Introduction
The epidermal growth factor receptor (EGFR) T790M
mutation has gradually become a research hotspot in the field of
targeted non-small cell lung cancer (NSCLC) therapy in recent
years. Secondary T790M mutation accounts for ~50% of acquired EGFR
tyrosine kinase inhibitor (TKI) resistance in originally highly
responsive NSCLC patients with EGFR activating mutations, including
exon 19 small in-frame deletions and exon 21 L858R mutations
(1). De novo T790M EGFR
mutations have been observed in TKI-naïve patients and could
predict a shorter response duration following EGFR-TKI treatment
(2,3).
Furthermore, the incidence of de novo T790M mutation may be
more prevalent than expected due to the limited sensitivity of
detection methods, such as direct sequencing (2–5). Next
generation EGFR-TKIs, including AZD9291, CO-1686 and HM61713, could
inhibit EGFR T790M and activating mutations, and have been proven
beneficial to NSCLC patients with EGFR-TKI (gefitinib or erlotinib)
resistance caused by secondary T790M mutation (6–8). Thus,
detecting the EGFR T790M mutation in NSCLC patients is important
for monitoring the presence of acquired resistance and for
selecting patients for treatment with next generation EGFR-TKIs
(1,6–8). However,
reliable methods for EGFR T790M mutation detection have not yet
been established and widely accepted. The major difficulty in
establishing such a method is that EGFR T790M mutant cells are
mixed with a large amount of wild-type cells derived from the site
of tissue sampling.
A number of methods to overcome this obstacle have
been reported, including mutant-enriched polymerase chain reaction
(PCR) (9,10), the amplification refractory mutation
system (ARMS) (4,11), peptide nucleic acid (PNA)-clamping PCR
(12–15), combining scorpion ARMS with whole
genome amplification (16), combining
co-amplification at lower denaturation temperature-PCR with TaqMan
technology (17), molecular
beacon-based quantitative PCR (18),
the beads, emulsion, amplification and magnetics (BEAMing) assay
(19), PCR-clone hybridisation
(5) and matrix-assisted laser
desorption/ionisation-time of flight mass spectrometry (2). Although these methods have significantly
improved the detection limit ranging from 1 to 0.01%, the majority
of these methods are unavailable and cannot be used easily in a
clinical setting. Additionally, certain methods involve a number of
steps of post-PCR processing, which may increase PCR-product
contamination.
In the present study, a sensitive and practical
method was developed that utilises an allele-specific competitive
blocker (ACB) coupled with ARMS TaqMan quantitative PCR in a
one-step reaction tube. This method allowed the preferential
amplification of the mutant DNA through use of an ACB to prohibit
wild-type allele elongation and was thus used to screen for the
T790M mutation in 27 TKI-naïve NSCLC specimens.
Patients and methods
Plasmid construction
Recombinant plasmids encoding wild-type and T790M
(2169 C>T) mutant EGFR exon 20 were constructed according to the
method previously reported by Board et al (20). Briefly, corresponding outer and mutant
primers (Sangon Biotech Co., Ltd., Shanghai, China) were used to
yield half fragments that each had complimentary ends and contained
a mutant base. The sequences of the outer primers were as follows:
Primer a, 5′-TTCACAGCCCTGCGTAAAC-3′; primer d,
5′-TTTCCACATGCAGATGGGAC-3′. The sequences of the mutant primers as
follows: Primer b, 5′-CGAAGGGCATGAGCTGCATGATGAGCTGCACGGTGG-3′;
primer c, 5′-CCACCGTGCAGCTCATCATGCAGCTCATGCCCTTC-3′. The PCR assay
was performed in a 20-µl mixture containing 2 µl of 10X PCR buffer,
0.5 units of HotStarTaq DNA polymerase (Qiagen China Co., Ltd.),
0.25 µM of each primer (Sangon China Co., Ltd.) and 2 µl of DNA in
a PCR instrument (ABI 2720; Applied Biosystems, Beijing, China).
The PCR conditions were as follows: Initial denaturation at 95°C
for 5 min, followed by 35 cycles at 95°C for 15 sec, 54°C for 30
sec and 72°C for 30 sec, then a hold at 72°C for 7 min and a final
permanent hold at 4°C. The PCR products were mixed and amplified
with outer primers and the PCR process was same as the
aforementioned method. The self-priming of the complementary half
fragments and subsequent amplification created a final product with
a mutant base. The product was ligated into the pMD19-T plasmid
(Tiangen Biotech Co., Ltd., Beijing, China) to generate recombinant
containing mutant alleles, which were confirmed by sequencing
(Sangon, Biotech Co., Ltd.). The plasmid DNA was extracted using
Tiangen Plasmids DNA kits (Tiangen Biotech Co., Ltd.).
Study population, sample collection
and processing
In total, 27 NSCLC patients with activating
mutations (19 Del or 21 L858R) were recruited for this study
between January 2008 and December 2012 at Peking Union Medical
College Hospital (PUMCH; Beijing, China). All patients received
gefitinib as a first-line or multiple-line therapy during the
course of cancer treatment. The clinical data of the patients,
including the demographics, pathological type, stage, smoking
status and treatment information, were obtained from each patient's
medical records and were reviewed by the physicians. The
progression-free survival (PFS) time was calculated based on the
time of gefitinib treatment initiation until disease progression or
mortality. Overall survival (OS) time was defined as the period
following the date of diagnosis, not the date of informed consent,
until mortality.
A tumour biopsy sample was obtained from each
patient (n=27) prior to gefitinib treatment. The samples were
formalin-fixed, paraffin-embedded (FFPE) and retrieved from the
Department of Pathology, PUMCH. The tumour tissues were isolated by
manual microdissection. The tumour content in each microdissected
sample was ≥75%. The DNA was extracted using a QIAamp DNA FFPE
Tissue kit (Qiagen China Co., Ltd.) according to the manufacturer's
protocols, and then stored at −20°C for further testing.
The study protocol was approved by the Institutional
Review Boards of PUMCH. All patients provided written informed
consent for the procurement of tumour specimens.
Mimic human genomic DNA panel of
different concentrations of T790M mutant EGFR
Serially diluted plasmids (10 µl) containing 10,000,
1,000, 100 or 10 copies/µl of the T790M mutation were each added to
90 µl of human genomic DNA (30 ng/µl; Sigma-Aldrich China Inc.,
Shanghai, China). As one copy of genomic DNA is equivalent to 3 pg
of DNA on average, this mimic human genomic DNA panel consisted of
10, 1, 0.1 and 0.01% mutants.
Genotyping by ACB-ARMS TaqMan
quantitative PCR (ACB-ARMS PCR) assay
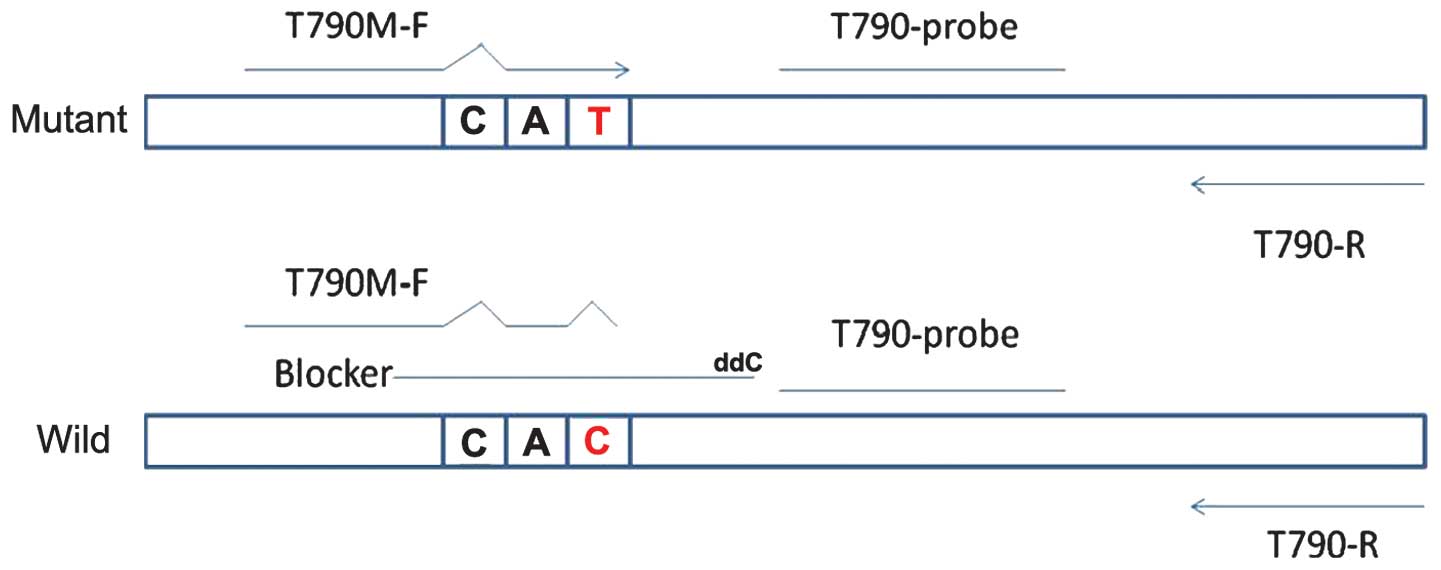
The ACB-ARMS PCR assay for the detection of the EGFR
T790M mutation was established in the molecular laboratories of the
Beijing BGI-GBI Biotech Co., Ltd. (Beijing, China). In the ACB-ARMS
PCR assay, dideoxynucleotide-labelled oligonucleotides were used as
a competitive blocker to suppress the amplification of the
wild-type allele via subtle differences in the melting temperature
between the blocker-wild-type and blocker-mutant DNA hybrids. The
principle of this assay is depicted in Fig. 1, and the sequences are shown in
Table I.
 | Table I.Sequences of primers, blocker and
probes. |
Table I.
Sequences of primers, blocker and
probes.
| Detection | Primers (5′-3′) | Probes (5′-3′) |
|---|
| T790M |
|
|
|
ARMS | F:
5′-CACCGTGCAGCTCATTAT-3′ |
5′FAM-CCTTCCCTGGACTATGT-BHQ3′ |
|
| R:
5′-CACACACCAGTTGAGCAGGTACT-3′ |
|
| Blocker |
5′-TGCAGCTCATCACGCAGCTCATG-ddC3′ |
|
| Internal
control | F:
5′-TGCCAAGGCACGAGTAACAAG-3′ |
5′FAM-TCTCAGCCTCCAGAGGATGTTCAA |
|
| R:
5′TCCAAATTCCCAAGGACCAC-3′ | TAACT-BHQ3′ |
The ACB-ARMS PCR assay was performed in a 20-µl
mixture containing 2 µl of 10X PCR buffer, 0.5 units of HotStarTaq
DNA polymerase (Qiagen China Co., Ltd.), 0.15 µM of probe, 0.15 µM
of blocker, 0.25 µM of each primer (Sangon China Co., Ltd.) and 2
µl of DNA in a fluorometric PCR instrument (ABI 7300 and StepOne;
Applied Biosystems). The detection includes two phases: i) Step one
enriches the T790M mutation with 5 cycles of 95°C for 15 sec, 68°C
for 20 sec, 60°C for 30 sec and 72°C for 20 sec after denaturing at
95°C for 5 min; ii) step two entails normal amplification with 40
cycles of 95°C for 15 sec, 68°C for 20 sec and 60°C for 45 sec
(fluorescence collection). The mimic human genomic DNA was used as
the positive control, RNase and DNase free water was used as the
negative control and β-actin was used as the reference control. The
assay was repeated three times.
Validation of T790M mutation by clone
sequencing
The T790M-positive samples detected by ACB-ARMS PCR
were confirmed with clone sequencing. The wild human genomic DNA
was used as the controls. The forward primer used here was similar
to T790M ARMS-F, but the mutated base was reduced and lacked the
third mismatched base. The sequences of the primers for PCR were as
follows: Forward, 5′-CACCGTGCAGCTCATCA-3′ and reverse,
5′-GATGGGACAGGCACTGATTT-3′. The length of the PCR product was 307
bp. The blocker remained unchanged. The PCR was performed in a
20-µl mixture containing 2 µl of 10X PCR buffer, 0.15 µM of probe,
0.15 µM of blocker, 0.25 µM of each primer, 0.5 units of HotStarTaq
DNA polymerase and 2 µl of DNA in a fluorometric PCR instrument
(ABI 7300 and StepOne; Applied Biosystems). The PCR procedure
included an initial denaturation at 95°C for 5 min followed by 35
cycles at 95°C for 30 sec, 68°C for 20 sec for blocker binding,
60°C for 30 sec and 72°C for 30 sec, then a hold at 72°C for 7 min
and a final permanent hold at 4°C. The product was ligated into the
pMD19-T plasmid (Tiangen Biotech Co., Ltd.). The positive
monoclonal colonies identified by colony PCR were sequenced (Sangon
Biotech Co., Ltd.).
Statistical analysis
For categorical data, Fisher's exact test was
performed to compare differences between groups. The Kaplan-Meier
method was used to estimate survival curves for PFS and OS.
Log-rank tests were used to compare survival curves between
different EGFR mutations. A two-sided P-value of <0.05 was
considered to indicate a statistically significant difference. All
analyses were performed using SPSS software, version 13.0 (SPSS
Inc., Chicago, IL, USA).
Results
Sensitivity of ACB-ARMS PCR for
detecting EGFR T790M mutation
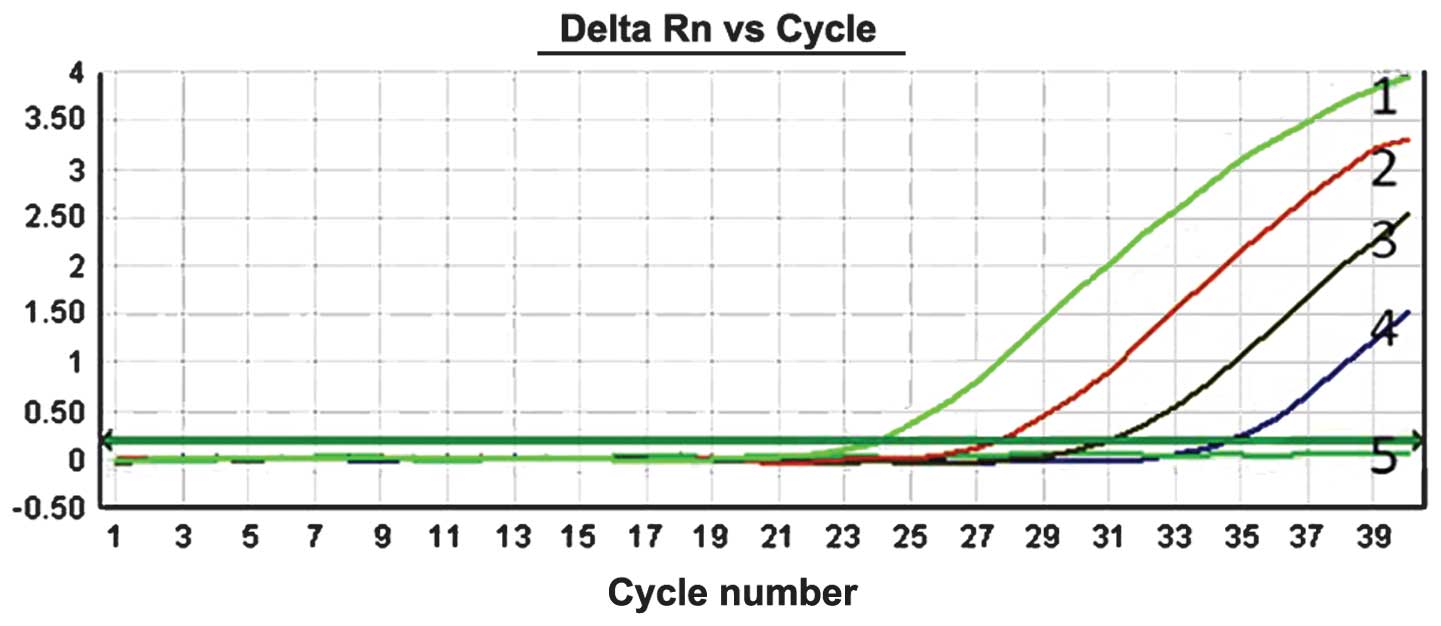
To evaluate the sensitivity of the ACB-ARMS PCR
assay, mimic human genomic DNA panels consisting of 10, 1, 0.1,
0.01 and 0% T790M mutant plasmids were used. The results of
detecting T790M mutation by this method showed that can ACB-ARMS
PCR definitely detect 10, 1, 0.1 and 0.01% mutant plasmids in mimic
samples (Fig. 2), which indicated
that the lower limit (sensitivity) of this method was 0.01% (one
mutant in the presence of 10,000 wild type genes), corresponding to
<10 copies.
EGFR T790M mutation detected by
ACB-ARMS PCR in clinical samples
Patient characteristics
Of the 27 patients with NSCLC, 15 were female
(55.6%), and adenocarcinoma was the most common pathological type
(26 patients; 96.3%). The majority of patients were non-smokers (20
patients; 74.1%). According to the TNM staging system (21), advanced disease at stages IIIB or IV
was identified in 81.5% of all enrolled patients. All patients had
EGFR activating mutations, including 19 Del in 13 patients (48.1%)
and 21 L858R in 14 patients (51.9%), and no T790M mutation was
detected by scorpion ARMS PCR (catalogue no. EG-04; Qiagen China
Co., Ltd.). The patient data are summarised in Table II.
 | Table II.Demographics and clinical
characteristics of enrolled patients. |
Table II.
Demographics and clinical
characteristics of enrolled patients.
|
|
| T790M detected by
ACB-ARMS PCR |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Total, n (%) | Positive, n
(%) | Negative, n
(%) | P-value |
|---|
| Age, years |
|
|
| 0.363 |
|
≤60 | 17 (63.0) | 5 (18.5) | 12 (44.4) |
|
|
>60 | 10 (37.0) | 1 (3.7) | 9 (33.3) |
|
| Gender |
|
|
| 1.000 |
|
Female | 15 (55.6) | 3 (11.1) | 12 (44.4) |
|
|
Male | 12 (44.4) | 3 (11.1) | 9 (33.3) |
|
| Smoking status |
|
|
| 0.290 |
|
Never-smoker | 20 (74.1) | 3 (11.1) | 17 (63.0) |
|
|
Smoker | 7 (25.9) | 3 (11.1) | 4 (14.8) |
|
| Pathology |
|
|
| 0.222 |
|
Squamous | 1 (3.7) | 1 (3.7) | 0 (0.0) |
|
|
Adenocarcinoma | 26 (96.3) | 5 (18.5) | 21 (77.8) |
|
| EGFR activating
mutation |
|
|
| 0.648 |
| 19
Del | 13 (48.1) | 2 (7.4) | 11 (40.7) |
|
| 21
L858R | 14 (51.9) | 4 (14.8) | 10 (37.0) |
|
| Stage |
|
|
| 1.000 |
|
IA-IIIA | 5 (18.5) | 1 (3.7) | 4 (14.8) |
|
|
IIIB-IV | 22 (81.5) | 5 (18.5) | 17 (63.0) |
|
| Line of TKI
therapy |
|
|
| 0.628 |
|
First | 9 (33.3) | 1 (3.7) | 8 (29.6) |
|
| Second
or multiple | 18 (66.7) | 5 (18.5) | 13 (48.1) |
|
T790M mutation is not rare in NSCLC patients with
activating mutations prior to TKI treatment
The sensitive ACB-ARMS PCR assay was used to analyse
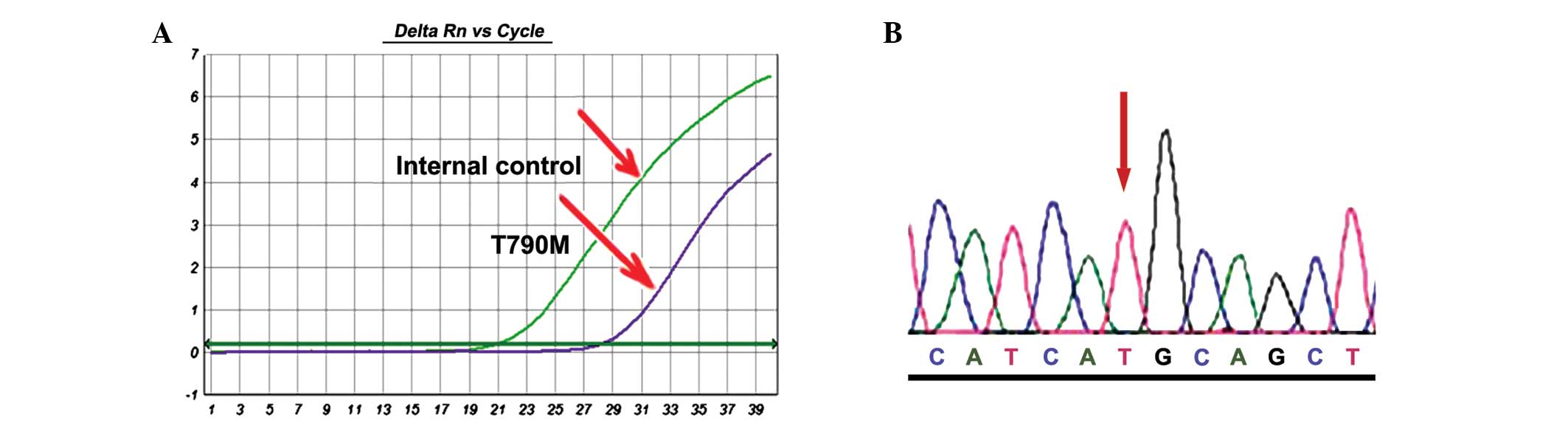
the T790M mutation. Of the 27 specimens pre-treated with EGFR-TKIs,
6 patients (22.2%) with T790M were identified (Fig. 3A). These results were confirmed by
performing the ACB-PCR assay in triplicate and employing clone
sequencing (Fig. 3B). The clone
sequencing showed that all mutant cases harboured T790M mutant
colonies in different proportions, which also suggested that the
ACB-PCR assay is highly specific.
Fisher's exact test was used to evaluate the
association between the T790M mutation and clinical variables, such
as gender, age, smoking status, tumour stage, EGFR activating
mutation and pathological type. However, none of these clinical
factors were found to be associated with the occurrence of T790M in
the NSCLC patients with EGFR-activating mutations.
PFS time, OS time and T790M mutation in NSCLC
patients with activating mutations
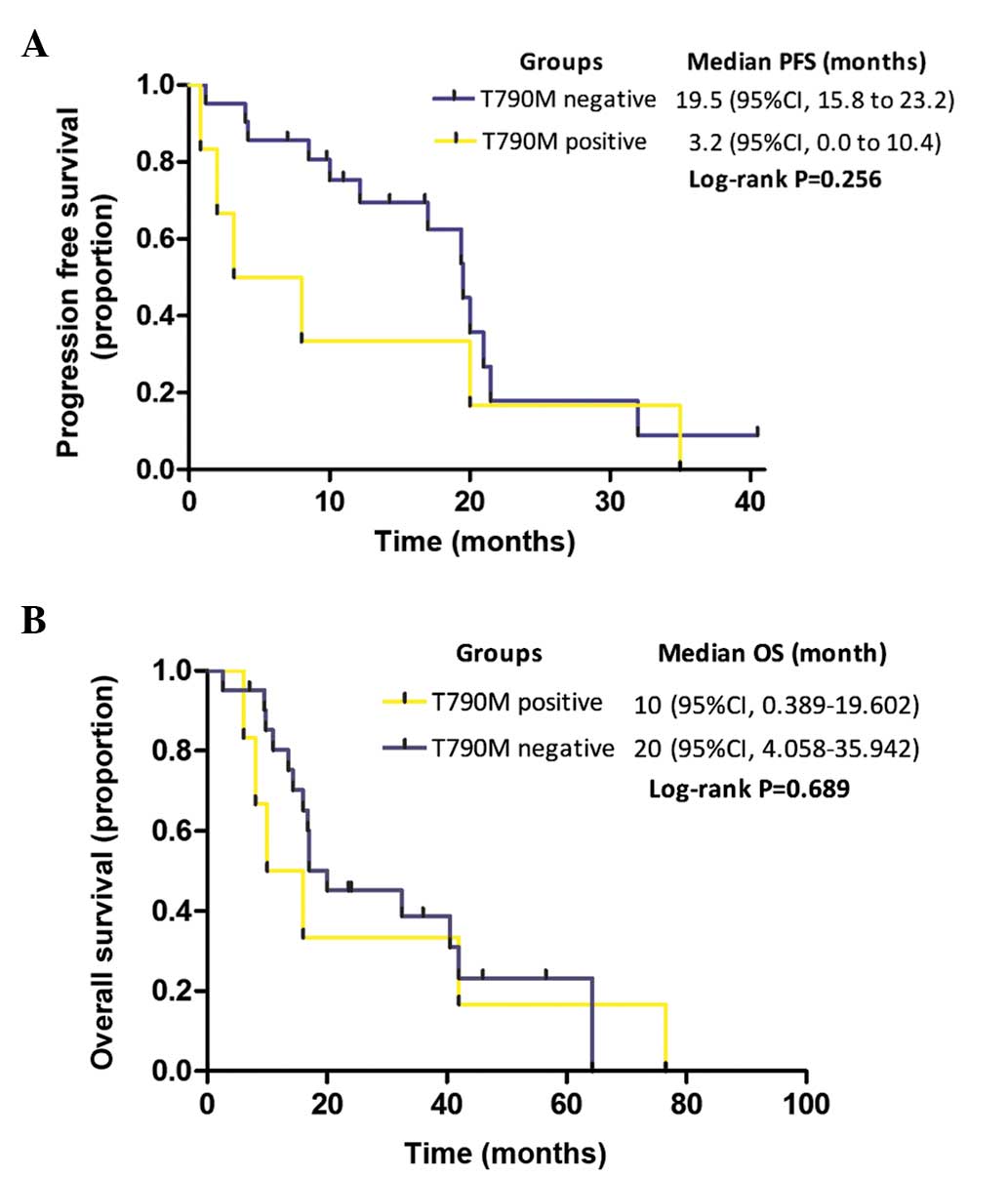
Among the present cohort of 27 patients with
EGFR-activating mutations, the median PFS time in the TKI-naïve
patients with a T790M mutation (n=6) was shorter than in those
without a T790M mutation (n=21), but the difference was not
significant (3.2 vs. 19.5 months, respectively; P=0.256; Fig. 4A). Furthermore, the median OS time in
the groups with or without T790M mutation did not significantly
differ (10 vs. 20 months, respectively; P=0.689; Fig. 4B).
Discussion
The detection of the EGFR T790M mutation has become
increasingly important, as it could be used to monitor acquired
resistance to EGFR-TKIs (1), predict
EGFR-TKI response duration and patient prognosis (2,3,5), and identify appropriate patients for
treatment with next-generation EGFR-TKIs (6–8). Direct
sequencing is a straightforward method that is widely used to
detect the EGFR T790M mutation. However, the detection limit of
direct sequencing is generally 25–30%, and homogeneous tumour
samples may not be available in a clinical setting. To date,
several strategies to improve the detection limit of the EGFR T790M
mutation have been proposed (as summarised in Table III). Although the sensitivities of
these methods are greatly improved, each method features unique
advantages and disadvantages or difficulties in practical
situations, such as the requirement for special equipment and
expensive reagents or the involvement of complicated procedures. Of
these strategies, the various PCR-based methods are the most
suitable and easy to use in order to detect this known mutation in
clinical practice. Hence, a PCR-based assay was developed in the
present study. This method was sensitive, cost-effective and
clinically applicable for the detection of the EGFR T790M
mutation.
 | Table III.Documented methods for T790M mutation
detection. |
Table III.
Documented methods for T790M mutation
detection.
|
| T790M mutation
detection rate, % (sample type) |
|
|---|
|
|
|
|
|---|
| First author,
year | Methods | Detection range,
% | Disadvantages | Pre-treatment | TKI-resistance | (Ref.) |
|---|
| Sequist et
al, 2008 | Direct
sequencing | 25–35 | Insensitive | 2.04 | NA | (22) |
| Chen et al,
2009 | Scorpion ARMS | 1 | Expensive | 0 | 48.3 | (11) |
| Maheswaran et
al, 2008 |
|
|
| 38 (CTC) | 64 (CTC) | (4) |
| Taniguchi et
al, 2011 | BEAMing |
0.01 | Complicated,
time-consuming | 4.8
(plasma) | 43.5 (plasma) | (19) |
| He et al,
2013 | Direct
sequencing | NA | Time-consuming | NA | 6.1
(plasma) | (9) |
|
| Mutant-enriched
PCR |
0.1 |
|
| 36.4 (plasma) |
|
| Arcila et
al, 2011 |
PCR-sequencing/FA | 12.5 | Post-PCR
procedure | NA | 52 | (12) |
|
|
LNA-PCR-sequencing |
0.1 |
|
| 68 |
|
| Rosell et
al, 2011 | PNA-TaqMan PCR | NA | Acceptable | 35–38 | NA | (3) |
| Miyazawa et
al, 2008 | PNA-LNA PCR |
0.1 | Acceptable | 0 | NA | (13) |
| Oh et al,
2011 | PNA-clamping
PCR |
0.01 | Acceptable | 8.2 | NA | (14) |
| Oh et al,
2010 | Molecular
beacon-PCR | 2 | Insensitive | NA | NA | (18) |
| Li et al,
2009 | COLD-PCR |
0.8 | Acceptable | NA | NA | (17) |
| Kim et al,
2013 | Pyrosequencing | NA | Insensitive | 0.49 | NA | (23) |
| Inukai et
al, 2006 | Direct
sequencing | NA | Acceptable | 0.36 | NA | (10) |
|
| Mutant-enriched
PCR |
0.1 |
| 3.2 |
|
|
| Guha et al,
2013 | DISSECT-PNA-LNA
PCR |
0.01 | Complicated | NA | NA | (15) |
| Fujita et
al, 2012 | PCR-clone
hybridisation | NA | Complicated | 79 | NA | (5) |
| Su et al,
2012 | Direct
sequencing | 25–35 | Special
equipment | 2.7–2.8 | 33.3 | (2) |
|
| MALDI-TOF-MS |
1.5 | required | 25.2–31.5 | 83.3 |
|
The method used to screen such a small fraction of
mutant alleles as the template should be highly specific and
sensitive. Based on the mimic human genomic DNA panel that
consisted of serially diluted plasmids containing T790M mutation,
the present study results revealed that the ACB-ARMS PCR assay can
detect 0.01% mutant alleles in total genomic DNA, which corresponds
to <10 copies. This method is comparable to PNA-clamping PCR
(14,15) or the BEAMing assay (20), and is more sensitive than any other
documented method (Table III)
(2–5,9–15,17–19,22,23).
Regarding specificity, the purine/purine and pyrimidine/pyrimidine
mismatches are considerably more selective than the
purine/pyrimidine mismatches (24).
Thus, the relatively weak G/T mismatch of the wild-type
template/primer leads to base misincorporation during PCR and
false-positive results when the ARMS method is used. Hence, in the
present study, another mismatched site (C>T) was designed at the
third base of the 3′-ends of the forward primer of the T790M
mutation to decrease the non-specific combination of the primer
with wild-type template alleles. This method was used to screen 10
different concentrations of human wild genomic DNA (Sigma-Aldrich
China Inc.), and no specific amplifications were found, which
suggested that this assay is highly specific (data not shown).
In clinical practice, 27 TKI-naïve clinical samples
were successfully screened in the present study using this method
and 6 mutant samples of T790M (22.2%) were found. These results
were confirmed by performing ACB-ARMS PCR in triplicate. The
positive examples were further identified by clone sequencing, and
all mutant cases harboured T790M mutant colonies in different
proportions. These results also strongly suggested the high
specificity of this method. Furthermore, the result was consistent
with the majority of previous studies (2–4), in which
the incidence of de novo T790M mutation ranged from 25.2 to
38% in TKI pre-treated specimens, as detected by relatively
sensitive methods. These findings suggested that T790M mutation is
more predominant than expected in pre-treated samples, and
indicated the high practicability of this protocol.
The present study showed that the incidence of T790M
mutations was not associated with any other clinical factors,
including gender, age, smoking status, pathological type and
EGFR-activating mutations, which was similar to results reported in
a study by Oxnard et al (25).
However, certain studies have suggested that the T790M mutation is
associated with a late disease stage (10) and EGFR 19 Del mutations (3), which was not confirmed by the present
study due to the limited sample size. In TKI-naïve specimens, the
predicted value of T790M mutation for TKI response duration was
contradictory. In the studies of Su et al (2) or Rosell et al (3), patients with the T790M mutation
experienced a shorter PFS time in response to EGFR-TKI treatment in
comparison to patients without T790M in TKI-naïve samples. However,
Fujita et al (5) reported that
a high proportion of T790M alleles may define a clinical subset
with a relatively favourable prognosis. However, the present study
also did not reveal significant differences in the PFS time based
on the T790M mutation status in NSCLC patients harbouring
EGFR-activating mutations (3.2 vs. 19.5 months in patients with or
without the mutation, respectively; P=0.256). Certain important
issues regarding the association between T790M mutation and PFS
time should to be considered in the present study. First, this
retrospective study examined a limited simple size, and a selection
bias did exist. Second, a number of additional factors regulate the
TKI response duration beyond EGFR T790M mutation. Intratumoural
EGFR mutational heterogeneity (26)
and other EGFR-related genetic aberrances and downstream pathways
(27), such as C-Met amplification,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
mutation and EGFR amplification (1),
have been identified as associated with TKI resistance, which
confounded the effect of T790M on PFS time. Although the start time
for OS was defined as the time of diagnosis, not the time of
informed consent or EGFR-TKI treatment, in the present study, the
median OS time in the different groups did not significantly differ
(10 vs. 20 months, respectively; P=0.689), which was consistent
with the study by Su et al (2). However, more recent studies have also
revealed contradictory effects of T790M mutation on OS time. Oxnard
et al (25) and Hata et
al (28) reported that patients
who acquired TKI resistance and harboured the T790M mutation
experienced a significantly longer post-progression survival time
than patients without the T790M mutation (19 vs. 12 months;
P=0.036). However, Lee et al (29) reported that the patients with
T790M-positive tumours experienced a shorter overall survival time
than those with T790M-negative tumours (median, 9.1 vs. 18.7
months; P=0.018). Numerous factors affect the OS, including the
patient's performance status score, cancer therapy, disease stage,
and importantly, organ metastasis. In basic studies, it has been
revealed that tumour cells carrying the T790M mutation grow slowly
and present with indolent biological behaviours (30). Therefore, the true prognostic value of
T790M mutation should be further investigated. Furthermore, large
prospective randomised clinical trials with excellent designs to
balance the confounding factors in different groups are warranted
to validate the clinical significance of T790M mutation in
TKI-naïve specimens.
In summary, the ACB-ARMS PCR assay considerably
improves the sensitivity and specificity of T790M mutation
detection, and could be a promising method to screen for this
mutation in lung cancer samples that contain only a small number of
mutant cells. The clinical significance of de novo T790M
mutation should be further investigated in future studies.
References
|
1
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su KY, Chen HY, Li KC, Kuo ML, Yang JC,
Chan WK, Ho BC, Chang GC, Shih JY, Yu SL and Yang PC: Pretreatment
epidermal growth factor receptor (EGFR) T790M mutation predicts
shorter EGFR tyrosine kinase inhibitor response duration in
patients with non-small-cell lung cancer. J Clin Oncol. 30:433–440.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosell R, Molina MA, Costa C, Simonetti S,
Gimenez-Capitan A, Bertran-Alamillo J, Mayo C, Moran T, Mendez P,
Cardenal F, et al: Pretreatment EGFR T790M mutation and BRCA1 mRNA
expression in erlotinib-treated advanced non-small-cell lung cancer
patients with EGFR mutations. Clin Cancer Res. 17:1160–1168. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maheswaran S, Sequist LV, Nagrath S, Ulkus
L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ,
Bell DW, et al: Detection of mutations in EGFR in circulating
lung-cancer cells. N Engl J Med. 359:366–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujita Y, Suda K, Kimura H, Matsumoto K,
Arao T, Nagai T, Saijo N, Yatabe Y, Mitsudomi T and Nishio K:
Highly sensitive detection of EGFR T790M mutation using colony
hybridization predicts favorable prognosis of patients with lung
cancer harboring activating EGFR mutation. J Thorac Oncol.
7:1640–1644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janne PA, Ramalingam SS, Yang JC, et al:
Clinical activity of the mutant-selective EGFR inhibitor AZD9291 in
patients (pts) with EGFR inhibitor-resistant non-small cell lung
cancer (NSCLC). J Clin Oncol. 32(Suppl 15): 508s Abstract
80092014.
|
|
7
|
Sequist LV, Soria J, Gadgeel SM, et al:
First-in-human evaluation of CO-1686, an irreversible, highly
selective tyrosine kinase inhibitor of mutations of EGFR
(activating and T790M). J Clin Oncol. 32(Suppl 15): 508s Abstract
80102014.
|
|
8
|
Kim D, Lee D, Kang J, et al: Clinical
activity and safety of HM61713, an EGFR-mutant selective inhibitor,
in advanced non-small cell lung cancer (NSCLC) patients (pts) with
EGFR mutations who had received EGFR tyrosine kinase inhibitors
(TKIs). J Clin Oncol. 32(Suppl 15): 508s Abstract 80112014.
|
|
9
|
He C, Zheng L, Xu Y, Liu M, Li Y and Xu J:
Highly sensitive and noninvasive detection of epidermal growth
factor receptor T790M mutation in non-small cell lung cancer. Clin
Chim Acta. 425:119–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inukai M, Toyooka S, Ito S, Asano H,
Ichihara S, Soh J, Suehisa H, Ouchida M, Aoe K, Aoe M, et al:
Presence of epidermal growth factor receptor gene T790M mutations
as a minor clone in non-small cell lung cancer. Cancer Res.
66:7854–7858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen HJ, Mok TS, Chen ZH, Guo AL, Zhang
XC, Su J and Wu YL: Clinicopathologic and molecular features of
epidermal growth factor receptor T790M mutation and c-MET
amplification in tyrosine kinase inhibitor-resistant Chinese
non-small cell lung cancer. Pathol Oncol Res. 15:651–658. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arcila ME, Oxnard GR, Nafa K, Riely GJ,
Solomon SB, Zakowski MF, Kris MG, Pao W, Miller VA and Ladanyi M:
Rebiopsy of lung cancer patients with acquired resistance to EGFR
inhibitors and enhanced detection of the T790M mutation using a
locked nucleic acid-based assay. Clin Cancer Res. 17:1169–1180.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyazawa H, Tanaka T, Nagai Y, Matsuoka M,
Huqun Sutani A, Udagawa K, Zhang J, Hirama T, Murayama Y, et al:
Peptide nucleic acid-locked nucleic acid polymerase chain reaction
clamp-based detection test for gefitinib-refractory T790M epidermal
growth factor receptor mutation. Cancer Sci. 99:595–600. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oh JE, An CH, Yoo NJ and Lee SH: Detection
of low-level EGFR T790M mutation in lung cancer tissues. APMIS.
119:403–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guha M, Castellanos-Rizaldos E and
Makrigiorgos GM: Dissect method using PNA-LNA clamp improves
detection of EGFR T790m mutation. PLoS One. 8:e677822013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuang Y, Rogers A, Yeap BY, Wang L,
Makrigiorgos M, Vetrand K, Thiede S, Distel RJ and Jänne PA:
Noninvasive detection of EGFR T790M in gefitinib or erlotinib
resistant non-small cell lung cancer. Clin Cancer Res.
15:2630–2636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Wang L, Jänne PA and Makrigiorgos
GM: Coamplification at lower denaturation temperature-PCR increases
mutation-detection selectivity of TaqMan-based real time PCR. Clin
Chem. 55:748–756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oh YH, Kim Y, Kim YP, Seo SW, Mitsudomi T,
Ahn MJ, Park K and Kim HS: Rapid detection of the epidermal growth
factor receptor mutation in non-small-cell lung cancer for analysis
of acquired resistance using molecular beacons. J Mol Diagn.
12:644–652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taniguchi K, Uchida J, Nishino K, Kumagai
T, Okuyama T, Okami J, Higashiyama M, Kodama K, Imamura F and Kato
K: Quantitative detection of EGFR mutations in circulating tumor
DNA derived form lung adenocarcinomas. Clin Cancer Res.
17:7808–7815. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Board RE, Thelwell NJ, Ravetto PF, Little
S, Ranson M, Dive C, Hughes A and Whitcombe D: Multiplexed assays
for detection of mutations in PIK3CA. Clin Chem. 54:757–760. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sequist LV, Martins RG, Spigel D, Grunberg
SM, Spira A, Jänne PA, Joshi VA, McCollum D, Evans TL, Muzikansky
A, et al: First-line gefitinib in patients with advanced non-small
cell lung cancer harboring somatic EGFR mutations. J Clin Oncol.
26:2442–2449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HJ, Oh SY, Kim WS, Kim SJ, Yoo GH, Kim
WD and Lee KY: Clinical investigation of EGFR mutation detection by
pyrosequencing in lung cancer patients. Oncol Lett. 5:271–276.
2013.PubMed/NCBI
|
|
24
|
Newton CR, Graham A, Heptinstall LE,
Powell SJ, Summers C, Kalsheker N, Smith JC and Markham AF:
Analysis of any point mutation in DNA. The amplification refractory
mutation system (ARMS). Nucleic Acids Res. 17:2503–2516. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oxnard GR, Arcila ME, Sima CS, Riely GJ,
Chmielecki J, Kris MG, Pao W, Ladanyi M and Miller VA: Acquired
resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung
cancer: Distinct natural history of patients with tumors harboring
the T790M mutation. Clin Cancer Res. 17:1616–1622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bai H, Wang Z, Wang Y, Zhuo M, Zhou Q,
Duan J, Yang L, Wu M, An T, Zhao J and Wang J: Detection and
clinical significance of intratumoral EGFR mutational heterogeneity
in Chinese patients with advanced non-small cell lung cancer. PLoS
One. 8:e541702013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim HR, Cho BC, Shim HS, Lim SM, Kim SK,
Chang J, Kim DJ and Kim JH: Prediction for response duration to
epidermal growth factor receptor-tyrosine kinase inhibitors in EGFR
mutated never smoker lung adenocarcinoma. Lung Cancer. 83:374–782.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hata A, Katakami N, Yoshioka H, Takeshita
J, Tanaka K, Nanjo S, Fujita S, Kaji R, Imai Y, Monden K, et al:
Rebiopsy of non-small cell lung cancer patients with acquired
resistance to epidermal growth factor receptor-tyrosine kinase
inhibitor: Comparison between T790M mutation-positive and
mutation-negative populations. Cancer. 119:4325–4332. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee Y, Lee GK, Lee YS, Zhang W, Hwang JA,
Nam BH, Kim SH, Kim JH, Yun T, Han JY, et al: Clinical outcome
according to the level of preexisting epidermal growth factor
receptor T790M mutation in patients with lung cancer harboring
sensitive epidermal growth factor receptor mutations. Cancer.
120:2090–2098. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chmielecki J, Foo J, Oxnard GR, Hutchinson
K, Ohashi K, Somwar R, Wang L, Amato KR, Arcila M, Sos ML, et al:
Optimization of dosing for EGFR-mutant non-small cell lung cancer
with evolutionary cancer modeling. Sci Transl Med. 3:90ra592011.
View Article : Google Scholar : PubMed/NCBI
|