Introduction
Mitogen-activated protein kinases (MAPKs) mediate
intracellular signals transduced by a number of cell surface
receptors, including epidermal growth factor receptor (EGFR). Among
the MAPK-mediated pathways, the Raf-MEK-ERK signaling pathway has
been well characterized and its importance in oncogenesis has been
previously demonstrated (1,2). Aberrant activation of the Raf-MEK-ERK
pathway is frequently observed in human cancers and contributes to
oncogenic properties, such as enhanced cellular proliferation,
independent of growth factors and their receptors (3). The activating mutation of BRAF that
constitutively activates the Ras-MEK-ERK pathway has been
identified in 8% of all human cancers and in 59% of malignant
melanomas (MMs) (4).
Cluster of differentiation 147 (CD147)/basigin, an
integral plasma membrane protein belonging to the immunoglobulin
superfamily, is a multipotential molecule expressed widely in
various tissues (5–7). The protein is particularly enriched on
the surface of malignant tumor cells (8–13),
including MM cells (14–17). We previously reported that CD147
expressed on MM cells played an important role in cellular
proliferation and tumor growth in vitro and in vivo
(14,17). Our previous in vitro study
demonstrated that CD147 promotes the cellular proliferation,
invasiveness, metastasis and angiogenesis of MM cells by regulating
tumor cell glycolysis (17). CD147 is
a multifunctional molecule, and in addition to the regulating of
glycolysis, it has a wide range of pathophysiological functions
(7). Previous studies have
demonstrated that CD147 promotes the growth of human breast
carcinoma cells by stimulating hyaluronan production (18) and that it confers resistance to
apoptosis (19). In these
pathophysiological processes, CD147 directly activates ERK.
The present study investigated the correlation
between CD147 and the Raf-MEK-ERK pathway using the A375 MM cell
line that harbors the activating mutation of BRAF (20). Considering that CD147 and EGFR promote
tumor cell proliferation, it was initially anticipated that EGFR
phosphorylation would be reduced by CD147 silencing. However, our
preliminary studies identified that CD147 silencing enhances the
phosphorylation of EGFR and, therefore, the present study aimed to
investigate the molecular mechanism(s) underlying this finding.
Materials and methods
Cell Culture
The A375 human MM cell line was purchased from the
American Type Culture Collection (Manassas, VA, USA). All cells
were grown in Dulbecco's modified Eagle's medium supplemented with
10% fetal bovine serum and 1% penicillin-streptomycin-amphotericin
B solution (Invitrogen Life Technologies, Carlsbad, CA, USA). The
cells were maintained at 37°C in a 5% CO2 humidified
atmosphere.
CD147 silencing
A U6-vshRNA-CMV-PUR lentivirus encoding CD147 short
hairpin (sh)RNA (CD147 shRNA lentivirus) was constructed and
produced by Shanghai Genechem Co., Ltd. (Shanghai, China) (21). The CD147 shRNA sequences were as
follows: Sequence 1,
5′-TGTCGTCAGAACACATCAACTTCAAGAGAGTTGATGTGTTCTGACGACTTTTTTC-3′ and
sequence 2,
5′-TCGAGAAAAAAGTCGTCAAACACATCAACTCTCTTGAAGTTGATGTGTTCTGACGACA-3′.
Lentiviral infection
The A375 cells were seeded in 12-well plates in 1 ml
of growth medium without antibiotics. Once the cells had reached
70% confluency (after ~24 h), they were infected with 5×108 TU/ml
CD147 shRNA lentivirus or empty lentivirus (control), as described
previously (21), for 4 h at 37°C.
The A375 cells were then grown for 7–10 days in 2 ml of medium
containing 400 ng/ml puromycin (Sigma-Aldrich, St. Louis, MO, USA)
and then for ≥2 weeks in medium containing 200 ng/ml puromycin
until stable cell clones could be harvested. Infected cells were
selected based on their resistance to puromycin.
Western blot
EGF (100 ng/ml; Wako Chemicals USA, Inc., Richmond,
VA, USA) was added to the A375 cell cultures for 0,2,5 and 10 min
to induce EGFR phosphorylation. Following this, total protein was
isolated by detaching and suspending the cells in lysis buffer (150
mmol/l NaCl, 10 mmol/l Tris, 0.1% sodium dodecylsulfate, 1.0%
Triton X-100, 1% deoxycholates, 5 mmol/l ethylene diaminetetra
acetate) on ice for 30 min and then centrifuging (4,500 × g for 10
min). Protein concentration was determined using the bicinchoninic
acid assay (Pierce Biotechnology, Inc., Rockford, IL, USA)
according to the manufacturer's instructions. Equal amounts of
proteins were separated by SDS polyacrylamide gel electrophoresis
and then electrotransferred to polyvinylidene fluoride membranes.
Non-specific antibody binding sites were blocked overnight at 4°C
in Tris-buffered saline containing 5% skimmed dry milk and 0.1%
Tween-20. The membranes were subsequently incubated for 1 h at room
temperature (RT) with polyclonal rabbit anti-human CD147 (cat. no.
34–5600; 1:2,000; Invitrogen Life Technologies), polyclonal rabbit
anti-human GAPDH (cat. no. GTX100118; 1:500; GeneTex Inc., Irvine,
CA, USA), polyclonal rabbit anti-human phosphorylated EGFR
(Tyr1068; cat. no. 2234; 1:200; Cell Signaling Technology, Inc.,
Danvers, MA, USA), monoclonal rabbit anti-human phosphorylated
mitogen-activated protein kinase kinase (MEK; cat. no. 9154;
1:1,000; Cell Signaling Technology Inc.), and polyclonal rabbit
anti-human phosphorylated cell division cycle 25C (cdc25C; cat. no.
9527S; 1:1,000; Cell Signaling Technology Inc.) primary antibodies.
Secondary horseradish peroxidase-conjugated polyclonal goat
anti-rabbit IgG antibodies (cat. no. sc-2004; 1:2,000; Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) were then incubated with
the membranes for 30 min at RT. The membranes were washed three
times with Tris-Buffered Saline containing 0.1% Tween-20 for 5 min.
The signal from antibody-conjugated horseradish peroxidase was
visualized by applying SuperSignal™ West Pico Chemiluminescent
Substrate (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
exposing to X-ray film.
Cell growth assay
Cell growth was assayed with the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The cells (100 µl at 3×103/ml per well) were seeded in
96-well flat bottom plates and incubated with 1,000 nM of the EGFR
inhibitor, erlotinib (ChemieTek, Indianapolis, IN, USA). At 24, 48,
72 and 96 h, the cells were incubated for 3 h with 0.5 mg/ml MTT
(10 µl per well). Subsequently, dimethylsulfoxide (100 µl) was
added to all wells and the plates were kept overnight at RT in the
dark. Following this, the absorbance of each well was measured with
a microplate reader (iMark™ microplate absorbance reader; Bio-Rad
Laboratories, Hercules, CA, USA). The test and reference
wavelengths were 570 and 630 nm, respectively.
Statistical analysis
All data are presented as the mean ± standard
deviation. Differences between two groups were evaluated using
Student's t-test. Dunnett's procedure was used for the statistical
analysis of differences between multiple groups and the control.
All reported P-values are two-tailed, and P<0.05 was considered
to indicate a statistically significant difference.
Results
shRNA-mediated silencing of CD147
expression in A375 cells
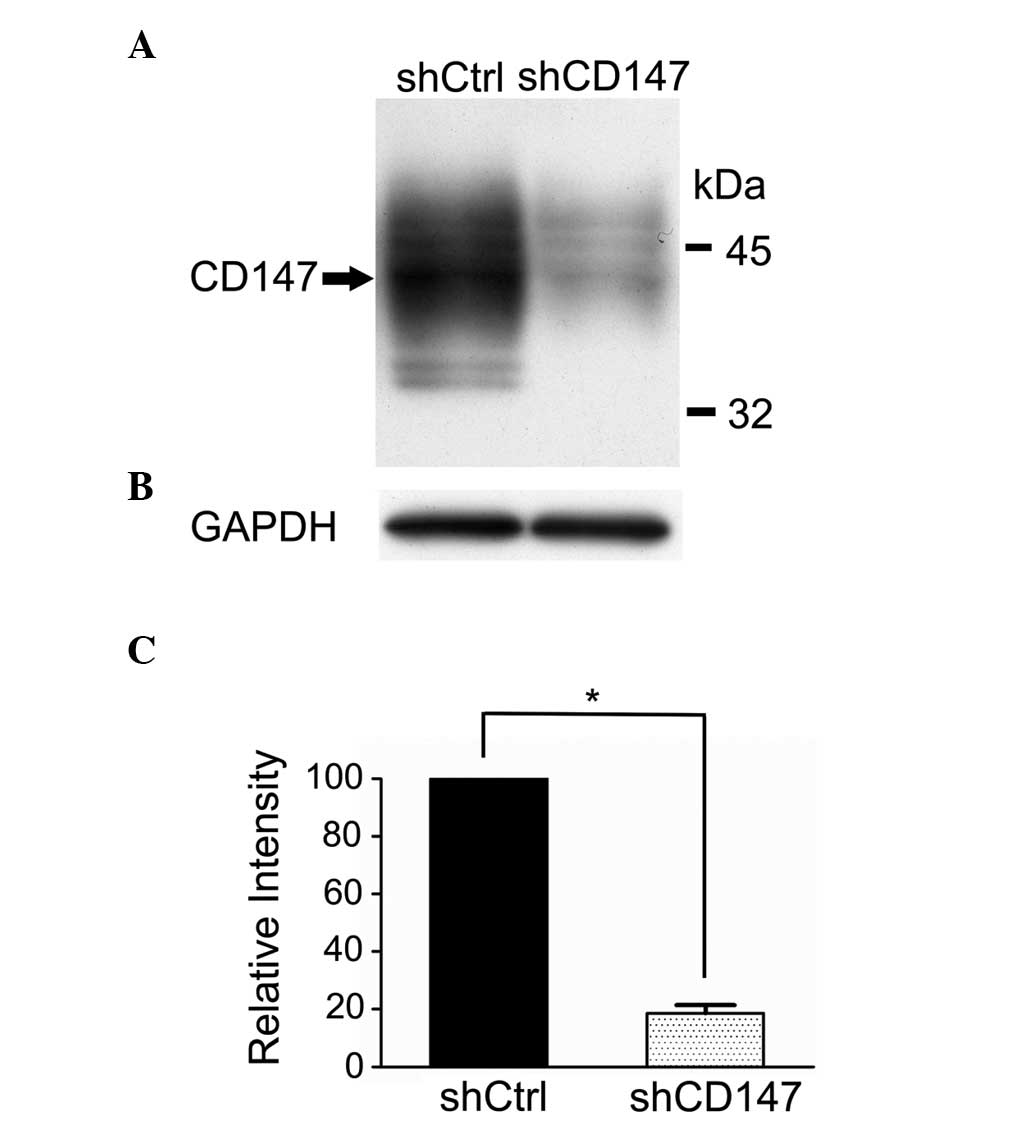
The expression of CD147 was knocked down with
specific shRNA-targeting of CD147 mRNA, and CD147 protein levels
were determined using western blotting. CD147 protein levels were
decreased in the A375 cells transfected with CD147 shRNA lentivirus
(A375 shCD147) compared with the cells transfected with the empty
lentivirus control (A375 shCtrl; Fig.
1).
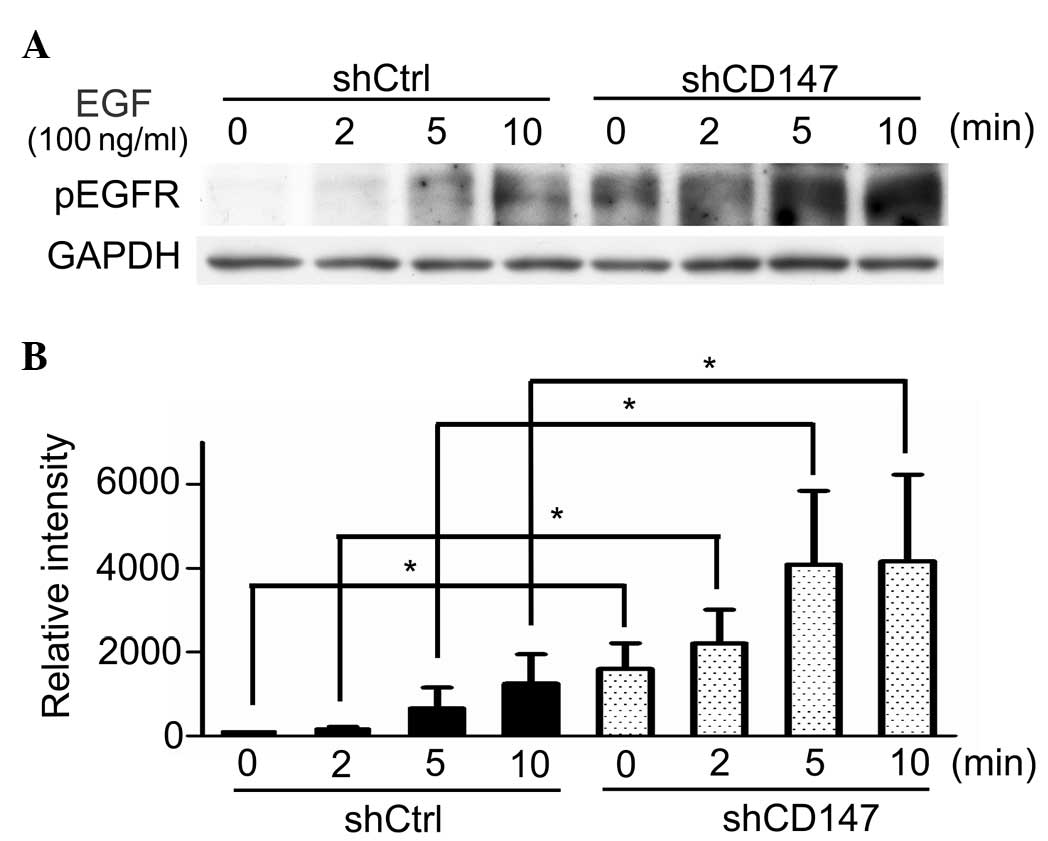
Phosphorylation of EGFR is upregulated
by silencing CD147 in A375 cells
The effects of CD147 silencing on EGF (100
ng/ml)-induced EGFR phosphorylation were investigated. The results
demonstrated that knockdown of CD147 enhances the phosphorylation
of EGFR in a time-dependent manner, when stimulated by its ligand,
EGF. The highest level of EGFR phosphorylation was recorded at 10
min (Fig. 2).
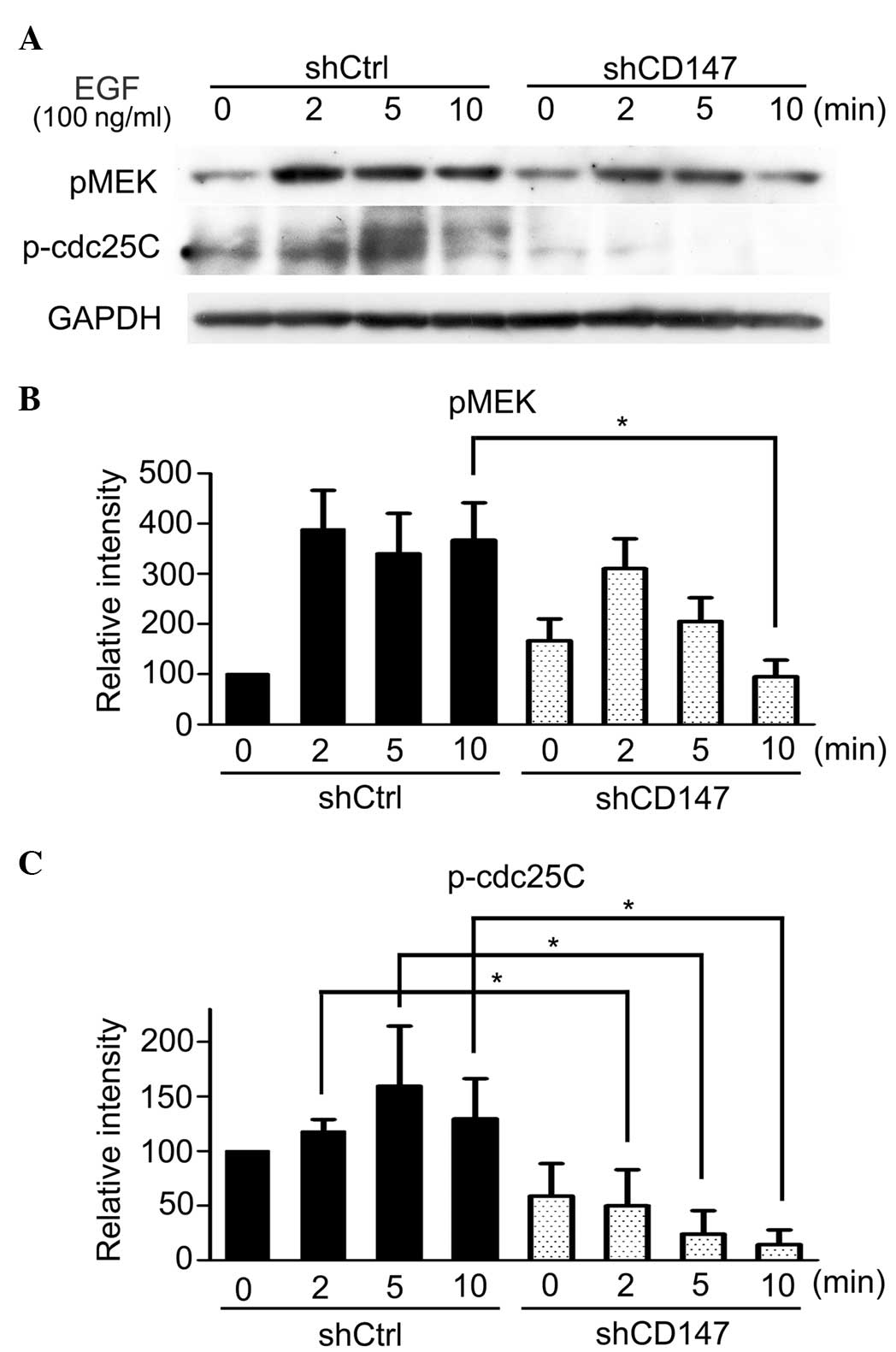
Phosphorylation of MEK and cdc25C is
downregulated by silencing CD147 in A375 cells
To investigate the underlying mechanism(s) of
CD147-regulated EGFR phosphorylation, the effects of CD147
silencing on the phosphorylation of MEK, a downstream effector of
B-Raf was examined. In line with previous studies reporting that
CD147 promotes ERK activity (18,19), the
results of the present study demonstrated that CD147 was required
for high levels of MEK activation, and that MEK phosphorylation was
inhibited by CD147 silencing (Fig.
3). As previous studies have demonstrated that ERK
phosphorylates cdc25C in Xenopus and that the cdc25
homologue, cdc25A, binds to and dephosphorylates EGFR (24,25), the
phosphorylation of cdc25C was investigated. Fig. 3 shows that the phosphorylation of
cdc25C was downregulated by knockdown of CD147 in the present
study.
CD147 silencing and the EGFR inhibitor
in combination confers an additive effect on the reduced growth of
A375 cells
We previously demonstrated that MM cell growth is
inhibited by CD147 silencing (14,17), and
that EGFR phosphorylation is upregulated by CD147 silencing. To
investigate these findings further, the effect of the
small-molecule kinase inhibitor of EGFR, erlotinib, on the growth
of shCtrl and shCD147 A375 cells was examined. Cell growth
inhibition was significantly higher in the shCD147 cells compared
with the shCtrl cells (P<0.01). Growth inhibition by erlotinib
was also significantly higher in the shCD147 cells compared with
the shCtrl cells (P<0.01), indicating the additive inhibition of
A375 cell growth by silencing CD147 and treatment with erlotinib
(Fig. 4).
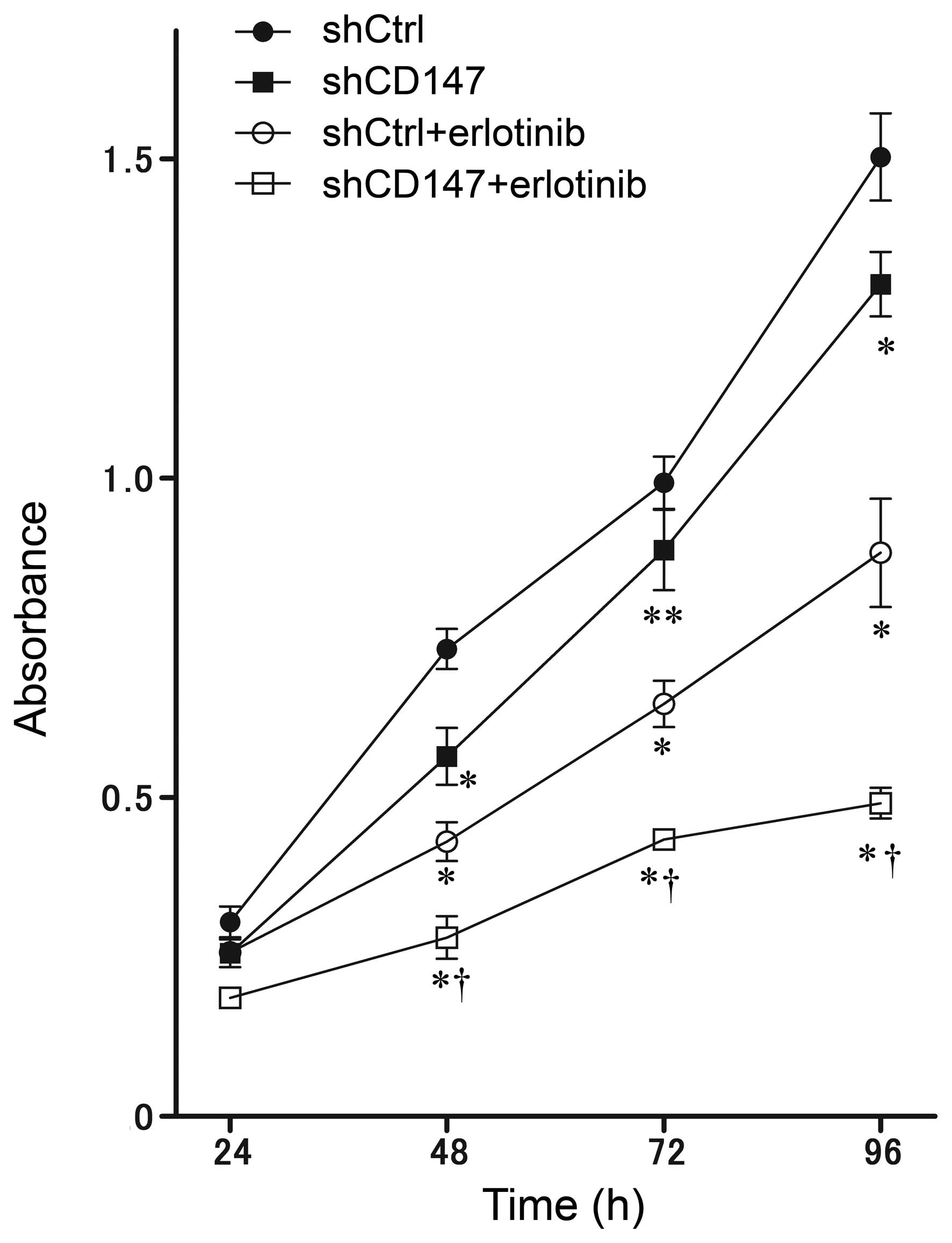 | Figure 4.Additive growth inhibition of A375
human malignant melanoma cells by CD147 knockdown (shCD147),
treatment with erlotinib (an EGFR inhibitor), or the two techniques
in combination. Inhibition of growth was measured using the MTT
assay, and the absorbances at 570 nm and 630 nm were recorded for
test and reference wavelengths, respectively. Growth inhibition was
significantly lower in shCD147 cells than control (shCtrl) cells.
Erlotinib significantly inhibited the growth of shCtrl cells.
Growth inhibition by erlotinib was significantly higher in shCD147
cells than shCtrl cells. *P<0.01 and **P<0.05 vs. shCtrl;
†P<0.01 vs. shCtrl plus erlotinib. MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; EGFR,
epidermal growth factor receptor; CD147, cluster of differentiation
147; sh, short hairpin. |
Discussion
MM, a highly aggressive cutaneous tumor, is
characterized by rapid growth and a high potential for invasiveness
and metastasis. An activating mutation of the BRAF oncogene (V600E)
is observed in ~70% of primary MM (4). The Raf-MEK-ERK pathway plays a
significant role in oncogenesis. The pathway is activated by either
ligand-dependent stimulation of membrane-associated receptor
tyrosine kinases or by ligand-independent mechanisms, such as
increased expression or mutational activation of Raf. The
activating mutation of BRAF triggers the sequential activation of
MEK and ERK, resulting in constitutive activation of tumor cell
proliferation (2). BRAF has emerged
as a therapeutic target due to the high incidence of its mutation
in MM. The effects of a highly selective BRAF (V600E) kinase
inhibitor, vemurafenib, have been previously studied, and
preclinical investigations have revealed potent and selective
activity in MM cell lines containing the V600E BRAF mutation
(22). Higher overall and
progression-free survival rates were demonstrated in a phase 3
randomized clinical trial that compared vemurafenib with
dacarbazine in 675 previously untreated metastatic MM patients with
the BRAF V600E mutation (23).
Despite the marked progress in signal-transduction therapy
targeting the MAPK cascade, clinical effectiveness has remained
limited.
We previously reported a series of studies on the
role of CD147 in MM progression (14–17). CD147
is highly expressed on MM cells and plays an important role in cell
proliferation, tumor invasiveness, metastasis and angiogenesis by
promoting tumor cell glycolysis. The protein facilitates lactate
transport in combination with monocarboxylate transporters (MCTs)
(17), and is involved in multidrug
resistance by regulating the expression of P-glycoprotein (P-gp) on
cell membranes (16). CD147 appears
to have chaperone-like functions in the correct folding,
trafficking and cell surface expression of membrane proteins such
as MCTs and P-gp. These findings strongly implicated CD147 as a
possible therapeutic target for MM. In addition to these MAPK
pathway-independent functions, CD147 was demonstrated to directly
activate ERK. In MDA-MB-231 and MCF7 breast cancer cells, ERK was
phosphorylated by CD147 transfection (18,19). As
the A375 MM cells harbor the V600E BRAF activating mutation
(20), the correlation between CD147
and the RAF-MEK-ERK pathway was examined in the present study. The
phosphorylation level of EGFR in A375-shCtrl and A375-shCD147 cells
was investigated. EGFR phosphorylation was anticipated to be
reduced by CD147 silencing, as CD147 and EGFR promote tumor cell
proliferation. Unexpectedly, EGFR phosphorylation was upregulated
by CD147 silencing. Following this result, the phosphorylation of
MEK, a downstream effector of B-Raf, was examined. In agreement
with previous studies (18,19), the present study demonstrated that MEK
phosphorylation was inhibited by CD147 silencing.
Wang et al (24) documented that in Xenopus,
cdc25C was activated by MEK, and the cdc25 homologue, cdc25A,
interacted with and dephosphorylated EGFR in human Hep3B hepatoma
cells (25). Therefore, we
hypothesized that the downregulation of MEK by silencing CD147
would lead to the inhibition of cdc25C and the subsequent
phosphorylation of EGFR. In support of this hypothesis, the results
of the present study demonstrated that cdc25C phosphorylation was
downregulated by CD147 silencing. The results herein constitute the
first documentation of the functional interaction between CD147 and
membrane-associated tyrosine kinase receptors. A similar molecular
mechanism has been identified in colon cancer harboring the V600E
BRAF mutation (26). Unlike MM, colon
cancer with the activating mutation is resistant to vemurafenib, a
phenomenon attributed to feedback activation of EGFR. When B-Raf
was inhibited by vemurafenib, MEK and cdc25C were downregulated,
resulting in EGFR activation (26).
The current study demonstrated the additive effects
of CD147 silencing and EGFR inhibition on MM cell growth. The
results of the present study suggest that the combination of EGFR
and CD147 inhibition may be useful for the treatment of
BRAF-mutated MM. Anti-EGFR drugs such as erlotinib, gefinitib,
cetuximab and panitumumab are clinically available, and
translational research studies on the clinical applicability of
CD147 are warranted.
References
|
1
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Montagut C and Settleman J: Targeting the
RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 283:125–134.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davies H, Bignell GR, Cox C, et al:
Mutations of the BRAF gene in human cancer. Nature. 417:949–954.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanekura T, Miyauchi T, Tashiro M and
Muramatsu T: Basigin, a new member of the immunoglobulin
superfamily: Genes in different mammalian species, glycosylation
changes in the molecule from adult organs and possible variation in
the N-terminal sequences. Cell Struct Funct. 16:23–30. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyauchi T, Kanekura T, Yamaoka A, Ozawa
M, Miyazawa S and Muramatsu T: Basigin, a new, broadly distributed
member of the immunoglobulin superfamily, has strong homology with
both the immunoglobulin V domain and the beta-chain of major
histocompatibility complex class II antigen. J Biochem.
107:316–323. 1990.PubMed/NCBI
|
|
7
|
Muramatsu T: Basigin: A multifunctional
membrane protein with an emerging role in infections by malaria
parasites. Expert Opin Ther Targets. 16:999–1011. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bordador LC, Li X, Toole B, Chen B, Regezi
J, Zardi L, Hu Y and Ramos DM: Expression of emmprin by oral
squamous cell carcinoma. Int J Cancer. 85:347–352. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishibashi Y, Matsumoto T, Niwa M, Suzuki
Y, Omura N, Hanyu N, Nakada K, Yanaga K, Yamada K, Ohkawa K, et al:
CD147 and matrix metalloproteinase-2 protein expression as
significant prognostic factors in esophageal squamous cell
carcinoma. Cancer. 101:1994–2000. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muraoka K, Nabeshima K, Murayama T, Biswas
C and Koono M: Enhanced expression of a tumor-cell-derived
collagenase-stimulatory factor in urothelial carcinoma: Its
usefulness as a tumor marker for bladder cancers. Int J Cancer.
55:19–26. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nabeshima K, Suzumiya J, Nagano M, Ohshima
K, Toole BP, Tamura K, Iwasaki H and Kikuchi M: Emmprin, a cell
surface inducer of matrix metalloproteinases (MMPs), is expressed
in T-cell lymphomas. J Pathol. 202:341–351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Polette M, Gilles C, Marchand V, Lorenzato
M, Toole B, Tournier JM, Zucker S and Birembaut P: Tumor
collagenase stimulatory factor (TCSF) expression and localization
in human lung and breast cancers. J Histochem Cytochem. 45:703–709.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sameshima T, Nabeshima K, Toole BP,
Yokogami K, Okada Y, Goya T, Koono M and Wakisaka S: Glioma cell
extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147)
stimulates production of membrane-type matrix metalloproteinases
and activated gelatinase A in co-cultures with brain-derived
fibroblasts. Cancer Lett. 157:177–184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Lin J, Kanekura T, Su J, Lin W,
Xie H, Wu Y, Li J, Chen M and Chang J: A small interfering
CD147-targeting RNA inhibited the proliferation, invasiveness, and
metastatic activity of malignant melanoma. Cancer Res.
66:11323–11330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanekura T, Chen X and Kanzaki T: Basigin
(CD147) is expressed on melanoma cells and induces tumor cell
invasion by stimulating production of matrix metalloproteinases by
fibroblasts. Int J Cancer. 99:520–528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanekura T and Chen X: CD147/basigin
promotes progression of malignant melanoma and other cancers. J
Dermatol Sci. 57:149–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su J, Chen X and Kanekura T: A
CD147-targeting siRNA inhibits the proliferation, invasiveness, and
VEGF production of human malignant melanoma cells by
down-regulating glycolysis. Cancer Lett. 273:140–147. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marieb EA, Zoltan-Jones A, Li R, Misra S,
Ghatak S, Cao J, Zucker S and Toole BP: Emmprin promotes
anchorage-independent growth in human mammary carcinoma cells by
stimulating hyaluronan production. Cancer Res. 64:1229–1232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang JM, O'Neill P, Jin W, Foty R, Medina
DJ, Xu Z, Lomas M, Arndt GM, Tang Y, Nakada M, et al: Extracellular
matrix metalloproteinase inducer (CD147) confers resistance of
breast cancer cells to Anoikis through inhibition of Bim. J Biol
Chem. 281:9719–9727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eskandarpour M, Kiaii S, Zhu C, Castro J,
Sakko AJ and Hansson J: Suppression of oncogenic NRAS by RNA
interference induces apoptosis of human melanoma cells. Int J
Cancer. 115:65–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie W, Xie H, Liu F, Li W, Dan J, Mei Y,
Dan L, Xiao X, Li J and Chen X: Propranolol induces apoptosis of
human umbilical vein endothelial cells through downregulation of
CD147. Br J Dermatol. 168:739–748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sala E, Mologni L, Truffa S, Gaetano C,
Bollag GE and Gambacorti-Passerini C: BRAF silencing by short
hairpin RNA or chemical blockade by PLX4032 leads to different
responses in melanoma and thyroid carcinoma cells. Mol Cancer Res.
6:751–759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chapman PB, Hauschild A, Robert C, Haanen
JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et
al: BRIM-3 Study Group: Improved survival with vemurafenib in
melanoma with BRAF V600E mutation. N Engl J Med. 364:2507–2516.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang R, He G, Nelman-Gonzalez M, Ashorn
CL, Gallick GE, Stukenberg PT, Kirschner MW and Kuang J: Regulation
of Cdc25C by ERK-MAP kinases during the G2/M transition. Cell.
128:1119–1132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Wang M, Lazo JS and Carr BI:
Identification of epidermal growth factor receptor as a target of
Cdc25A protein phosphatase. J Biol Chem. 277:19470–19475. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prahallad A, Sun C, Huang S, Di
Nicolantonio F, Salazar R, Zecchin D, Beijersbergen RL, Bardelli A
and Bernards R: Unresponsiveness of colon cancer to BRAF (V600E)
inhibition through feedback activation of EGFR. Nature.
483:100–103. 2012. View Article : Google Scholar : PubMed/NCBI
|