Introduction
Lung cancer is the leading cause of
cancer-associated mortality and the most common cancer type
worldwide (1). In total, ~80% of all
lung cancer cases are non-small cell lung cancer (NSCLC) (2), for which surgery, chemotherapy and
radiotherapy remain the standard treatments (3). The majority of patients with NSCLC are
not recommended for surgery, since patients are often diagnosed at
an advanced stage of disease (stage IIIb or IV). Patients that do
undergo radical surgery may eventually develop locoregional
recurrence or distant metastasis (4).
Characterized by inexorable disease progression, advanced NSCLC
usually has a high malignant potential. Its prognosis is poor and
the clinical onset is extensive, despite treatment with
chemotherapy and radiation (5).
Systemic chemotherapy is the recommended first-line treatment for
patients with advanced stage and metastatic NSCLC, but it is often
considered ineffective or excessively toxic (6).
Multidisciplinary approaches are required to develop
novel treatments for advanced NSCLC (7). One of these multidisciplinary approaches
is immunotherapy (7,8), an important and effective method in
cancer treatment, particularly for advanced-stage disease (9). Immune cells, including cytokine-induced
killer (CIK) cells and dendritic cells (DCs), aid in mounting an
effective immune response against cancer cells and kill cancer
cells, including residual cells (10–13). A
combination of conventional methods, such as surgery, chemotherapy
and radiotherapy, combined with immunotherapy is a novel approach
to reducing mortality from cancer (3,14).
In the present study, the immune response induced by
a DC vaccine and CIK cell therapy (DC-CIK), the adverse effects of
the vaccine, and improvements in the quality of life (QOL) and
overall survival (OS) time were evaluated in patients with advanced
NSCLC.
Patients and methods
Patients and immunotherapy
All patients were enrolled at the Department of
Oncology, Tianjin Union Medicine Centre (Tianjin, China). The
present study evaluated two groups of patients, consisting of
patients that received DC-CIK [immunotherapy group (group I)] and
matched patients that did not receive DC-CIK [non-immunotherapy
group (group NI)], who acted as the control. Group I consisted of
hospitalized patients with NSCLC that underwent DC-CIK between
January 2012 and October 2012, and who possessed stage IIIb or IV
disease and locoregional recurrence or distant metastasis following
surgery for NSCLC. The immunotherapy patients underwent blood tests
and met the following requirements: Lymphyocytes and monocytes,
>0.7×109/l; white blood cells, >3.0×109
cells/l; hemoglobin, >75.0 g/l; platelets,
>50.0×109 platelets/l; coagulation: prothrombin time,
<22.5 sec; activated partial thromboplastin time, <65 sec;
thrombin time, <32 sec; cardiac function, ≤III level (15); liver function, <Child-Pugh
Class/Score C (16); no renal failure
or uremia. The requirements indicated adequate kidney, liver,
coagulation and bone marrow function. Group NI consisted of NSCLC
patients with matched characteristics and treatments to the
patients in group I, but did not receive DC-CIK.
The present study was performed at the Department of
Oncology of Tianjin Union Medicine Centre (Tianjin, China).
Following the policy outlined by the Ministry of Health of China,
the present study was approved as a class III medical technique,
and was listed in Treatment with Autologous Immune Cells (T cells,
NK cells) (17,18). All patients provided written informed
consent prior to treatment, as required by the Ethics Committee of
Tianjin Union Medicine Centre. The present study was a
retrospective study using data obtained from the medical records of
patients that received a standard treatment for NSCLC. The clinical
data were collected with permission from the Inpatients Electronic
Medical Records of Tianjin Union Medicine Centre.
Preparation of DCs and CIK cells
On the first day of treatment, leukocyte fractions
were collected from the patients via leukapheresis using the
Fresenius KABI System (COM.TEC; Fresenius SE & Co. KGaA,
Homburg, Germany) under electrocardiographic monitoring. Peripheral
blood mononuclear cells (PBMCs) were isolated via Ficoll-Hypaque
gradient centrifugation and cultured for 2 h [DCs, RPMI-1640
(Gibco®; Thermo Fisher Scientific, Inc., Waltham, MA, USA); CIK,
GT-T551 medium (Takara Bio, Inc., Beijing China)] in a Forma™
Series II 3111 CO2 Waterjacket Incubator (Thermo Fisher
Scientific, Inc.). The human lung squamous cell carcinoma SK-MES-1
and human lung adenocarcinoma SPC-A-1 cell lines (Chinese Academy
of Sciences, Shanghai, China) were cultured in Gibco Cell Culture
Media (Thermo Fisher Scientific, Inc.) for 24 h at 37°C (19–21). The
cells were lysed by ultrasound and centrifuged (Heraeus Fresco 17;
Thermo Fisher Scientific, Inc.) at 600 × g for 30 min. The
supernatants were collected and used as a tumor lysate for DC
preparation. DCs and CIK cells were prepared as described in a
previous study (22–24). The supernatants of cultured PBMCs were
removed for the additional preparation of CIK cells and the
remaining cells were cultured for 7 days in the presence of
granulocyte-macrophage colony stimulating factor, interleukin
(IL)-4 and tumor necrosis factor (PeproTech EC, Ltd., London, UK),
and pulsed with the tumor lysate (PeproTech EC, Ltd.) (25). On the ninth day, the DCs were
harvested for immediate vaccinations (12,26) or
frozen for subsequent reinfusion. The supernatants were washed, and
were cultured in the presence of interferon-γ, rat anti-human
anti-cluster of differentiation 3 monoclonal antibody (catalog no.,
T210; dilution, 1:100; Takara Bio, Inc.) and IL-2 (PeproTech EC,
Ltd.) (27). The cells were harvested
between days 13 and 15.
Clinical regimen
The viability of DCs and CIK cells was >70 and
90%, respectively, detected using flow cytometry (FC500; Beckman
Coulter, Miami, FL, USA). The cells were subjected to a bacterial
gram stain, bacterial culture, fungal culture and bacterial
endotoxin test to ensure they were not contaminated with bacteria,
fungi or mycoplasma, and endotoxin levels in the cells were >5
EU/kg prior to infusion. On the ninth day, 1×107 vaccine
DCs suspended in 100 ml normal saline (NS) were infused
intravenously once a week for 3 weeks. Subsequently, 1.0×107 DCs
suspended in 4 ml NS in 2 syringes were infused intradermally once
a week for an additional 3 weeks. On the twelfth day,
1×109 CIK cells suspended in 100 ml NS were infused
intravenously once a day for 4 consecutive days.
Immune response
In total, 1 week subsequent to the last DC
vaccination, delayed-type hypersensitivity (DTH) of the patients
was tested as an immune response marker. A total of
1×107 DCs in 0.1 ml NS was administrated intradermally
into the forearm of each patient. The patients were asked to
measure the diameter of the skin erythema around the injection
point following 24, 48 and 72 h. The DTH reaction was defined as
follows: >10 mm in diameter, strongly positive; 5–10 mm,
positive; 2–5 mm, weakly positive; and <2 mm, negative (22,28).
QOL
The QOL of the patients was evaluated based on
physical strength, appetite, amount of sleep and body weight
(29). The patients were provided a
clinical response questionnaire, on which they recorded feedback
regarding the effect of the treatment. QOL score was determined by
the number of improved factors, ranging between 0, indicating no
improvement, and 4, indicating all factors improved.
Safety
Fever was the most common adverse effect of DC-CIK
and was of considerable concern. Fever was defined as a cold-like
symptom, with varying temperatures defining the severity of the
fever, as follows: <38°C, Low fever; 38–39°C, moderate fever;
and >39°C, high fever. Skin rashes were also observed during
therapy.
Adverse effects
Adverse reactions, including insomnia, anorexia and
joint soreness were monitored during DC-CIK. A slight increase in
body temperature was regarded as a cold-like symptom. Fever, skin
rashes and other symptoms previously observed during DC-CIK were
recorded (22). Adverse effects known
to be associated with chemotherapy and radiotherapy, such as
nausea, vomiting, bone marrow suppression and organ function
impairment, including the kidneys, liver, lungs and heart, were
also monitored.
OS time
The OS time was calculated as the date from which
the patients were enrolled in the present study to the date that
the patient succumbed to NSCLC or any other cause. The OS time for
the two groups was analyzed and compared using Kaplan-Meier
estimates.
Data collection and statistical
analysis
The primary end-points for the present study were
the immune response and QOL of the patients, and the safety of the
therapy. The secondary end-point was the OS time of the patients
(30). Clinical data were collected
using EpiData software version 3.02 (EpiData Association, Odense,
Denmark). Statistical analyses were performed using SPSS software
version 19.0 (IBM SPSS, Armonk, NY, USA). The Student's
t-test was used for measurement data and the χ2
test was used for count data. The OS curves were calculated using
the Kaplan-Meier method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
A total of 507 patients with advanced NSCLC were
enrolled in the present study, consisting of 311 male and 196
female patients, with a mean age of 66.28 years (range, 29–93
years). The tumor diagnoses of the patients were as follows:
Squamous cell carcinoma, 219 patients; adenocarcinoma, 256
patients; and undefined, 32 patients. The degree of NSCLC
differentiation was high in 36 patients, medium in 207 patients and
low in 263 patients. Among the 507 patients, 44 (8.7%) underwent
primary tumor resection, 96 (18.9%) received radiotherapy and 107
(21.1%) received chemotherapy within 3 months prior to the start of
immunotherapy (Table I).
 | Table I.Characteristics of patients with
advanced non-small cell lung cancer. |
Table I.
Characteristics of patients with
advanced non-small cell lung cancer.
| Characteristics | Group I, n (%) | Group NI, n (%) | Total, n (%) | P-value |
|---|
| Total | 99 (100.0) | 408 (100.0) | 507 (100.0) |
|
| Age, years |
|
|
| 0.39 |
|
Range | 31–87 | 29–93 | 29–93 |
|
| Mean ±
SD | 64.7±10.4 | 68.1±10.6 | 66.28±8.9 |
|
| Gender |
|
|
| 0.33 |
| Male | 65 (65.7) | 246 (60.3) | 311 (61.3) |
|
|
Female | 34 (34.3) | 162 (39.7) | 196 (38.7) |
|
| Degree of
differentiationa |
|
|
| 0.99 |
|
High | 7 (7.1) | 29
(7.1) | 36 (7.1) |
|
|
Middle | 40 (40.4) | 167
(40.9) | 207 (40.8) |
|
|
Low | 52 (52.5) | 211
(51.7) | 263 (51.9) |
|
| Tumor types |
|
|
|
|
|
Squamous cell carcinoma | 35 (35.4) | 184 (45.1) | 219 (43.2) | 0.21 |
|
Adenocarcinoma | 57 (57.6) | 199 (48.7) | 256 (50.5) |
|
|
Undefined | 7 (7.1) | 25 (6.1) | 32 (6.3) |
|
| Combined
therapies |
|
|
|
|
|
Surgery | 11 (11.1) | 33 (8.1) | 44 (8.7) | 0.34 |
|
Radiotherapy | 25 (25.3) | 71
(17.4) | 96
(18.9) | 0.07 |
|
Chemotherapy | 25 (25.3) | 82
(20.1) | 107 (21.1) | 0.26 |
The 507 patients were divided into two groups. A
total of 408 patients that received a routine treatment regimen
consisting of surgery, radiotherapy and chemotherapy were enrolled
into the non-immunotherapy group (control group; group NI). In
addition, 99 patients that received DC-CIK in addition to the
routine treatments were classified as the immunotherapy group
(group I). The characteristics of the patients, including age,
gender, diagnostic methods and tumor type, were similar between the
two groups. With the exception of the use of DC-CIK, the treatment
strategies did not differ significantly between the two groups
(Table I).
DTH skin test
The DTH skin test results were available for 97 out
of the 99 patients in group I, of whom 59 (60.8%) possessed a
positive result. In total, 10 of these patients (10.3%) had a
strongly positive result; 25 (25.8%) had a positive result; 24
(24.7%) had a weakly positive result; and 38 (39.2%) had a negative
result (Table II). The results from
2 patients were unknown.
 | Table II.Grade of DTH, fever, allergy and QOL
in patients with advanced non-small cell lung cancer treated with
dendritic cell vaccine and cytokine-induced killer cell
therapy. |
Table II.
Grade of DTH, fever, allergy and QOL
in patients with advanced non-small cell lung cancer treated with
dendritic cell vaccine and cytokine-induced killer cell
therapy.
| Response grade | DTH, n (%) | Fever, n (%) | Allergy, n (%) | QOL, n (%) |
|---|
| Total | 97 (98.0) | 98 (99.0) | 98 (99.0) | 98 (99.0) |
| Data loss | 2 (2.0) | 1 (1.0) | 1 (1.0) | 1 (1.0) |
| Grade |
|
|
|
|
| 0 | 38 (39.2) | 62 (63.3) | 91 (92.9) | 31 (31.6) |
| 1 | 24 (24.7) | 14 (14.3) | 5 (5.1) | 26 (26.5) |
| 2 | 25 (25.8) | 16 (16.3) | 2 (2.0) | 34 (34.7) |
| 3 | 10 (10.3) | 6 (6.1) | – | 7 (7.1) |
QOL
The 4 QOL factors assessed were physical strength,
appetite, amount of sleep and body weight. QOL scores were
available for 98 out of the 99 patients in group I, as follows: 7
patients (7.1%) demonstrated improvements in all 4 QOL factors; 34
patients (34.7%) demonstrated improvements in 2–3 factors; and 26
patients (26.5%) demonstrated improvements in a single factor.
Overall, 68.4% of the group I patients (67 out of 98) possessed an
improved QOL following immunotherapy, while the remaining 31.6% of
patients (31 out of 98) had no improvement in QOL (Table II).
Adverse effects
Body temperature was recorded in 98 out of the 99
patients in group I. In these 98 patients, cold-like symptoms with
a low fever were observed in 14 patients (14.3%). A moderate fever
was observed in 16 patients (16.3%) and a high fever was observed
in 6 patients (6.1%). In total, 36 of the 98 patients (36.7%)
experienced fever, including cold-like symptoms. No fever was
detected in the remaining 62 patients (63.3%) during DC-CIK
(Table II). The presence or absence
of allergy was recorded in 7 of the 98 patients in group I, 2 of
whom experienced a skin rash (2.0%) and 5 of whom experienced local
erythema around the injection site. No additional toxicities were
observed in patients receiving DC-CIK (Table II) (31).
Association between DTH and tumor
type
In group I, 27 of the 35 patients (77.1%) with
squamous cell carcinoma of the lung developed DTH. By contrast, 23
of the 57 patients (40.4%) with adenocarcinoma of the lung
developed DTH (P=0.0013; Table
IV).
 | Table IV.Association between DTH and tumor
type in patients with advanced non-small cell lung cancer that
underwent dendritic cell vaccine and cytokine-induced killer cell
therapy therapy. |
Table IV.
Association between DTH and tumor
type in patients with advanced non-small cell lung cancer that
underwent dendritic cell vaccine and cytokine-induced killer cell
therapy therapy.
| Tumor types | Total, n | DTH+,
n | DTH−,
n | Incidence of DTH,
% | P-value |
|---|
| Total, n | 92 | 50 | 42 | 54.3 |
|
| Squamous cell
carcinoma | 35 | 27 | 8 | 77.1 | 0.0013 |
| Adenocarcinoma | 57 | 23 | 34 | 40.4 |
|
Effect of radiotherapy and
chemotherapy on DTH
The association between radiotherapy, chemotherapy
and DTH was assessed in 93 out of the 96 patients with NSCLC in
group I. The remaining 3 patients received radiotherapy and
chemotherapy concurrently and were excluded from the analysis.
There was no significant difference between the rate
of DTH in the 49 patients in group I that did not undergo
radiotherapy (79.6%) compared with the 22 patients that did
(72.7%). However, the incidence of DTH was significantly lower
amongst the patients that underwent chemotherapy (18.2%) compared
with those patients that did not (79.6%; P=0.22; Table III).
 | Table III.Effect of radiotherapy and
chemotherapy on DTH in patients with advanced non-small cell lung
cancer that underwent DC-CIK. |
Table III.
Effect of radiotherapy and
chemotherapy on DTH in patients with advanced non-small cell lung
cancer that underwent DC-CIK.
| Groups | Total, n | DTH+,
n | DTH−,
n | Incidence of DTH,
% | P-value |
|---|
| Total, n | 93 | 57 | 36 | 61.3 |
|
| Radiotherapy +
DC-CIK | 22 | 16 | 6 | 72.7 | 0.213 |
| Chemotherapy +
DC-CIK | 22 | 4 | 18 | 18.2 | 0.001 |
| DC-CIK | 49 | 39 | 10 | 79.6 | – |
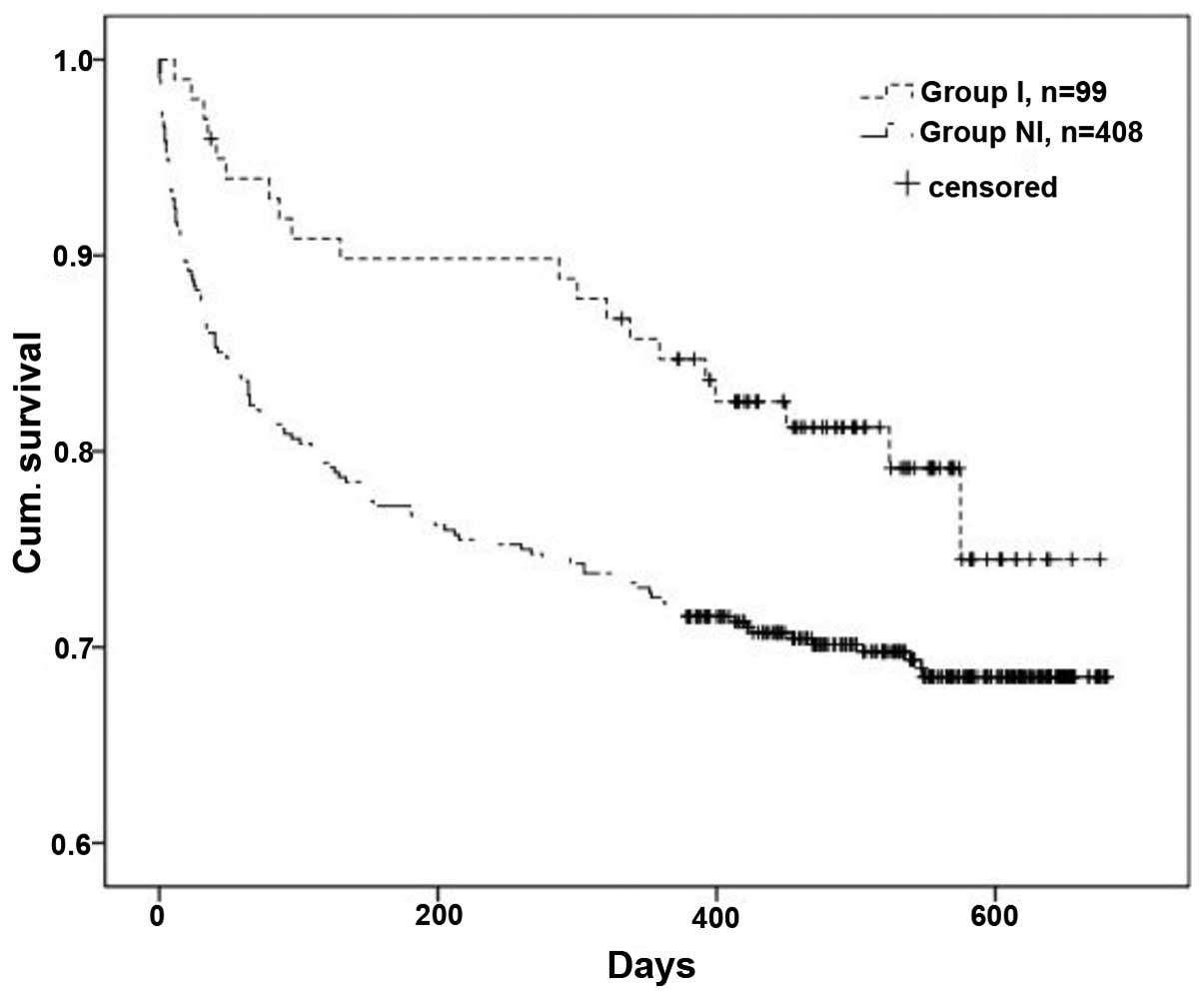
OS time
The OS time of patients in group I was estimated and
compared to the OS time of patients in group NI using the
Kaplan-Meier method. In total, 20 out of 99 patients (20.2%) in
group I and 125 out of 408 patients (30.6%) in group NI succumbed
to NSCLC or other causes during the present study. The OS time of
patients in group I was significantly improved compared with group
NI (P=0.03; Fig. 1) (32).
Discussion
Despite rapid advances in surgery, radiotherapy and
chemotherapy, which are effective modalities in cancer treatment,
the prognosis following treatment for NSCLC remains poor (33). To improve the clinical outcome for
patients with NSCLC, novel treatment modalities are urgently
required. One such alternative is immunotherapy with DC-CIK, where
DCs have previously been revealed to activate CIK cells, which
enhances antitumor effects in NSCLC patients (34,35). An
adoptive transfer of CIK cells with DCs has proven to be an
effective strategy for extending the OS time in patients (7,36),
enhancing host cellular immune responses and improving the QOL of
patients (8,37). Furthermore, no severe adverse effects
were reported during DC-CIK cell transfusion, indicating that this
therapy is safe for clinical use. Adverse effects were mild and
were effectively managed.
The present study used the DTH skin test to assess
the cell-mediated cytotoxicity response induced by DC-CIK, and
identified that 61% of DC-CIK-treated patients (59 out of 97) had a
positive reaction. In addition, 68% of patients (67 out of 98)
demonstrated improved physical strength, appetite, sleep and body
weight. No toxicity other than fever and skin rash was associated
with DC-CIK. Fever was observed in 26 of the 98 patients (27%) in
group I and was generally mild and temporary. During these
episodes, body temperature was rarely >39°C and was relieved by
cooling or antipyretic analgesics. Cold-like symptoms, regarded as
a mild form of fever, were observed in 14 out of the 98 patients
(14%). In these cases, patients complained of symptoms, including
fatigue and sore joints, but their temperatures were normal
(36–37°C). Allergic reactions, indicated by a skin rash, were rare,
occurring in only 7 out of the 98 patients (7.1%), and usually mild
and self-resolving without requiring special treatments (Table II). The rate of DTH in patient with
squamous cell carcinoma of the lung was significantly higher
compared with patients with adenocarcinoma of the lung (77.1 vs.
40.4%; P=0.0013; Table IV).
Radiotherapy had no effect on DTH induced by DC-CIK (72.7 vs.
79.6%; P=0.02), but chemotherapy significantly reduced the
incidence of DTH (18.2 vs. 79.6%; P=0.001; Table III).
The OS time of group I patients was estimated and
compared to the OS time of group NI patients using the Kaplan-Meier
method. In total, 20 out of the 99 patients in group I, and 125 out
of the 408 patients in group NI succumbed to NSCLC or other causes
during the present study. The improvement in the OS time of
patients in group I was significant (P=0.03; Fig. 1) (11).
The present findings suggest that immunotherapy with
DC-CIK has antitumor effects and, in combination with conventional
treatment therapies, may prolong the OS time of advanced NSCLC
patients (32,34).
Severe treatment-associated adverse effects and
inadequate outcomes associated with conventional treatments may
account for the poor prognosis of patients with advanced NSCLC. The
present study revealed that there were no severe adverse effects
resulting from DC-CIK therapy. Furthermore, this therapy mounted an
immune response against NSCLC, and consequently lengthened the OS
time and improved the QOL of patients (35). The present study concludes that DC-CIK
may be beneficial for patients with advanced NSCLC.
Acknowledgements
The present study was partially supported by the
Tianjin Municipal Science and Technology Commission (grant no.,
12ZCDZSY17100).
References
|
1
|
Gupta N, Hatoum H and Dy GK: First line
treatment of advanced non-small-cell lung cancer - specific focus
on albumin bound paclitaxel. Int J Nanomedicine. 9:209–221.
2014.PubMed/NCBI
|
|
2
|
Zhong R, Han B and Zhong H: A prospective
study of the efficacy of a combination of autologous dendritic
cells, cytokine-induced killer cells, and chemotherapy in advanced
non-small cell lung cancer patients. Tumour Biol. 35:987–994. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zarogoulidis K, Zarogoulidis P, Darwiche
K, Boutsikou E, Machairiotis N, Tsakiridis K, Katsikogiannis N,
Kougioumtzi I, Karapantzos I, Huang H and Spyratos D: Treatment of
non-small cell lung cancer (NSCLC). J Thorac Dis. 5(Suppl 4):
S389–S396. 2013.PubMed/NCBI
|
|
4
|
Hirsh V: Review of the treatment of
metastatic non small cell lung carcinoma: A practical approach.
World J Clin Oncol. 2:262–271. 2011.PubMed/NCBI
|
|
5
|
Fiala O, Pesek M, Finek J, Svaton M,
Sorejs O, Bortlicek Z, Kucera R and Topolcan O: Prognostic
significance of serum tumor markers in patients with advanced-stage
NSCLC treated with pemetrexed-based chemotherapy. Anticancer Res.
36:461–466. 2016.PubMed/NCBI
|
|
6
|
Choi MK, Hong JY, Chang W, Kim M, Kim S,
Jung HA, Lee SJ, Park S, Chung MP, Sun JM, et al: Safety and
efficacy of gemcitabine or pemetrexed in combination with a
platinum in patients with non-small-cell lung cancer and prior
interstitial lung disease. Cancer Chemother Pharmacol.
73:1217–1225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang L, Ren B, Li H, Yu J, Cao S, Hao X
and Ren X: Enhanced antitumor effects of DC-activated CIKs to
chemotherapy treatment in a single cohort of advanced
non-small-cell lung cancer patients. Cancer Immunol Immunother.
62:65–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelly RJ, Gulley JL and Giaccone G:
Targeting the immune system in non-small-cell lung cancer: Bridging
the gap between promising concept and therapeutic reality. Clin
Lung Cancer. 11:228–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aktipis CA and Nesse RM: Evolutionary
foundations for cancer biology. Evol Appl. 6:144–159. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schürch CM, Riether C and Ochsenbein AF:
Dendritic cell-based immunotherapy for myeloid leukemias. Front
Immunol. 4:4962013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu H, Song J, Yang Z and Zhang X: Effects
of cytokine-induced killer cell treatment combined with FOLFOX4 on
the recurrence and survival rates for gastric cancer following
surgery. Exp Ther Med. 6:953–956. 2013.PubMed/NCBI
|
|
12
|
Linn YC and Hui KM: Cytokine-induced
NK-like T cells: From bench to bedside. J Biomed Biotechnol.
2010:4357452010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma YJ, He M, Han JA, Yang L and Ji XY: A
clinical study of HBsAg-activated dendritic cells and
cytokine-induced killer cells during the treatment for chronic
hepatitis B. Scand J Immunol. 78:387–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sangha R, Price J and Butts CA: Adjuvant
therapy in non-small cell lung cancer: Current and future
directions. Oncologist. 15:862–872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Severo M, Gaio R, Lourenço P, Alvelos M,
Bettencourt P and Azevedo A: Indirect calibration between clinical
observers - application to the New York Heart Association
functional classification system. BMC Res Notes. 4:2762011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ribeiro JP, Matuguma SE, Cheng S, Herman
P, Sakai P, D'Albuquerque LA and Maluf-Filho F: Results of
treatment of esophageal variceal hemorrhage with endoscopic
injection of n-butyl-2-cyanoacrylate in patients with Child-Pugh
class C cirrhosis. Endosc Int Open. 3:E584–E589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Yin J, Li T, Huang S, Yan H,
Leavenworth J and Wang X: NK cell-based cancer immunotherapy: From
basic biology to clinical application. Sci China Life Sci.
58:1233–1245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai H, Wang Y, Lu X and Han W: Chimeric
antigen receptors modified T-cells for cancer therapy. J Natl
Cancer Inst. 108:djv4392016.PubMed/NCBI
|
|
19
|
Yu D, Du K, Liu T and Chen G: Prognostic
value of tumor markers, NSE, CA125 and SCC, in operable NSCLC
Patients. Int J Mol Sci. 14:11145–11156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang R, Wang G, Zhang N, Li X and Liu Y:
Clinical evaluation and cost-effectiveness analysis of serum tumor
markers in lung cancer. Biomed Res Int. 2013:1956922013.PubMed/NCBI
|
|
21
|
Pujol JL, Molinier O, Ebert W, Daurès JP,
Barlesi F, Buccheri G, Paesmans M, Quoix E, Moro-Sibilot D,
Szturmowicz M, et al: CYFRA 21–1 is a prognostic determinant in
non-small-cell lung cancer: Results of a meta-analysis in 2063
patients. Br J Cancer. 90:2097–2105. 2004.PubMed/NCBI
|
|
22
|
Cui Y, Yang X, Zhu W, Li J, Wu X and Pang
Y: Immune response, clinical outcome and safety of dendritic cell
vaccine in combination with cytokine-induced killer cell therapy in
cancer patients. Oncol Lett. 6:537–541. 2013.PubMed/NCBI
|
|
23
|
Mitchell DA and Sampson JH: Toward
effective immunotherapy for the treatment of malignant brain
tumors. Neurotherapeutics. 6:527–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Zhang W, Zhang B, Yin X and Pang Y:
DC vaccine therapy combined concurrently with oral capecitabine in
metastatic colorectal cancer patients. Hepatogastroenterology.
60:23–27. 2013.PubMed/NCBI
|
|
25
|
Wang M, Shi SB, Qi JL, Tang XY and Tian J:
S-1 plus CIK as second-line treatment for advanced pancreatic
cancer. Med Oncol. 30:7472013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sabado RL and Bhardwaj N: Directing
dendritic cell immunotherapy towards successful cancer treatment.
Immunotherapy. 2:37–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mantia-Smaldone GM, Corr B and Chu CS:
Immunotherapy in ovarian cancer. Hum Vaccin Immunother.
8:1179–1191. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Minidis NM, Mesner O, Agan BK and Okulicz
JF: Delayed-type hypersensitivity (DTH) test anergy does not impact
CD4 reconstitution or normalization of DTH responses during
antiretroviral therapy. J Int AIDS Soc. 17:187992014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Timmermann C: 'Just give me the best
quality of life questionnaire': The Karnofsky scale and the history
of quality of life measurements in cancer trials. Chronic Illn.
9:179–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoos A, Eggermont AM, Janetzki S, Hodi FS,
Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L and Wolchok
J: Improved endpoints for cancer immunotherapy trials. J Natl
Cancer Inst. 102:1388–1397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pandolfi F, Cianci R, Pagliari D, Casciano
F, Bagalà C, Astone A, Landolfi R and Barone C: The immune response
to tumors as a tool toward immunotherapy. Clin Dev Immunol.
2011:8947042011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuanying Y, Lizhi N, Feng M, Xiaohua W,
Jianying Z, Fei Y, Feng J, Lihua H, Jibing C, Jialiang L and
Kecheng X: Therapeutic outcomes of combining cryotherapy,
chemotherapy and DC-CIK immunotherapy in the treatment of
metastatic non-small cell lung cancer. Cryobiology. 67:235–240.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma X, Lin C and Zhen W: Cancer care in
China: A general review. Biomed Imaging Interv J. 4:e392008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kirkwood JM, Butterfield LH, Tarhini AA,
Zarour H, Kalinski P and Ferrone S: Immunotherapy of cancer in
2012. CA Cancer J Clin. 62:309–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thomas AA, Ernstoff MS and Fadul CE:
Immunotherapy for the treatment of glioblastoma. Cancer J.
18:59–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maggi E: T-cell responses induced by
allergen-specific immunotherapy. Clin Exp Immunol. 161:10–18.
2010.PubMed/NCBI
|