Introduction
Non-gestational ovarian choriocarcinoma (NGCO),
which is not associated with pregnancy, is a rare germ cell tumor
of high-grade malignancy that most commonly occurs in pre-pubescent
females (1,2). The rate of occurrence in pure primary
NGOC is <1% (3). The clinical
manifestations of NGCO include vaginal bleeding, abdominal pain and
pelvic masses, so the majority of cases are misdiagnosed and are
initially treated for ovarian tumor torsion or ovarian cancer
(3,4).
The majority of NGCO cases have been confirmed by post-surgical
pathological diagnoses (4). The major
treatment for NGOC is surgery combined with chemotherapy, but
patients often have a poor prognosis (3,4).
Sex cord tumor with annular tubules (SCTAT) is a
subtype of sex cord-stromal tumor (SCST) that is also extremely
rare, accounting for 2.3% of SCSTs (5). The clinical manifestations of SCTAT
mainly include pelvic masses and irregular uterine bleeding, and in
children SCTAT is presented as the early onset of pseudo-puberty
(6,7).
SCTAT often occurs in women aged between 20–60 years old, and the
majority of patients receive surgery combined with chemotherapy as
treatment (8). In total, ~21.9% cases
are clinically malignant, but patients with SCTAT usually have
relatively good prognosis (9). To the
best of our knowledge, the co-existence of these two uncommon tumor
types has not been reported in the literature to date. In the
present study, two cases of NGCO are presented, one of which
synchronously occurred with SCTAT of the contralateral ovary. The
clinical and pathological features of these tumors, as well as
patient prognosis, are presented, and the current literature is
reviewed. The present study was approved by The Medical Research
Ethics Committee, Chinese People's Liberation Army General Hospital
(Beijing, China).
Case report
Case one
A 13 year-old female patient presented on June 5,
2012, to the Chinese People's Liberation Army General Hospital
(Bejing, China) with a 20-day history of abdominal pain that had
increased in severity in the previous 5 days. Menarche had not yet
occurred, however, a small amount of vaginal bleeding was observed
following admission. B-mode ultrasound (iU22; Phillips, Amsterdam,
Netherlands) identified a 16.6×10.8×12.0-cm heterogeneous solid
mass in the left ovary with a clear boundary to the left of uterus.
Color Doppler flow imaging (CDFI) revealed an abundant blood flow
signal, as well as a small amount of free fluid in the pleural and
peritoneal cavities. Positron emission tomography-computed
tomography revealed soft tissue density in both lungs and multiple
nodules in the greater omentum and concurrent increases in
metabolism; thus, metastases were suspected. Intraoperative
exploration revealed a left ovarian cystic mass, measuring 16×15×10
cm, that exhibited a close association with the greater omentum,
sigmoid colon and rectum, without clear boundaries at the uterine
body. Furthermore, a 2×2-cm nodule was observed on the surface of
the right fallopian tube and the right ovary measured 3.0×3.0×1.5
cm in size with a rough surface. The uterus, left ovary and
fallopian tube and a portion of the right ovary were resected.
Gross examination revealed a gray-red neoplastic mass measuring
12×10×8 cm in the left ovary and fallopian tube, which dark red and
brown in color, and solid on the cut surface. Hemorrhage and
necrosis were also evident. The tumor involved the serosa of the
left wall of the uterus. The additional 15×7×3-cm pelvic mass
exhibited the same features as the adnexal mass. The right ovarian
tumor measured 1.0×0.5×0.3 cm in size and was gray-white in color
with medium hardness. Eight days postoperatively, serum β-human
chorionic gonadotropin (β-hCG) levels were 2,045 U/l (normal range,
0–5 U/l). Two cycles of chemotherapy (cisplatin, 20 mg via
intravenous drip (VD), days 1–5; bleomycin, 15 mg, VD, days 1–5;
vincristine sulphate, 1 mg, VD, days 1–5) were administered.
Follow-up examination performed 3 months after surgery revealed
that β-hCG levels had increased to 79,102 µ/l. In addition, liver,
kidney and spleen metastases were identified. The patient
eventually succumbed due to multiple organ failure on September 22,
2012.
Case two
A 13 year-old female patient presented on August 6,
2012, to the Chinese People's Liberation Army General Hospital with
a 1-month history of abdominal pain. A mass with a rough surface
and poor mobility was identified, with light tenderness on the left
side. Anal examination revealed a palpable mass in the front left
of the uterus and that the corpus uteri was of normal size,
however, no abnormalities were identified on the right adnexal
zone. B-mode ultrasonography revealed an irregularly shaped mass in
the left ovary with heterogeneous internal echo. CDFI revealed a
mass with a small blood flow signal. During surgery, 200 ml bloody
ascites were observed within the left ovary by ultrasonography. In
addition, a dark red nodular tumor, 12×10×9 cm in size with a rough
surface, no envelope and no adhesions, was identified on the left
ovary. The uterus, right fallopian tube, omental and abdominal
viscera, and pelvic lymph node were normal in appearance. The left
ovary and fallopian tube and tumor mass were resected. Gross
examination revealed that the tumor was nodular with no envelope,
and hemorrhage and necrosis were observed at the resected surface.
The patient was followed up for 1 year postoperatively prior to
being lost to follow-up, so the final outcome is unknown.
Microscopic examination
The resected tumors were fixed with 4% formalin
(Beijing Yili Fine Chemicals Co., Ltd., Beijing, China), routinely
sampled, dehydrated and embedded in paraffin (Leica Biosystems
Richmond, Inc., Richmond, USA), sliced into 4-µm thick sections and
stained with Harris hematoxylin eosin staining and eosin staining
solutions (Beijing Yili Fine Chemicals Co., Ltd.). The sections
were observed under the BX53 microscope (Olympus Corporation,
Tokyo, Japan).
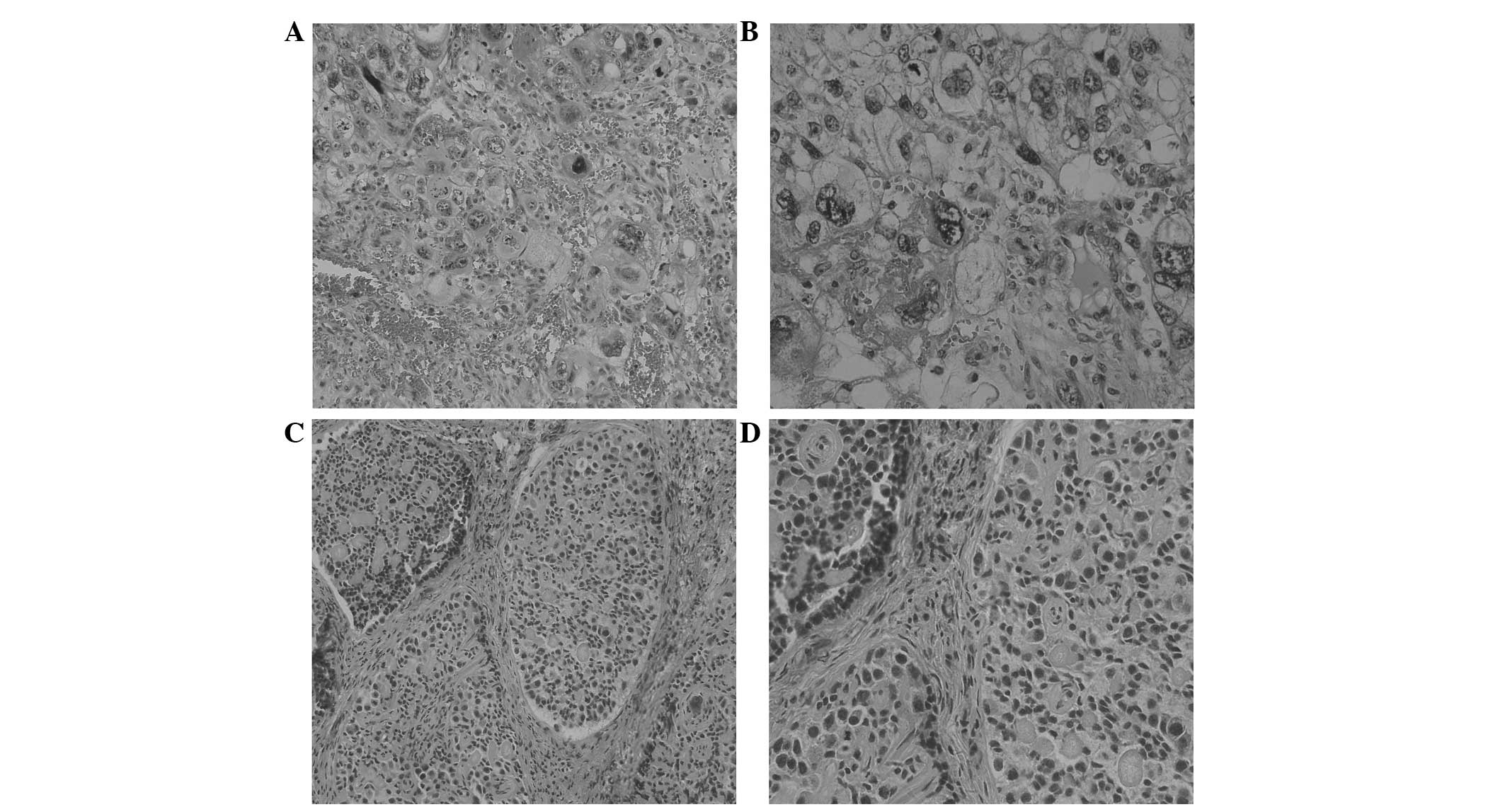
The left ovarian tumors in cases one and two
exhibited extensive hemorrhage and necrosis, and were composed of
cytotrophoblasts, syncytiotrophoblasts and intermediate trophoblast
cells arranged in papillary, cribriform and nest patterns. The
cytotrophoblasts exhibited clear or amphophilic cytoplasms, and
were oval and polygonal in shape with clear boundaries. The
syncytiotrophoblasts exhibited eosinophilic or basophilic cytoplasm
with cytoplasmic vacuoles and dark nuclei (Fig. 1A and B). In case one, the right
ovarian tumor cells surrounding single or multiple eosinophilic
hyaline bodies were arranged in a tubular formation and consisted
of well-defined round or oval calcified epithelial nests. The tumor
cells exhibited pale cytoplasm with nuclei palisading around the
transparent bodies or cell nests (Fig. 1C
and D).
Immunohistochemical staining
Resected specimens were fixed with 4% formalin
(Beijing Yili Fine Chemicals Co., Ltd.), routinely sampled,
embedded in paraffin (Leica Biosystems Richmond, Inc.), sliced, and
stained with the hematoxylin and eosin staining solution package
reagent box (Beijing Yili Fine Chemicals Co., Ltd.).
Immunohistochemistry (IHC) was performed using the EnVision
FLEX+ (DK-2600; Dako Denmark A/S, Glostrup, Denmark).
The hCG (mouse monoclonal antibody; dilution, 1:150; catalog no.,
ZM-0134), placental alkaline phosphatase (PLAP; rabbit monoclonal
antibody; dilution, 1:150, catalog no., ZA-0513), α-fetoprotein
(AFP; rabbit monoclonal antibody; dilution, 1:150, catalog no.,
ZM-0009), glypican-3 (GPC-3; mouse monoclonal antibody; dilution,
1:150, catalog no., ZM-0146), human placental lactogen (hPL; mouse
monoclonal antibody; dilution, 1:150, catalog no., ZM-0216), CD99
(mouse monoclonal antibody; dilution, 1:150, catalog no., ZA-0577)
and α-inhibin (mouse monoclonal antibody; dilution, 1:100, catalog
no., ZM-0460) antibodies were purchased from Zhongshan Jinqiao
Biological Technology Co., Ltd. (Beijing, China). The CD30 (mouse
monoclonal antibody; dilution, 1:100, catalog no., NCL-L-CD30-591)
was purchased from Leica Biosystems Newcastle, Ltd. (Newcastle Upon
Tyne, UK); cytokeratin (CK; mouse monoclonal antibody; dilution,
1:100, catalog no., M3515), CD117 (rabbit polyclonal antibody;
dilution, 1:400, catalog no., A4502), CD56 (mouse monoclonal
antibody; dilution, 1:100, catalog no., M7304) and smooth muscle
actin (SMA; mouse monoclonal antibody; dilution, 1:100, catalog
no., M0851) were purchased from Dako.
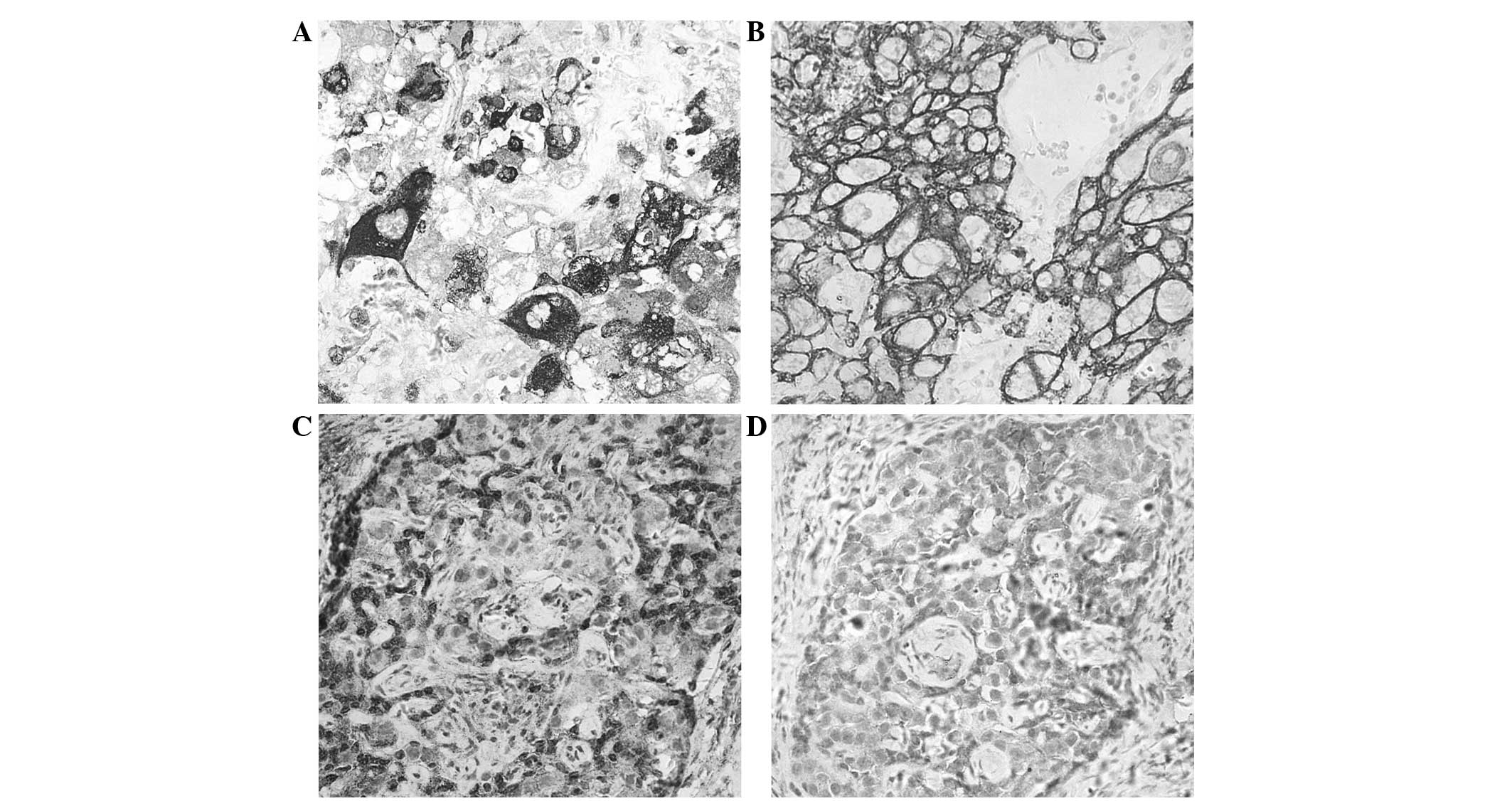
The major IHC findings of case one were as follows:
i) Left ovarian tumor cells: hCG(+) (Fig.
2A), CK(+) (Fig. 2B), PLAP(−),
AFP(−), CD30(−), CD117(−), GPC-3(−), hPL(−); ii) right ovarian
tumor cells: CD99(+) (Fig. 2C),
CD56(+) (Fig. 2D), SMA(−), CK (range
+) and α-inhibin(−). The IHC findings of the left ovarian tumor
cells of case two were as follows: hCG(+), CK(+), PLAP(−), AFP(−),
CD30(−), CD117(−) and GPC-3(−).
Pathological diagnosis
In case one, non-gestational choriocarcinoma of the
left ovary with a large degree of necrosis, invasion of the uterine
serosa and outer muscular wall, and metastases to the omentum were
diagnosed. SCTAT was also diagnosed in the right ovary. In case
two, left NGCO with necrosis was diagnosed.
Discussion
Non-gestational choriocarcinoma, also known as
primary choriocarcinoma, differs from gestational choriocarcinoma
(10). Non-gestational
choriocarcinoma is not associated with pregnancy, originates in the
primordial germ cells, and occurs in the reproductive organs and
the extragonadal midline areas of males and females, including the
pineal gland, mediastinum and retroperitoneum, as well as other
organs, such as the stomach (11),
lung (12) and pancreas (13). NGCO is rare, accounting for <1% of
ovarian germ cell tumors, most frequently occurring in adolescents
and young females, and occasionally in postmenopausal women
(14–16). Clinical manifestations include
abdominal pain and pelvic masses, tumoral secretion of hCG and, in
certain cases, precocious puberty and endocrine abnormalities. The
present study reported two cases in which a 13 year-old female was
admitted to hospital with abdominal pain with no evident endocrine
abnormalities (with the exception of a small amount of vaginal
bleeding following admission in case one).
SCTAT is derived from immature cord cells that may
differentiate into granular layer cells with Sertoli cell
potential. SCTAT is classified as a mixed sex cord stromal tumor
according to the 2014 World Health Organization classification
(17).
Ovarian SCTAT, which is typically identified in
young females, is relatively rare and may be divided into two
subtypes: One subtype is associated with familial Peutz Jeghers
syndrome (PJS; 36%) or cervical malignant adenoma (14%) (7), and the other subtype occurs in the
absence of PJS or cervical malignant adenoma. The most common
clinical manifestation of ovarian SCTAT is an abdominal mass and
40% of patients exhibit pseudo-precocious puberty or abnormal
estrogen levels. In the present study, the small SCTAT tumor
(1×0.5×0.3 cm) in the right ovary in case one was identified during
intraoperative exploration of the left ovarian choriocarcinoma. The
vaginal bleeding observed after admission may have been caused by
hormonal abnormalities, however, it remains unclear whether this
was due to the choriocarcinoma, SCTAT or both.
To date, only a small number of NGCO cases have been
reported (Table I). Contralateral
mature teratoma (18) or dysgerminoma
(19) have been reported, whereby the
bilateral tumors were of germ cell origin. Previously, endometrioid
carcinoma, clear cell carcinoma and small cell carcinoma epithelial
carcinoma with choriocarcinoma have also been reported, however,
only a small proportion of these tumors exhibited a choriocarcinoma
component (20). To the best of our
knowledge, no cases of left NGCO with right ovarian SCTAT, as
observed in case one, have been reported previously. The
association between the two types of cancer remains unclear.
 | Table I.Summary of reported cases of
non-gestational ovarian choriocarcinoma. |
Table I.
Summary of reported cases of
non-gestational ovarian choriocarcinoma.
| Author, year | Age, years | Tumor size, cm | Ovary | Type of ovarian
cancer | Metastatic sites | Chemotherapy | Follow-up
results | Ref. |
|---|
| Goswami et al,
2001 | 18 | 10×12 | Left | Contralateral mature
cystic teratoma | – | Yes | No recurrence after 5
months | (18) |
| Gungor et al,
1999 | 16 | 5×4.3 | Left | Contralateral
dysgerminoma | Lung and pelvis | Yes | Succumbed after 6
months | (19) |
| Case one | 13 | 16×15×10 | Left | Contralateral sex
cord tumor with annular tubules | Lung and pelvis | Yes | Succumbed after 3
months | – |
| Case two | 13 | 12×10×9 | Left | Without accompanying
tumor | – | Yes | Follow-up
discontinued after 1 year | – |
Large NGCOs often occur unilaterally, and exhibit
extensive hemorrhage and necrosis (21). The disease is similar to gestational
choriocarcinoma (22).
Non-gestational choriocarcinomas are predominantly composed of two
cell types: Cytotrophoblasts, which are medium in size and rounded
with lightly stained cytoplasm, clear cell boundaries and small,
round, dark, central nuclei; and syncytiotrophoblasts, which
exhibit cytoplasmic vacuoles, nuclei with coarse chromatin and
unclear cellular boundaries, and occasionally form nodules. These
two types of cells are typically arranged in cribriform, plexiform
and pseudopapillary patterns within blood. Furthermore, NGCO tumors
are commonly associated with dysgerminoma, teratoma, yolk sac
tumors and other germ cell tumor components (18,19), or
may be a single component of choriocarcinoma; however, this is
extremely rare. In the present study, no other germ cell tumors
were identified in the two patients. The tumor cells of both cases
were immunohistochemically positive for hCG and CK.
SCTAT with PJS often occur bilaterally, and are
typically <3 cm in diameter or only identified microscopically.
In addition, calcifications are common in these tumors. By
contrast, SCTAT without PJS are typically unilateral and larger in
size. The tumor sections were solid or cystic and grayish-yellow in
color. Microscopically, tumor cells surrounding the eosinophilic
hyaline bodies formed simple or complex annular tubule epithelial
nests. The tumor cell cytoplasms were lightly stained and the
cellular boundaries were not clear. The nuclei were round and the
hyaline bodies were in a palisade arrangement around the
surrounding epithelial nests. Previously, IHC staining has revealed
that the tumor cells express inhibin, CD99 and Melan-A (23). In case one, although the SCTAT of the
right ovary was small with focal calcifications, no PJS or cervical
lesions were present. Due to extensive tissue damage as a result of
the choriocarcinoma, it was unclear whether a SCTAT component was
present in the left ovary; thus, the association between SCTAT and
PJS in the present case was difficult to determine.
Although NGCO primarily occurs in children, it may
also occur in adults (24).
Histologically, gestational choriocarcinoma and non-gestational
choriocarcinoma share similar morphological features; therefore,
for adolescents with no sexual history, the disease may be
diagnosed according to the pathological features (25) and immunohistochemical phenotype. By
contrast, for females of childbearing age, the difference between
gestational and non-gestational choriocarcinoma is more difficult
to confirm, however, DNA polymorphism analysis may aid diagnosis
(14,26–29).
Fisher et al (26) first used
site-specific microsatellite probes to analyze DNA restriction
fragment length polymorphisms of tumor tissue by comparing blood
samples obtained from patients and their spouses. The results of
were as follows: If the tumor components only originate from the
patients, non-gestational choriocarcinoma may be diagnosed, whereas
if a patrilineal component exists, gestational choriocarcinoma may
be diagnosed.
NGCO must also be differentiated from embryonal
carcinoma, dysgerminoma and intermediate trophoblastic tumors
[placental site trophoblastic tumor (PSTT) and epithelioid
trophoblastic tumor (ETT)]. Although embryonal carcinoma is
composed of syncytiotrophoblast-like giant cells (30), it does not exhibit the bidirectional
property of trophoblast cells in choriocarcinoma. Furthermore, upon
IHC, embryonal carcinoma exhibits CD30 and AFP positivity, while
choriocarcinoma exhibits CD30 and AFP negativity. A small number of
dysgerminoma cases demonstrate syncytiotrophoblast differentiation
without cytotrophoblasts, while tumor cell components are
relatively simple, with positive PLAP and CD117, and negative hCG
expression.
PSTTs do not contain cytotrophoblasts or
syncytiotrophoblasts, however, the do exhibit pure intermediate
trophoblastic cells. Furthermore, IHC analysis of choriocarcinoma
shows diffuse positivity for hCG, while PSTTs exhibit only focal
and weak positivity for hCG, and strong positivity for hPL. ETTs
are composed of epithelioid trophoblasts without cytotrophoblasts
and syncytiotrophoblasts, while only a small number of tumor cells
exhibit focal hCG positivity (31,32).
SCTAT must be differentiated from Sertoli cell
tumors and microfollicular granulosa cell tumors. Microfollicular
granulosa cell tumors containing Call-Exner bodies resemble SCTAT
of the tubular lumen, however, such tumors are small with no
visible nuclear debris or palisading nuclei. Highly differentiated
Sertoli cell tumors may also form simple tubular structures with a
hollow lumen (33).
NGCO often invades the adjacent organs and commonly
metastasizes to distant organs (30),
particularly the brain and lung. Treatment predominantly consists
of surgery combined with chemotherapy, however, the efficacy of
this strategy is not as high as that for gestational
choriocarcinoma (34,35). Jiao et al (36) reported 21 cases of NGCO with a mean
follow-up period of 71.4 months and an overall 5-year survival rate
of 79.4%. Goswami et al (18)
summarized 30 case reports of NGCO and revealed that the 2 year
survival rate of patients who accepted surgery combined with
chemotherapy was 81%, while that of patients who underwent surgery
alone was 28%. Although cases of SCTAT with PJS are clinically
benign, recurrence and metastasis have been reported (37); ~25% of SCTAT patients without PJS
exhibit a malignant clinical course in which invasive growth of the
tumor occurs (38). In the present
study, the prognosis of case one was predominantly determined by
the choriocarcinoma. Bilateral pulmonary metastases were identified
following chemotherapy and the hCG level did not declined
significantly. However, the treatment exhibited poor efficacy and
the patient succumbed 3 months later. As indicated in Table I, pulmonary metastasis identified
after surgery indicated the poor prognosis.
In conclusion, NGCO is a rare malignant germ cell
tumor of which <100 cases have been reported worldwide (21,36,38);
therefore, limited clinical data is available. At present, no
optimum treatment has been identified, and although the prognosis
is worse than that of gestational choriocarcinoma, early detection,
diagnosis and treatment are important factors for patient
prognosis.
References
|
1
|
Heo EJ, Choi CH, Park JM, Lee JW, Bae DS
and Kim BG: Primary ovarian choriocarcinoma mimicking ectopic
pregnancy. Obstet Gynecol Sci. 57:330–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Serno J, Zeppernick F, Jäkel J, Schrading
S, Maass N, Meinhold-Heerlein I and Bauerschlag DO: Primary
pulmonary choriocarcinoma: Case report and review of the
literature. Gynecol Obstet Invest. 74:171–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rao KV, Konar S, Gangadharan J, Vikas V
and Sampath S: A pure non-gestational ovarian choriocarcinoma with
delayed solitary brain metastases: Case report and review of the
literature. J Neurosci Rural Pract. 6:578–581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu T, Yang M, Zhu H, Shi G and Wang H:
Pure non-gestational ovarian choriocarcinoma in a 45,XO/46,XX
SRY-negative true hermaphrodite. J Obstet Gynaecol Res.
37:1900–1905. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown J, Sood AK, Deavers MT, Milojevic L
and Gershenson DM: Patterns of metastasis in sex cord-stromal
tumors of the ovary: Can routine staging lymphadenectomy be
omitted? Gynecol Oncol. 113:86–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scully RE: Sex cord tumor with annular
tubules a distinctive ovarian tumor of the Peutz-Jeghers syndrome.
Cancer. 25:1107–11211970. View Article : Google Scholar
|
|
7
|
Young RH, Welch WR, Dickersin GR and
Scully RE: Ovarian sex cord tumor with annular tubules: Review of
74 cases including 27 with Peutz-Jeghers syndrome and four with
adenoma malignum of the cervix. Cancer. 50:1384–1402. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han Y, Li S, Wu L, Zhang X and Cao D:
Non-Peutz-Jeghers syndrome-associated ovarian sex cord tumor with
annular tubules: Report of a malignant case. J Obstet Gynaecol Res.
42:224–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kulkarni N: Recurrence of non-syndromic
sex cord stromal tumor with annular tubules of ovary: Case report.
Iran J Pathol. 10:61–64. 2015.PubMed/NCBI
|
|
10
|
Choi YJ, Chun KY, Kim YW and Ro DY: Pure
nongestational choriocarcinoma of the ovary: A case report. World J
Surg Oncol. 11:72013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Waseda Y, Komai Y, Yano A, Fujii Y,
Noguchi N and Kihara K: Pathological complete response and two-year
disease-free survival in a primary gastric choriocarcinoma patient
with advanced liver metastases treated with germ cell tumor-based
chemotherapy: A case report. Jpn J Clin Oncol. 42:1197–1201. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Yang J, Ren T, Zhao J, Feng F, Wan
X and Xiang Y: The encouraging prognosis of nongestational ovarian
choriocarcinoma with lung metastases. J Reprod Med. 59:221–226.
2014.PubMed/NCBI
|
|
13
|
Ramachandran BS, Murugesan M, Ali M and
Padmanabhan P: Primary pancreatic choriocarcinoma presenting as
pancreatitis. JOP. 13:217–218. 2012.PubMed/NCBI
|
|
14
|
Hirata Y, Yanaihara N, Yanagida S, Fukui
K, Iwadate K, Kiyokawa T and Tanaka T: Molecular genetic analysis
of nongestational choriocarcinoma in a postmenopausal woman: A case
report and literature review. Int J Gynecol Pathol. 31:364–368.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiao LZ, Xiang Y, Feng FZ, Wan XR, Zhao J,
Cui QC and Yang XY: Clinical analysis of 21 cases of nongestational
ovarian choriocarcinoma. Int J Gynecol Cancer. 20:299–302. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park SH, Park A, Kim JY, Kwon JH and Koh
SB: A case of non-gestational choriocarcinoma arising in the ovary
of a postmenopausal woman. J Gynecol Oncol. 20:192–194. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zaloudek CJ, States PN, Mooney EE and
Young RH: Sex cord-stromal tumours - pure sex cord tumours. WHO
classification of tumours of female reproductive organs. Kurman RJ,
Carcangiu ML, Herrington CS and Young RH: (6). (4th). IARC Press.
(Lyon). 532014.
|
|
18
|
Goswami D, Sharma K, Zutshi V, Tempe A and
Nigam S: Nongestational pure ovarian choriocarcinoma with
contralateral teratoma. Gynecol Oncol. 80:262–266. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gungor T, Ekin M, Zergeroĝlu S and Gokmen
O: Primary choriocarcinoma of the ovary with coexisting
dysgerminoma of the contralateral ovary. J Obstet Gynaecol.
19:325–326. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirabayashi K, Yasuda M, Osamura RY,
Hirasawa T and Murakami M: Ovarian nongestational choriocarcinoma
mixed with various epithelial malignancies in association with
endometriosis. Gynecol Oncol. 102:111–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong B, Tian YJ, Zhu WW and Qin YJ: A pure
nongestational ovarian choriocarcinoma in a 10-year-old girl: Case
report and literature review. J Obstet Gynaecol Res. 35:574–578.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Naniwadekar MR, Desai SR, Kshirsagar NS,
Angarkar NN, Dombale VD and Jagtap SV: Pure choriocarcinoma of
ovary diagnosed by fine needle aspiration cytology. Indian J Pathol
Microbiol. 52:417–420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan LD and Tang T: Application of
immunohistochemistry in ovarian tumors and tumor-like lesions.
Zhenduan Binglixue Zazhi. 11:369–372. 2004.(In Chinese).
|
|
24
|
Oladipo A, Mathew J, Oriolowo A, Lindsay
I, Fisher R, Seckl M and Yiannakis D: Nongestational
choriocarcinoma arising from a primary ovarian tumour. BJOG.
114:1298–1300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Corakçi A, Ozeren S, Ozkan S, Gürbüz Y,
Ustün H and Yücesoy I: Pure nongestational choriocarcinoma of
ovary. Arch Gynecol Obstet. 271:176–177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fisher RA, Newlands ES, Jeffreys AJ, Boxer
GM, Begent RH, Rustin GJ and Bagshawe KD: Gestational and
nongestational trophoblastic tumors distinguished by DNA analysis.
Cancer. 69:839–845. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsujioka H, Hamada H, Miyakawa T,
Hachisuga T and Kawarabayashi T: A pure nongestational
choriocarcinoma of the ovary diagnosed with DNA polymorphism
analysis. Gynecol Oncol. 89:540–542. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koo HL, Choi J, Kim KR and Kim JH: Pure
non-gestational choriocarcinoma of the ovary diagnosed by DNA
polymorphism analysis. Pathol Int. 56:613–616. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamamoto E, Ino K, Yamamoto T, Sumigama S,
Nawa A, Nomura S and Kikkawa F: A pure nongestational
choriocarcinoma of the ovary diagnosed with short tandem repeat
analysis: Case report and review of the literature. Int J Gynecol
Cancer. 17:254–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khuu HM, Crisco CP, Kilgore L, Rodgers WH
and Conner MG: Carcinosarcoma of the uterus associated with a
nongestational choriocarcinoma. South Med J. 93:226–228. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng X, Liu X, Tian Q, Xue Y and An R:
Placental site trophoblastic tumor: A case report and literature
review. Intractable Rare Dis Res. 4:147–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leow WQ, Loh HL, Lee LS and Goh CH: A rare
case of combined placental site trophoblastic tumour with mature
cystic teratoma and mixed germ cell tumour in the testis. Malays J
Pathol. 37:145–147. 2015.PubMed/NCBI
|
|
33
|
Duhig EE, Riha RL and Clarke BE: Test and
teach. An unusual tumour presenting in the lungs. Metastatic adult
granulosa cell tumour of the ovary, microfollicular patterns.
Pathology. 34:78–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gangadharan VP, Mathew BS, Kumar KS and
Chitrathara K: Primary choriocarcinoma of the ovary. Report of two
cases. Indian J Cancer. 36:213–215. 1999.PubMed/NCBI
|
|
35
|
Pentheroudakis G, White J, Davis J, Brown
I and Vasey P: Concurrent ovarian-type primary peritoneal
adenocarcinoma and peritoneal choriocarcinoma. A case report and
review of the literature. Gynecol Oncol. 92:697–700. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiao LZ, Xiang Y, Feng FZ, Wan XR, Zhao J,
Cui QC and Yang XY: Ovarian non-gestational choriocarcinoma
clinical analysis of 21 cases. Chin J Prac Gynecol Obstet.
25:359–361. 2009.(In Chinese).
|
|
37
|
Barker D, Sharma R, McIndoe A, Blair E,
Hall M, Gabra H and El-Bahrawy M: An unusual case of sex cord tumor
with annular tubules with malignant transformation in a patient
with Peutz-Jeghers syndrome. Int J Gynecol Pathol. 29:27–32. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lv L, Yang K, Wu H, Lou J and Peng Z: Pure
choriocarcinoma of the ovary: A case report. J Gynecol Oncol.
22:135–139. 2011. View Article : Google Scholar : PubMed/NCBI
|