Introduction
Giant cell tumor of bone (GCTB) is a relatively
uncommon primary bone tumor that exhibits an aggressive character
and a high risk of local recurrence following surgery. GCTB
accounts for 4–5% of all primary bone tumors and 13–20% of all
benign bone tumors (1,2). These tumors frequently occur in
skeletally mature persons, with a peak incidence in the third to
fourth decade of life, while rarely arise in patients with an open
growth plate. In addition, GCTBs display a slight female
preponderance (3). The majority of
GCTBs follow a benign course. However, GCTBs often exhibit local
recurrence following surgery (3), and
a previous study reported that pulmonary metastases develop despite
the presence of benign histological features in 3% of patients with
GCTB (4). GCTBs may undergo malignant
transformation (3). Rock et al
(5) reported that this may occur as a
result of dedifferentiation of the primary tumor, or secondary to
prior radiation therapy.
The majority of GCTBs are located at the end of long
bones, and ~50~60% of them are located around the knee, distal
femur and proximal tibia, being the distal femur the bone most
frequently involved (6–8).
A previous study described that GCTB arises in the
epiphyseal region of long tubular bones (9). The recent literature states that the
majority of GCTBs exhibit a typical metaphyseal/epiphyseal location
(10), whereas GCTBs may be centered
in the metaphysis in children with open physes (11). However, no studies have ever been
conducted to determine precisely the site of origin of GCTB. Thus,
the purpose of the present study was to determine the site of
origin of GCTB of the extremities and to analyze the pattern of
progression in GCTB of long bones.
Materials and methods
A total of 128 patients were diagnosed with GCTB at
Nagoya University Graduate School of Medicine (Nagoya, Japan)
between October 1977 and September 2011. Of these, GCTB cases with
location in the pelvis, vertebrae and small long bones (11 cases),
as well as rare sites such as the distal tibia, distal humerus and
proximal radius (7 cases) were excluded. Recurrent cases at initial
referral (21 cases) and cases with insufficient X-ray data (18
cases) were also excluded. Metabolic bone diseases or brown tumors
based on hyperparathyroidism were not included in the study. In
total, 71 patients (50 males and 21 females) who were
pathologically diagnosed with GCTB and subsequently treated at
Nagoya University Graduate School of Medicine were enrolled in the
present study, which was approved by the Institutional Review Board
of Nagoya University Graduate School of Medicine (approval no.
2013–0134). Written informed consent was obtained from each patient
for participation in the study. Patients' X-ray scans (RADREX-i;
Toshiba Medical Systems Cororation, Otawara, Japan) conducted at
the initial referral were subjected to analysis. The mean age of
the patients at diagnosis was 35 years (range, 13–71 years). The
tumor locations were the distal femur in 31 cases, the proximal
femur in 11 cases, the proximal tibia in 13 cases, the distal
radius in 6 cases, the proximal humerus in 5 cases and the proximal
fibula in 5 cases.
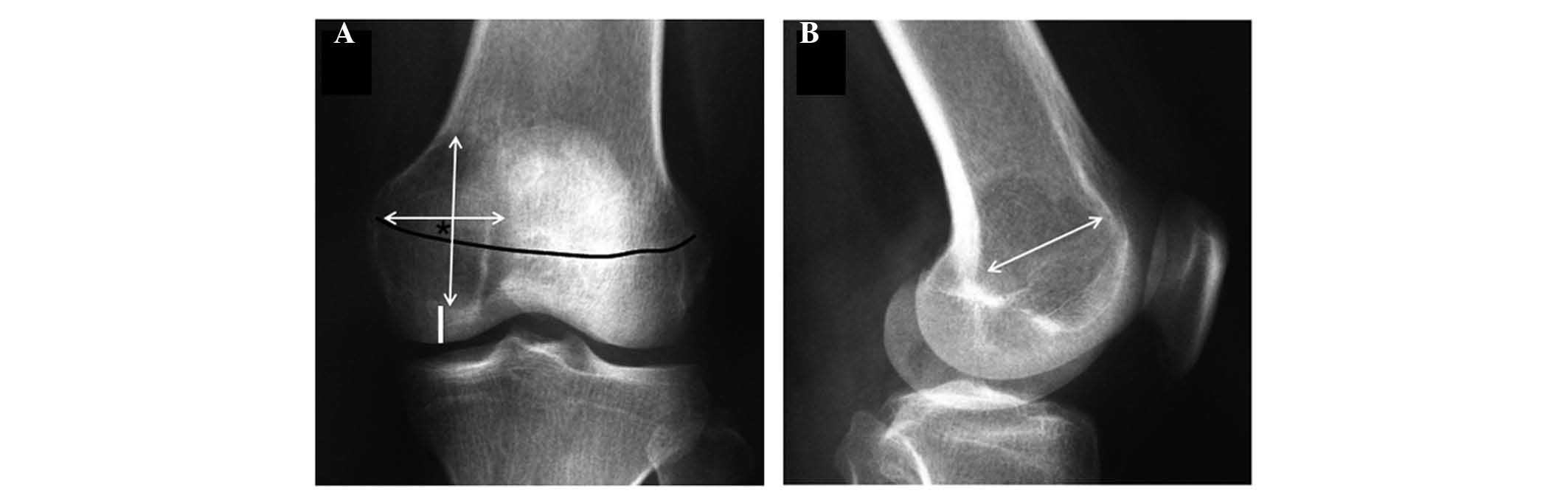
The size and volume of the tumor were estimated
according to the method previously described (12). Briefly, the largest dimensions of the
tumor (depth, width and height) were measured, and it was assumed
that the tumor was spherical in shape. The vertical center (VC) of
the tumor was determined as the center of tumor height on
anteroposterior (AP) X-ray views. The trace of the growth plate was
also determined with AP views (Fig.
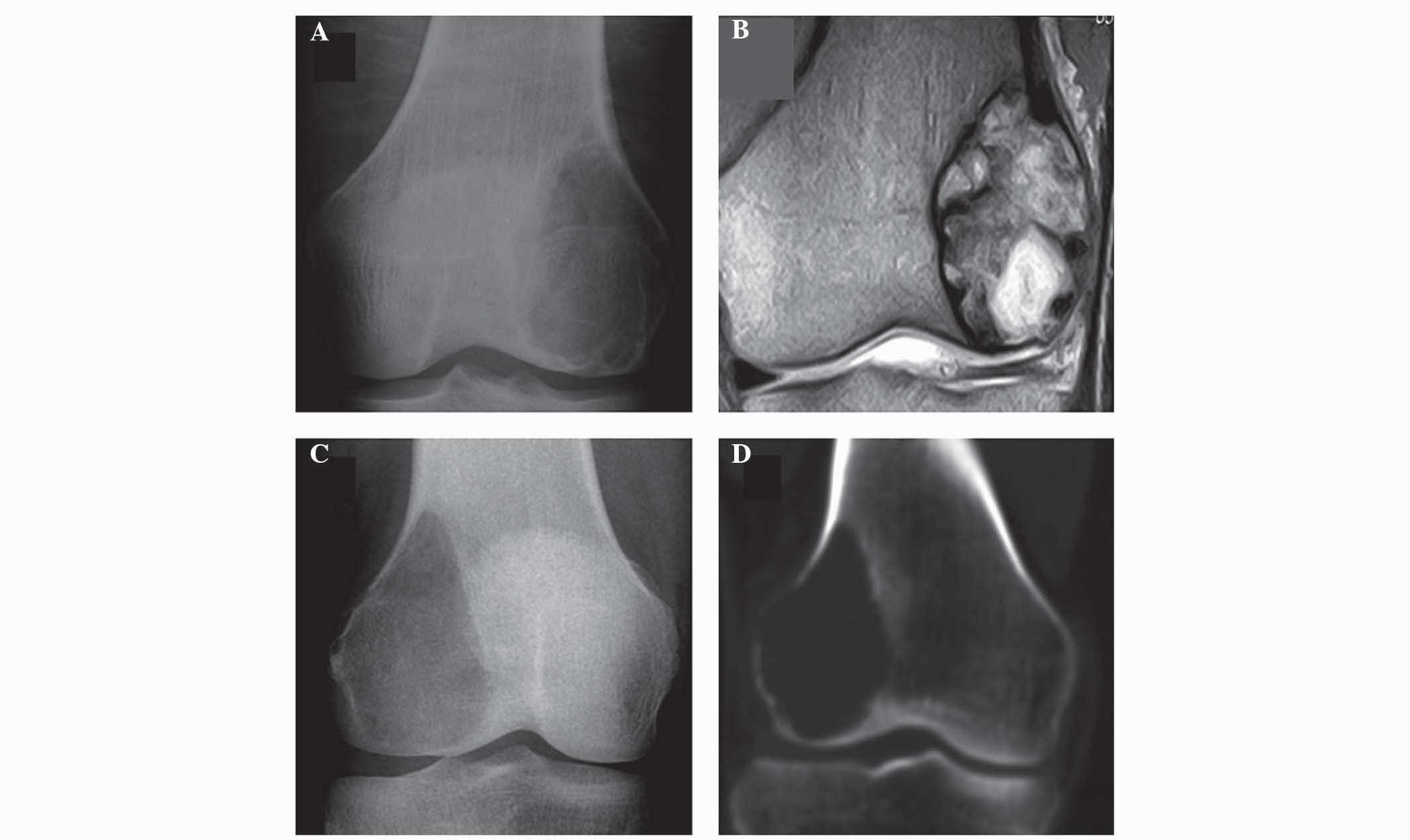
1). The accuracy of the observations conducted with AP X-ray
was confirmed to be adequate, since the same results were obtained
with computed tomography (CT; Aquilion™ ONE; Toshiba Medical
Systems Corporation) or magnetic resonance imaging (MRI; MAGNETOM
Verio; Siemens Healthcare, Erlangen, Germany) (Fig. 2). The absolute intraobserver and
interobserver differences were ≤1 mm in >90% of cases.
In cases where the VC was located in the metaphyseal
region, the distance from the epiphyseal line to the VC was
represented as a positive value, whereas a negative value was
assigned when the VC was located in the epiphyseal region. Joint
surface was defined as the roentgenographic border of long bones on
X-ray AP views. Using these data, a regression model equation was
derived from scatter plot diagrams using commercially available
software (Excel version 2013, Microsoft Corporation, Redmond, WA,
USA; Ekuseru-Toukei version 2012, Social Survey Research
Information Co. Ltd., Tokyo, Japan). Prediction of significant
correlations between each pair of variables was determined by the
value of the Pearsons correlation coefficient (r). The correlation
between the changes of a dependent variable (y) and an independent
variable (x) was ascertained by a simple linear regression, using
y=a+bx as the equation in the regression model, where a=y intercept
when x=0, and b is the regression coefficient. P<0.05 was
considered to indicate a statistically significant difference
(13).
Results
The VC of the tumor was located in the metaphyseal
region in 57 cases, in the epiphyseal line in 11 cases and in the
epiphyseal region in 3 cases (Table
I). The mean distance from the epiphyseal line to the VC was
13.1 mm (range, −20.0 to 50.0 mm). The mean tumor area and volume
were 17.8 cm2 (range, 2.4–62.8 cm2) and 45.7
cm3 (range, 2.4–209.3 cm3), respectively. The
mean distance from the joint space to the tumor border of the
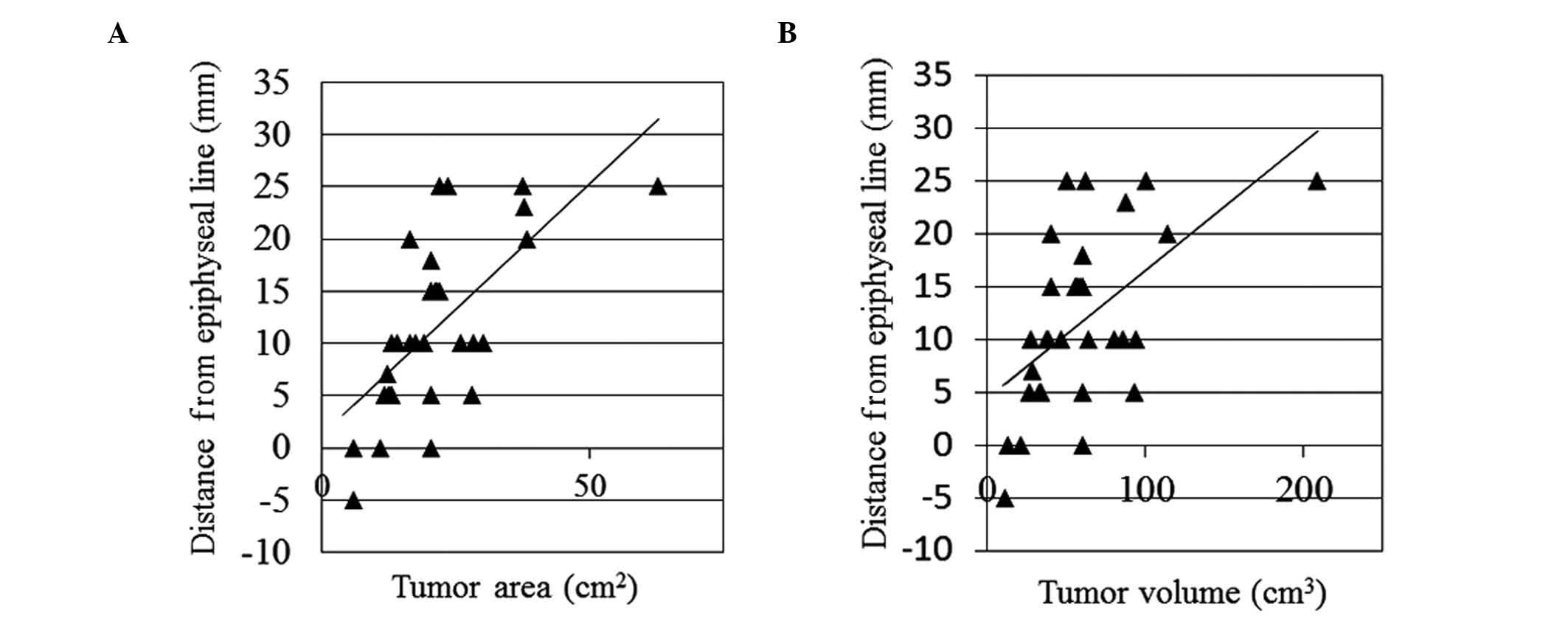
articular side was 6.2 mm (range, 1.0–35.0 mm) (Table II). In cases of distal femur and
proximal tibia, significant associations between the distance from
the epiphyseal line to the tumor VC and the tumor area or volume
were observed. The distance between the tumor VC and the epiphyseal
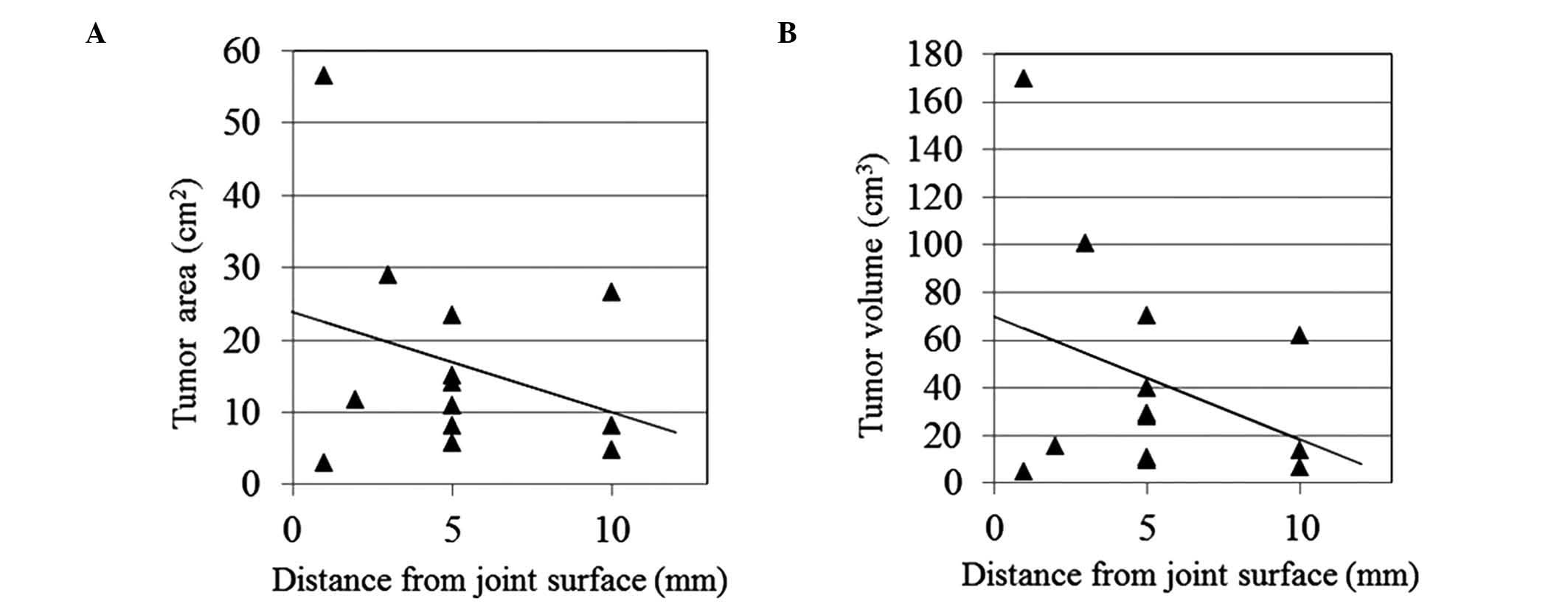
line increased with increasing tumor area or volume. In cases of
distal femur, the r values between the distance from the epiphyseal
line to the tumor VC and the tumor area or volume were 0.439
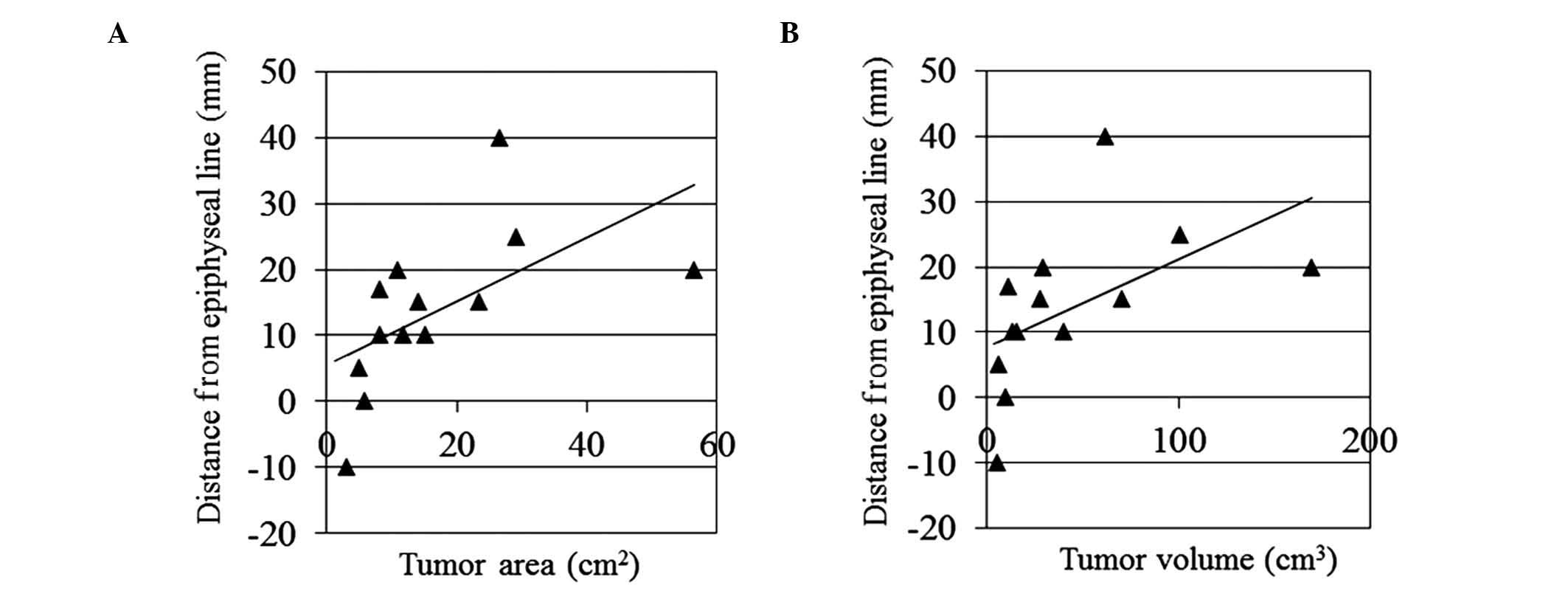
(P<0.001) and 0.313 (P=0.001), respectively (Fig. 3). Significant correlations were also
observed in cases of proximal tibia. The r values between the
distance from the epiphyseal line to the tumor VC and the tumor
area or volume were 0.332 (P=0.002) and 0.276 (P=0.002),
respectively (Fig. 4). A fitted line
corresponding to the regression model equation is represented in
the scatter plot diagrams shown in Figs.
3 and 4. The equations obtained
for the regression model corresponding to the correlation between
the distance from the epiphyseal line to the tumor VC and the tumor
area were y=1.2900+0.4812x in the cases of distal femur, and
y=5.5100+0.4825x in the cases of proximal tibia, where y is the
tumor VC in mm and × is the tumor area in cm2. The
equations obtained for the regression model evaluating the
correlation between the distance from the epiphyseal line to the
tumor VC and the tumor volume were y=4.4200+0.1209x for distal
femur and y=7.800+0.1339x for proximal tibia, where y is the tumor
VC in mm and × is the tumor volume in cm3. If the tumor
volume in the distal femur is hypothesized to be 0 cm3,
the tumor VC is assumed to be located in the metaphyseal region, at
4.4 mm distance from the growth plate. If the tumor volume in the
proximal tibia is hypothesized to be 0 cm3, the tumor VC
is assumed to be located in the metaphyseal region, at 7.8 mm
distance from the growth plate. These findings suggest the site of
origin of GCTB to be the metaphyseal region. No significant
associations between the distance from the epiphyseal line to the
tumor VC and the tumor area or volume were observed in cases of
GCBT located in the proximal femur (P=0.309 and P=0.32), distal
radius (P=0.512 and P=0.506), proximal humerus (P=0.089 and
P=0.172) or proximal fibula (P=0.505 and P=0.505). Regarding the
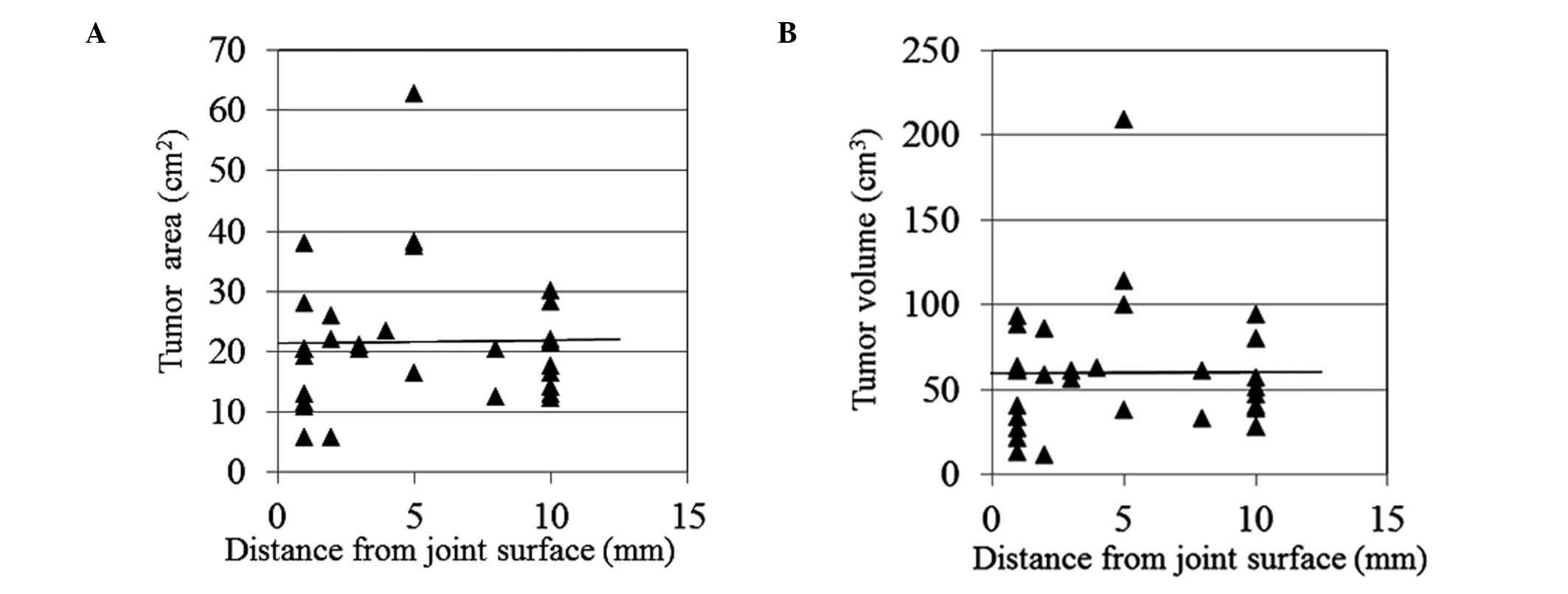
distance from the joint space to the tumor border, no associations
between the distance from the joint surface to the tumor border and
the tumor area or volume were observed in cases of distal femur
(P=0.536 and P=0.903, respectively, Fig.
5). Similarly, no associations were observed in cases of
proximal tibia (tumor area, P=0.526; tumor volume, P=0.555,
Fig. 6). No associations were
observed either in cases of proximal femur (P=0.785 and P=0.636),
distal radius (P=0.414 and P=0.543), proximal humerus (P=0.182 and
P=0.559) or proximal fibula (P=0.559 and P=0.559).
 | Table I.Distribution of the vertical center of
the tumor in patients with giant cell tumor of bone. |
Table I.
Distribution of the vertical center of
the tumor in patients with giant cell tumor of bone.
| Tumor site | Metaphysis | Epiphyseal line | Epiphysis |
|---|
| Distal femur | 27 | 3 | 1 |
| Proximal femur | 8 | 2 | 1 |
| Proximal tibia | 11 | 1 | 1 |
| Distal radius | 2 | 4 | 0 |
| Proximal humerus | 5 | 0 | 0 |
| Proximal fibula | 4 | 1 | 0 |
 | Table II.Mean distance from the epiphyseal line
to the tumor VC, tumor area, tumor volume and distance from the
joint surface to the tumor border in patients with giant cell tumor
of bone. |
Table II.
Mean distance from the epiphyseal line
to the tumor VC, tumor area, tumor volume and distance from the
joint surface to the tumor border in patients with giant cell tumor
of bone.
| Tumor site | Epiphyseal line to
VC, mm (range) | Tumor area,
cm2 (range) | Tumor volume,
cm3 (range) | Joint surface to
tumor border, mm (range) |
|---|
| Distal femur | 11.7 (−5.0 to
25.0) | 21.6 (5.9–62.8) | 60.3
(11.8–209.3) | 4.9 (1.0–10.0) |
| Proximal femur | 19.0 (−20.0 to
50.0) | 17.8 (4.7–42.4) | 37.8 (11.0–84.8) | 12.8 (1.0–35.0) |
| Proximal tibia | 13.6 (−10.0 to
40.0) | 16.8 (3.1–56.5) | 43.3 (5.2–169.6) | 5.2 (1.0–10.0) |
| Distal radius | 1.3 (0.0–5.0) | 4.6 (2.4–9.6) | 6.6 (2.4–16.0) | 1.7 (1.0–3.0) |
| Proximal humerus | 23.6 (20.0–30.0) | 18.0 (7.9–31.4) | 48.6 (13.1–91.6) | 10.6 (10.0–13.0) |
| Proximal fibula | 12.0 (0.0–25.0) | 12.3 (7.9–20.4) | 22.9 (10.5–40.8) | 2.8 (1.0–10.0) |
| All cases | 13.1 (−20.0 to
50.0) | 17.8 (2.4–62.8) | 45.7 (2.4–209.3) | 6.2 (1.0–35.0) |
In addition, a significant association between
patients' age and tumor area or volume was observed only in cases
of proximal tibia, whereas no association was observed in any of
the other cases.
Discussion
To the best of our knowledge, the results of the
present study suggest for the first time the site of origin of GCTB
to be the metaphyseal region, using equations fitted to regression
models. The exact site of origin of GCT remains controversial.
Murphey et al (14) reported
that in skeletally immature patients, GCTBs were located in
metaphyseal rather than meta-epiphyseal bone, with an open
epiphyseal plate acting as a barrier to tumor growth. Fain et
al (15) reported non-epiphyseal
GCTB of long bones. Of 1,682 cases of GCTB reported by the authors,
only 14 (0.8%) were located in exclusively metaphyseal or
diaphyseal regions. A notable finding of that study was the fact
that the majority of patients with non-epiphyseal GCTB were <15
year-old (15). These unusual cases
raised debate as to whether GCTB is capable of developing in the
epiphysis (6,9) or in the metaphysis, with subsequent
extension to the epiphysis following growth plate closure (16–18).
Gandhe et al (19) reported
cases of epiphyseal GCTB. However, considering that GCTB is
occasionally misdiagnosed or confused with other giant cell-rich
tumors, other giant cell-containing tumors such as giant cell-rich
osteosarcoma and chondroblastoma should be strictly differentiated
from epiphyseal GCTB by experienced pathologists (3).
The current study revealed that the distance between
the joint surface and the tumor border of the articular side is
short, even in cases of small tumors. These results are consistent
with those reported by Murphey et al (14), whereby 84–99% of lesions extended to
locations within 1 cm of subarticular bone (14). Suzuki et al (20) reported that less residual thickness of
subarticular bone correlated with higher recurrence following
surgery for GCTB, and tended to be associated with secondary
osteoarthritis. Thus, it may be hypothesized that early diagnosis
may lead to preservation of a sufficient quantity of subchondral
bone, and as a result, the rate of local recurrence may decrease
and the functional outcome may improve. However, the biological
character of GCTB, which easily extends to the articular side
(epiphyseal region) of the affected long bones, difficulties the
reduction of the recurrence rate and induces the development of
postoperative osteoarthritic changes.
Gandhe et al (19) and Kransdorf et al (21) reported that GCTB lesions involve the
metaphysis rather than the epiphysis in skeletally immature
patients, since the open epiphyseal plate acts as a barrier to
tumor growth. Puri et al (22)
noted that an open physis did not prevent GCTBs from penetrating
the epiphyseal cartilage. Campanacci et al (23) observed invasion of the joint in only
5% of GCTB cases. Based on these previous reports, it may be
proposed that the expansion of GCTBs is partly inhibited by the
presence of articular cartilage or an open growth plate. As
indicated in the current study, GCTB appears to arise at a
metaphyseal site and extend in a diaphyseal and epiphyseal
direction. There was observed to be no barrier in the diaphysis
against tumor extension, whereas tumor growth was inhibited by
articular cartilage. As a result, the tumor VC may shift slowly in
a diaphyseal direction. However, the site of origin of GCTB is
suggested to be the metaphyseal region, according to the regression
model equations discussed in the present study.
There are a number of limitations affecting the
current study. First, a relatively small number of patients were
included in the study, which may be underpowered to obtain more
meaningful results regarding the original site of GCTBs. Second, it
is not possible to analyze or explain the site-specific differences
observed in the present study, since the location of GCTBs in the
proximal femur, distal radius and proximal fibula should be
analyzed in a larger number of cases, based on multicenter
analyses. Third, other radiological modalities including CT or MRI
may evaluate more precisely the VC of GCTBs than X-rays, although,
as demonstrated in the present study, AP X-rays were able to
evaluate the center of tumor as adequately as CT or MRI. Fourth, a
tumor is unable to extend to the articular side once it reaches the
subchondral region, possibly biasing the results of the current
analyses. However, the significant correlations obtained with the
regression model equations suggest the reliability of the present
results.
In conclusion, the findings of the present study
indicate that the site of origin of GCTB is possibly the
metaphyseal region. The results of the current study provide useful
information regarding the clinical course of GCTB for physicians,
and suggest that early detection may be crucial to cure this
refractory benign tumor.
Acknowledgements
The authors would like to thank Miss Eri Ishihara
(Department of Orthopedic Surgery, Nagoya University Graduate
School of Medicine) for her secretarial assistance during the
present study, and Dr Satoshi Yamashita (Department of Orthopedic
Surgery, Nagoya University Graduate School of Medicine) for his
support with statistical analyses. The present study was partly
funded by the Ministry of Education, Culture, Sports, Science and
Technology of Japan [Tokyo, Japan; grant-in-aid no. 262933341 for
Scientific Research (B)] and partly by the National Cancer Center
Research and Development Fund (Tokyo, Japan; grant no. 26-A-4).
References
|
1
|
Chakarun CJ, Forrester DM, Gottsegen CJ,
Patel DB, White EA and Matcuk GR Jr: Giant cell tumor of bone:
Review, mimics, and new developments in treatment. Radiographics.
33:197–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niu X, Zhang Q, Hao L, Ding Y, Li Y, Xu H
and Liu W: Giant cell tumor of the extremity: Retrospective
analysis of 621 Chinese patients from one institution. J Bone Joint
Surg Am. 94:461–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campanacci M: Giant Cell Tumor. Bone and
Soft Tissue Tumors. Campanacci M (ed). (2nd). Springer. (Vienna).
99–142. 1990.
|
|
4
|
Tubbs WS, Brown LR, Beabout JW, Rock MG
and Unni KK: Benign giant-cell tumor of bone with pulmonary
metastases: Clinical findings and radiologic appearance of
metastases in 13 cases. AJR Am J Roentgenol. 158:331–334. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rock MG, Sim FH, Unni KK, Witrak GA,
Frassica FJ, Schray MF, Beabout JW and Dahlin DC: Secondary
malignant giant-cell tumor of bone. Clinicopathological assessment
of nineteen patients. J Bone Joint Surg Am. 68:1073–1079.
1986.PubMed/NCBI
|
|
6
|
Dahlin DC, Cupps RE and Johnson EW Jr:
Giant-cell tumor: A study of 195 cases. Cancer. 25:1061–1070. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang N, Qin CH, Tan CX, Wen SF, Ma YF,
Dong F, Diao XC, Zhang P and Yu B: A retrospective analysis of 140
patients with giant cell tumor in the extremity: A multicenter
study based on four hospitals in South China. Cancer Epidemiol.
37:294–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saikia KC, Bhuyan SK, Borgohain M, Saikia
SP, Bora A and Ahmed F: Giant cell tumour of bone: An analysis of
139 Indian patients. J Orthop Sci. 16:581–588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldenberg RR, Campbell CJ and Bonfiglio
M: Giant-cell tumor of bone. An analysis of two hundred and
eighteen cases. J Bone Joint Surg Am. 52:619–664. 1970.PubMed/NCBI
|
|
10
|
Turcotte RE: Giant cell tumor of bone.
Orthop Clin North Am. 37:35–51. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raskin KA, Schwab JH, Mankin HJ,
Springfield DS and Hornicek FJ: Giant cell tumor of bone. J Am Acad
Orthop Surg. 21:118–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeys LM, Suneja R, Chami G, Grimer RJ,
Carter SR and Tillman RM: Impending fractures in giant cell tumours
of the distal femur: Incidence and outcome. Int Orthop. 30:135–138.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mondal MK, Jana TK, Jana Giri S and Roy H:
Height prediction from ulnar length in females: A study in Burdwan
district of West Bengal (regression analysis). J Clin Diagn Res.
6:1401–1404. 2012.PubMed/NCBI
|
|
14
|
Murphey MD, Nomikos GC, Flemming DJ,
Gannon FH, Temple HT and Kransdorf MJ: From the archives of AFIP.
Imaging of giant cell tumor and giant cell reparative granuloma of
bone: Radiologic-pathologic correlation. Radiographics.
21:1283–1309. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fain JS, Unni KK, Beabout JW and Rock MG:
Nonepiphyseal giant cell tumor of the long bones. Clinical,
radiologic and pathologic study. Cancer. 71:3514–3519. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campanacci M, Giunti A and Olmi R:
Giant-cell tumors of bone. A study of 209 cases with long term
follow up in 130. Ital J Orthop Traumatol. 1:249–277. 1975.
|
|
17
|
Peison B and Feigenbaum J: Metaphyseal
giant-cell tumor in a girl of 14. Radiology. 118:145–146. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rietveld LA, Mulder JD Brûtel, de la
Rivière G and van Rijssel TG: Giant cell tumour: Metaphyseal or
epiphyseal origin? Diagn Imaging. 50:289–293. 1981.PubMed/NCBI
|
|
19
|
Gandhe A, Sankhe A, Aeron G and Joshi A:
Epiphyseal giant cell tumour in an immature skeleton. Br J Radiol.
81:e75–e78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki Y, Nishida Y, Yamada Y, Tsukushi S,
Sugiura H, Nakashima H and Ishiguro N: Re-operation results in
osteoarthritic change of knee joints in patients with giant cell
tumor of bone. Knee. 14:369–374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kransdorf MJ, Sweet DE, Buetow PC, Giudici
MA and Moser RP Jr: Giant cell tumor in skeletally immature
patients. Radiology. 184:233–237. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Puri A, Agarwal MG, Shah M, Jambhekar NA,
Anchan C and Behle S: Giant cell tumor of bone in children and
adolescents. J Pediatr Orthop. 27:635–639. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Campanacci M, Baldini N, Boriani S and
Sudanese A: Giant-cell tumor of bone. J Bone Joint Surg Am.
69:106–114. 1987.PubMed/NCBI
|