703 cells treated with different elemene
concentrations and compared the results with the untreated cells.
The results demonstrated that all the GSTP1 genes in the untreated
QGY7703 cells were methylated. However, after treatment with
elemene, unmethylated GSTP1 genes were found in the QGY7703 cells.
The GSTP1 gene is located at q13 of human chromosome 11 and encoded
an enzyme with detoxicating and protein-binding effects (37). The GSTP1 protein's main function is to
catalyze the reactions between glutathione and electron-containing
compounds, which could help metabolize carcinogens and exogenous
drugs into low- or non-toxic metabolites and thus exert
anti-cytotoxic and anti-carcinogen effects (37). Several previous studies showed that
GSTP1 inactivation induced by hypermethylation is mainly found in
several human tumors, including prostate, renal, breast, and liver
cancers (10–12). Tchou et al (38) found that GSTP1 in HCC tissues and cell
lines were hypermethylated, with the rate of methylation of 85%.
Additionally, GSTP1 protein levels reduced significantly, and its
absence was found in 90% of the tissues or cells. In our previous
studies, the GSTP1 gene methylation was investigated in 35 liver
cancer tissues and adjacent tissues, as well as in 20 normal liver
tissues (Wu et al, unpublished data). In that study, the
positive methylation rate was 57.1% in liver cancer tissues, which
was significantly higher than in the adjacent tissues (25.7%,
P<0.01). However, no methylation was observed in normal liver
tissues, suggesting that GSTP1 expression is highest in normal
liver tissues and lowest in liver cancer tissues. In the present
study, GSTP1 in the HCC cell line was completely methylated (100%),
which further confirmed that GSTP1 methylation could be involved in
HCC's development and progression. GSTP1 could also be used as a
promising molecular biomarker of great clinical significance in
helping early HCC screening and diagnosis. Unmethylated GSTP1 were
demonstrated in elemene-treated QGY7703 cells, suggesting that
elemene could reverse tumor suppressor gene methylation. However,
further studies are needed to investigate the exact mechanisms
involved. In summary, the present study provides insight into
elemene's anti-tumor mechanisms and provided a novel method to
identify novel demethylation drugs in TCM to treat tumors.
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bharadwaj M, Roy G, Dutta K, Misbah M,
Husain M and Hussain S: Tackling hepatitis B virus-associated
hepatocellular carcinoma-the future is now. Cancer Metastasis Rev.
32:229–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 65:1118–1127. 2011. View Article : Google Scholar
|
|
4
|
Bruix J and Llovet JM: Major achievements
in hepatocellular carcinoma. Lancet. 373:614–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi JD and Lee JS: Interplay between
epigenetics and genetics in cancer. Genomics Inform. 11:164–173.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rongrui L, Na H, Zongfang L, Fanpu J and
Shiwen J: Epigenetic mechanism involved in the HBV/HCV-related
hepatocellular carcinoma tumorigenesis. Curr Pharm Des.
20:1715–1725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sato K, Satoh K, Tsuchida S, Hatayama I,
Shen H, Yokoyama Y, Yamada Y and Tamai K: Specific expression of
glutathione S-transferase Pi forms in (pre) neoplastic tissues:
Their properties and functions. Tohoku J Exp Med. 168:97–103. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukushige S and Horii A: DNA methylation
in cancer: A gene silencing mechanism and the clinical potential of
its biomarkers. Tohoku J Exp Med. 229:173–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hessels D and Schalken JA: Urinary
biomarkers for prostate cancer: A review. Asian J Androl.
15:333–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
White DL, Li D, Nurgalieva Z and El-Serag
HB: Genetic variants of glutathione S-transferase as possible risk
factors for hepatocellular carcinoma: A HuGE systematic review and
meta-analysis. Am J Epidemiol. 167:377–389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoo CB and Jones PA: Epigenetic therapy of
cancer: Past, present and future. Nat Rev Drug Discov. 5:37–50.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saito Y, Hibino S and Saito H: Alterations
of epigenetics and microRNA in hepatocellular carcinoma. Hepatol
Res. 44:31–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Borthakur G, El Ahdab SE, Ravandi F,
Faderl S, Ferrajoli A, Newman B, Issa JP and Kantarjian H: Activity
of decitabine in patients with myelodysplastic syndrome previously
treated with azacitidine. Leuk Lymphoma. 49:690–695. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karahoca M and Momparler RL:
Pharmacokinetic and pharmacodynamic analysis of
5-aza-2′-deoxycytidine (decitabine) in the design of its
dose-schedule for cancer therapy. Clin Epigenetics. 5:32013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Mao Y, Hou L and Cui X: The
effect of beta-elemene on alpha-tubulin polymerization in human
hepatoma HepG2 cells. Chin J Cancer Res. 25:7702013.PubMed/NCBI
|
|
18
|
Peng X, Zhao Y, Liang X, Wu L, Cui S, Guo
A and Wang W: Assessing the quality of RCTs on the effect of
beta-elemene, one ingredient of a Chinese herb, against malignant
tumors. Contemp Clin Trials. 27:70–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, Liu G, Zhang Y, Zhu H, Ren Y and
Shen YM: Synthesis and in vitro anti-proliferative activity of
beta-elemene monosubstituted derivatives in HeLa cells mediated
through arrest of cell cycle at the G1 phase. Bioorg Med Chem.
17:1118–1124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bohari SP, Hukins DW and Grover LM: Effect
of calcium alginate concentration on viability and proliferation of
encapsulated fibroblasts. Biomed Mater Eng. 21:159–170.
2011.PubMed/NCBI
|
|
21
|
Wang J, Zhang H and Sun Y: Phase III
clinical trial of elemenum emulsion in the management of malignant
pleural and peritoneal effusions. Zhonghua Zhong Liu Za Zhi.
18:464–467. 1996.(In Chinese). PubMed/NCBI
|
|
22
|
Mao Y, Zhang J, Hou L and Cui X: The
effect of beta-elemene on alpha-tubulin polymerization in human
hepatoma HepG2 cells. Chin J Cancer Res. 25:770–776.
2013.PubMed/NCBI
|
|
23
|
Lee RX, Li QQ and Reed E: β-elemene
effectively suppresses the growth and survival of both
platinum-sensitive and -resistant ovarian tumor cells. Anticancer
Res. 32:3103–3113. 2012.PubMed/NCBI
|
|
24
|
Liu JS, He SC, Zhang ZL, Chen R, Fan L,
Qiu GL, Chang S, Li L and Che XM: Anticancer effects of β-elemene
in gastric cancer cells and its potential underlying proteins: A
proteomic study. Oncol Rep. 32:2635–2647. 2014.PubMed/NCBI
|
|
25
|
Chen J, Chen YJ and Wu MD: Herbal extract
elemene intrathoracic injection in the treatment of lung cancer
patients with malignant pleural effusion: A meta-anaylsis. J Cancer
Res Ther. 10(Suppl 1): 56–59. 2014.PubMed/NCBI
|
|
26
|
Guan C, Liu W, Yue Y, Jin H, Wang X and
Wang XJ: Inhibitory effect of β-elemene on human breast cancer
cells. Int J Clin Exp Pathol. 7:3948–3956. 2014.PubMed/NCBI
|
|
27
|
Jiang ZY, Qin SK, Yin XJ, Chen YL and Zhu
L: Synergistic effects of Endostar combined with β-elemene on
malignant ascites in a mouse model. Exp Ther Med. 4:277–284.
2012.PubMed/NCBI
|
|
28
|
Shi H, Liu L, Liu L, Geng J, Zhou Y and
Chen L: β-Elemene inhibits the metastasis of B16F10 melanoma cells
by downregulation of the expression of uPA, uPAR, MMP-2 and MMP-9.
Melanoma Res. 24:99–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo HQ, Zhang GN, Wang YJ, Zhang YK,
Sodani K, Talele TT, Ashby CR Jr and Chen ZS: β-Elemene, a compound
derived from Rhizoma zedoariae, reverses multidrug resistance
mediated by the ABCB1 transporter. Oncol Rep. 31:858–866.
2014.PubMed/NCBI
|
|
30
|
Yang H, Wang X and Yu L: The antitumor
activity of elemene is associated with apoptosis. Zhonghua Zhong
Liu Za Zhi. 18:169–172. 1996.(In Chinese). PubMed/NCBI
|
|
31
|
Zhu T, Zhao Y, Zhang J, Li L, Zou L, Yao Y
and Xu Y: β-Elemene inhibits proliferation of human glioblastoma
cells and causes cell-cycle G0/G1 arrest via mutually compensatory
activation of MKK3 and MKK6. Int J Oncol. 38:419–426.
2011.PubMed/NCBI
|
|
32
|
Dai ZJ, Tang W, Lu WF, Gao J, Kang HF, Ma
XB, Min WL, Wang XJ and Wu WY: Antiproliferative and apoptotic
effects of β-elemene on human hepatoma HepG2 cells. Cancer Cell
Int. 13:272013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang XS, Yang W, Tao SJ, Li K, Li M, Dong
JH and Wang MW: Effect of delta-elemene on Hela cell lines by
apoptosis induction. Yakugaku Zasshi. 126:979–990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng CP, Tong XM, Yao HP, Yang J, Xu J,
Cai XP and Liu Z: Beta-elemene enhances aclarubicin-induced
apoptotic effect in HL-60 cells and its mechanism. Zhonghua Xue Ye
Xue Za Zhi. 30:821–824. 2009.(In Chinese). PubMed/NCBI
|
|
35
|
Zhao YS, Zhu TZ, Chen YW, Yao YQ, Wu CM,
Wei ZQ, Wang W and Xu YH: B-elemene inhibits Hsp90/Raf-1 molecular
complex inducing apoptosis of glioblastoma cells. J Neurooncol.
107:307–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yao CC, Tu YR, Jiang J, Ye SF, Du HX and
Zhang Y: β-elemene reverses the drug resistance of lung cancer
A549/DDP cells via the mitochondrial apoptosis pathway. Oncol Rep.
31:2131–2138. 2014.PubMed/NCBI
|
|
37
|
Rezaei MK, Shobbar ZS, Shahbazi M, Abedini
R and Zare S: Glutathione S-transferase (GST) family in barley:
identification of members, enzyme activity, and gene expression
pattern. J Plant Physiol. 170:1277–1284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tchou JC, Lin X, Freije D, Isaacs WB,
Brooks JD, Rashid A, De Marzo AM, Kanai Y, Hirohashi S and Nelson
WG: GSTP1 CpG island DNA hypermethylation in hepatocellular
carcinomas. Int J Oncol. 16:663–676. 2000.PubMed/NCBI
|