Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide, and ~85% of lung cancer
diagnoses are of non-small cell lung cancer (NSCLC) (1,2). Although
great progress has been made in small molecular-targeted drugs for
treating NSCLC, particularly epidermal growth factor receptor
(EGFR) tyrosine kinase inhibitors such as gefitinib and erlotinib,
EGFR mutations are detected in only 10% of NSCLC patients in the
United States and in 35% of NSCLC patients in East Asia. Thus,
platinum-based combination chemotherapies remain the mainstay of
advanced NSCLC treatment, and cisplatin (DDP) is widely used in
clinical therapy (3). However, the
overall 5-year survival rate for lung cancer is ~15%, and this rate
has improved only slightly over the last 30 years despite the
advancement of modern chemotherapy, a problem which is mainly
caused by drug resistance to platinum (4).
The problem of resistance to DDP-based chemotherapy
remains one of the major obstacles to the treatment of lung cancer.
A number of mechanisms have been proposed to explain cancer cell
resistance to chemotherapy (5,6). These
mechanisms generally involve an increase in the level of multidrug
resistance-1/P-glycoprotein (P-gp) (7,8), and the
regulation of apoptosis-related genes and proteins such as tumor
protein p53 (p53) and B-cell lymphoma 2 (Bcl-2) family members
(9–11). However the underlying mechanisms are
not yet fully understood. Thus, there is an urgent requirement to
learn how to improve the efficacy of platinum or to identify a
novel generation of platinum agents.
Nedaplatin (NDP), which is a second-generation DDP
analog, was developed by the Shionogi Pharmaceutical Company
(Japan) in 1983, in order to provide a treatment with a level of
effectiveness similar to that of DDP, but with decreased
gastrointestinal and renal toxicities (12). A number of previous clinical studies
have demonstrated the efficacy of NDP to be higher than that of DDP
in patients with DDP-resistant lung cancer (13,14).
Conversely, there are comparatively few in vitro studies to
support the consensus. Moreover, it is unclear why NDP is not
completely cross-resistant with DDP.
The purpose of the present study was to demonstrate
the efficacy of NDP in DDP-resistant A549 (A549DDP) cells in
vitro directly. Moreover, the study aimed to detect the
expression of DDP resistance-related proteins, such as P-gp, p53,
Bcl-2-associated X protein (Bax) and Bcl-2, to investigate the
possible mechanisms behind NDP efficacy in the A549DDP cells.
Materials and methods
Cell culture
The human NSCLC A549 cell line and the human
DDP-resistant cell strain, A549DDP, were used in this study. The
cells were obtained from Shanghai Cell Biology, an Institute of the
Chinese Academy of Sciences (Shanghai, China). The A549 cell line
was cultured in RPMI-1640 medium supplemented with 100 U/ml
penicillin, 100 mg/ml streptomycin and 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
A549DDP cells were cultured in high glucose Dulbecco's modified
Eagle's medium supplemented with 100 U/ml penicillin, 100 mg/ml
streptomycin and 10% fetal bovine serum; 2 µg/ml DDP (Jiangsu
Haosen Pharmaceutical Co., Ltd., Lianyungang, China) was dissolved
into this solution in order to maintain drug resistance. However,
the A549DDP cells were grown in the absence of DDP 2 days prior to
treatment. These cells were incubated in a standard cell culture
incubator (Series 8000 Water-Jacketed CO2 Incubator; Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2, and passaged once or twice a
week. Cells in the algorithm growth phase were used in the
following experiments.
Cell proliferation and the
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The half maximal inhibitory concentration (IC50) of
the A549DDP and A549 cells was determined by the MTT assay
(Beyotime, Shanghai, China). The A549 and A549DDP cells were seeded
into 96-well plates (1×104-1×105 cells per well), and treated with
DDP and NDP (Jiangsu Aosaikang, Nanjing, China) at different
concentrations (A549 cells: 2, 4, 6, 8 and 10 µg/ml; A549DDP cells:
10, 15, 20, 25 and 30 µg/ml) for 48 h. Following incubation, 5
mg/ml MTT (20 µl/well) was added to the media and the cells were
further incubated in an atmosphere of 5% CO2 at 37°C for 4 h.
Dimethylsulfoxide (150 µl; Sigma-Aldrich, St. Louis, MO, USA) was
added to the cells in each of the wells after the media was
removed, and the cells were further incubated for 10 min. The
optical density (OD) of each well was measured using a microplate
reader (Multiskan™ GO Microplate Spectrophotometer; Thermo Fisher
Scientific, Inc.) at 560 nm. All experiments were performed in
triplicate according to the following formula: Cell inhibitory rate
(%) = (1 - OD of test group / OD of control group) × 100.
Apoptosis detection and cell cycle
analysis
The rate of apoptosis induced by the anticancer
regimens was analyzed by flow cytometry using an annexin
V-fluorescein isothiocyanate/propidium iodide kit (Kaijibio,
Nanjing, China). Adherent and floating cells were harvested and
gently disaggregated to a single-cell suspension. Staining was
performed according to the manufacturer's protocols. The data were
analyzed immediately by flow cytometry using CXP software (Beckman
Coulter, Inc., Brea, CA, USA).
Protein isolation and western blot
analysis
Subsequent to exposure to DDP and NDP for 48 h, cell
protein extracts were determined using 500 µl
radioimmunoprecipitation assay lysis buffer with 5 µl
phenylmethylsulfonyl fluoride and protease inhibitor (Abcam,
Cambridge, UK). Total proteins were quantified using the
bicinchoninic acid assay (Beyotime, Shanghai, China) according to
the manufacturer's protocols. Total protein (20 µg) was loaded onto
an 8% sodium dodecyl sulfate-polyacrylamide gel and transferred to
a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). The membrane was incubated for 1 h at 25°C in 5% (w/v)
skimmed dried milk and then washed three times for 5 min at 25°C
using blocking buffer [Tris-buffered saline with Tween 20 (TBST)
buffer: 0.1% Tween 20, 13.7 mM NaCl, 0.27 mM KCl and 2.4 mM Tris).
Next, the membrane was incubated overnight at 4°C with monoclonal
mouse anti-human primary antibodies for P-gp (clone, JSB-1; catalog
no., ab3366), p53 (clone, PAb 1801; catalog no., ab28), Bax (clone,
2D2; catalog no., ab77566) and Bcl-2 (clone, Bcl2/100; catalog no.,
ab117115). All antibodies were diluted to 1:1,000 and purchased
from Abcam. Subsequent to being washed three times with TBST for 5
min each, the membranes were incubated for 1 h with horseradish
peroxidase-conjugated secondary antibodies (goat anti-rabbit IgG;
1:5,000; catalog no., sc-2004; Santa Cruz Biotechnology Inc.,
Dallas, TX, USA). In order to evaluate of protein expression
accurately, β-actin (mouse monoclonal; clone, AC-15; catalog no.,
ab6276; Abcam) and histone H3 protein (mouse monoclonal; clone,
mAbcam 1220; catalog no., ab1220; Abcam) were used as an internal
standard. Band intensity was analyzed with an imaging and analysis
system (Peiqing JS-780; Hai Pei Qing Technology Co., Ltd.,
Shanghai, China), and protein expression was presented as the ratio
of the protein band intensity to β-actin or Histone H3 in the same
blot.
Statistical analysis
The values presented represent the mean ± standard
deviation calculated from the data. All analyses were performed
using the Statistical Package for Social Sciences, version 13 (SPSS
Inc., Chicago, IL, USA). Differences were evaluated using Student's
t-test or an analysis of variance.
Results
Cell inhibitory measurement by MTT
assay
An MTT assay was used to determine the sensitivity
of A549DDP cells to DDP, to investigate whether these cells are
resistant to DDP and to determine whether NDP has a stronger
inhibitory effect than DDP in A549DDP cells.
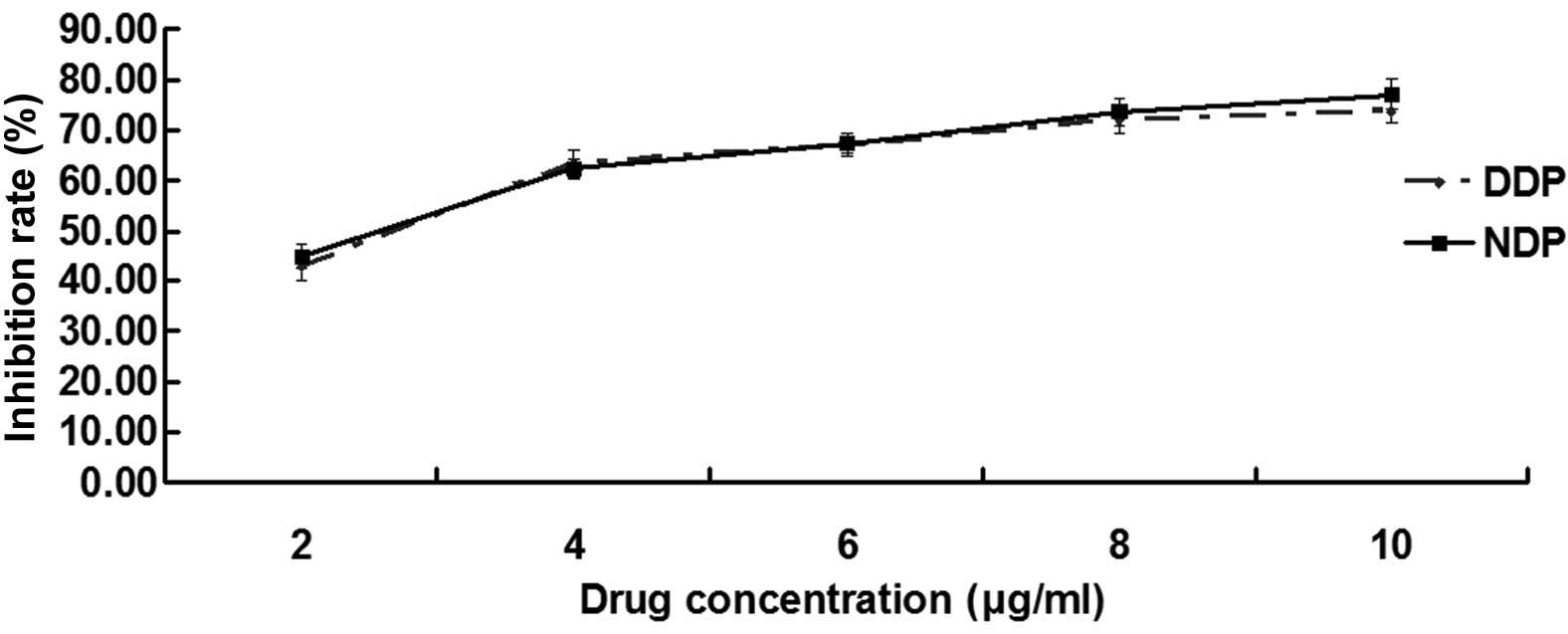
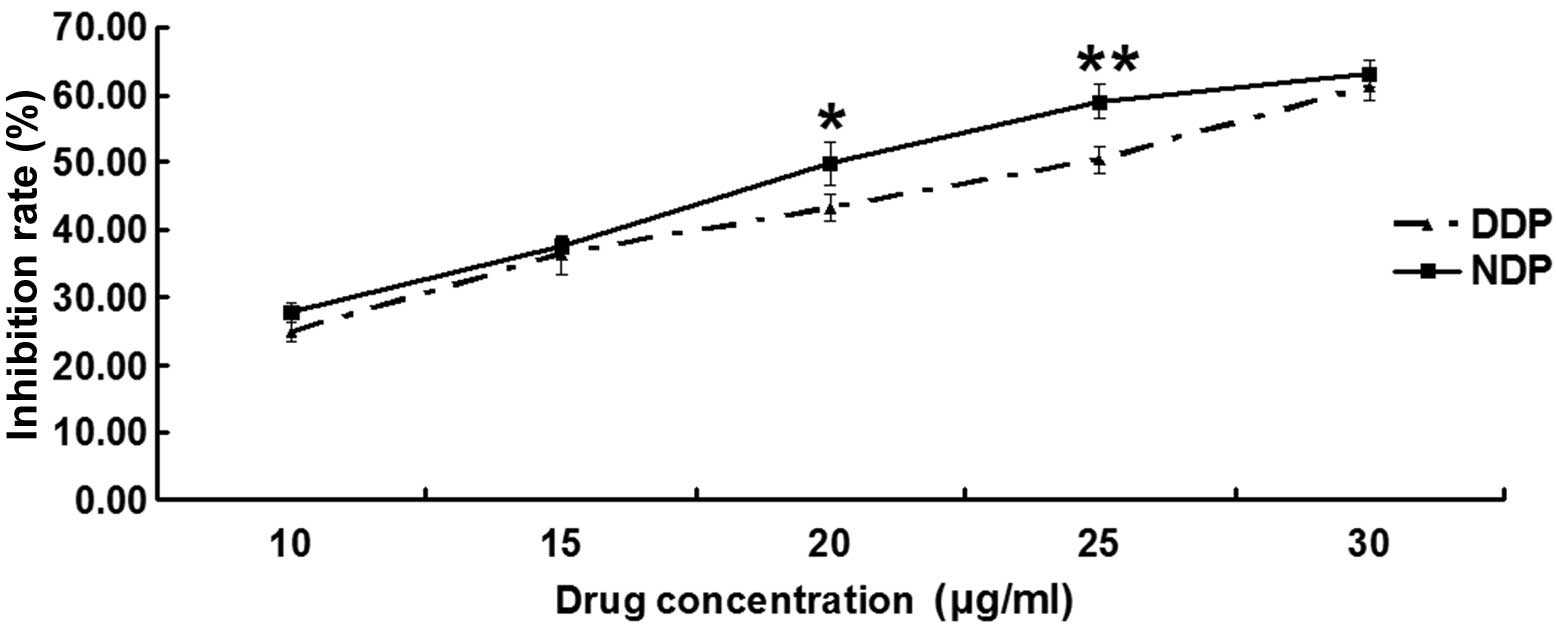
The inhibition rate is shown in Figs. 1 and 2,
and Tables I and II. For the first part of the MTT assay, the
IC50 values of the A549 and A549DDP cells treated with DDP were
2.53±0.12 and 23.36±1.41 µg/ml, respectively, and the difference
between them was significant (P<0.001), which verified that
A549DDP cells exhibit resistance to DDP.
 | Table I.Inhibition A549 cells after 48 h of
intervention with DDP and NDP at different concentrations. |
Table I.
Inhibition A549 cells after 48 h of
intervention with DDP and NDP at different concentrations.
|
| Drug concentration,
µg/ml |
|
|---|
|
|
|
|
|---|
| Group | 2 | 4 | 6 | 8 | 10 | IC50 |
|---|
| DDP | 42.78±2.50 | 63.21±2.73 | 66.79±1.76 | 72.06±2.83 | 73.68±2.51 | 2.53±0.12 |
| NDP | 44.84±2.32 | 62.34±1.97 | 67.28±1.96 | 73.56±2.63 | 76.88±3.04 | 2.49±0.78 |
| P-value | 0.354 | 0.677 | 0.765 | 0.537 | 0.232 | 0.834 |
In the second part, of the MTT assay, the IC50 of
the A549 cells treated with DDP and NDP was 2.53±0.12 and 2.49±0.78
µg/ml, respectively (Fig. 1; Table I), and the difference was not
significant (P=0.834). However, the IC50 values of the A549DDP
cells treated with DDP and NDP were 23.36±1.41 and 19.97±0.88 µg/ml
(Fig. 2; Table II); this difference was significant,
suggesting that NDP had a better effect than DDP on the A549DDP
cells.
 | Table II.Inhibition of A549DDP cells after 48 h
of intervention with DDP and NDP at different concentrations. |
Table II.
Inhibition of A549DDP cells after 48 h
of intervention with DDP and NDP at different concentrations.
|
| Drug concentration,
µg/ml |
|
|---|
|
|
|
|
|---|
| Group | 10 | 15 | 20 | 25 | 30 | IC50 |
|---|
| DDP | 24.79±1.53 | 36.28±2.85 | 43.25±2.04 | 50.38±1.95 | 60.54±1.66 | 23.36±1.41 |
| NDP | 27.74±1.48 | 39.20±2.91 | 49.93±3.22 | 59.05±2.56 | 63.21±1.93 | 19.97±0.88 |
| P-value | 0.074 | 0.283 | 0.038a | 0.009a | 0.144 | 0.024a |
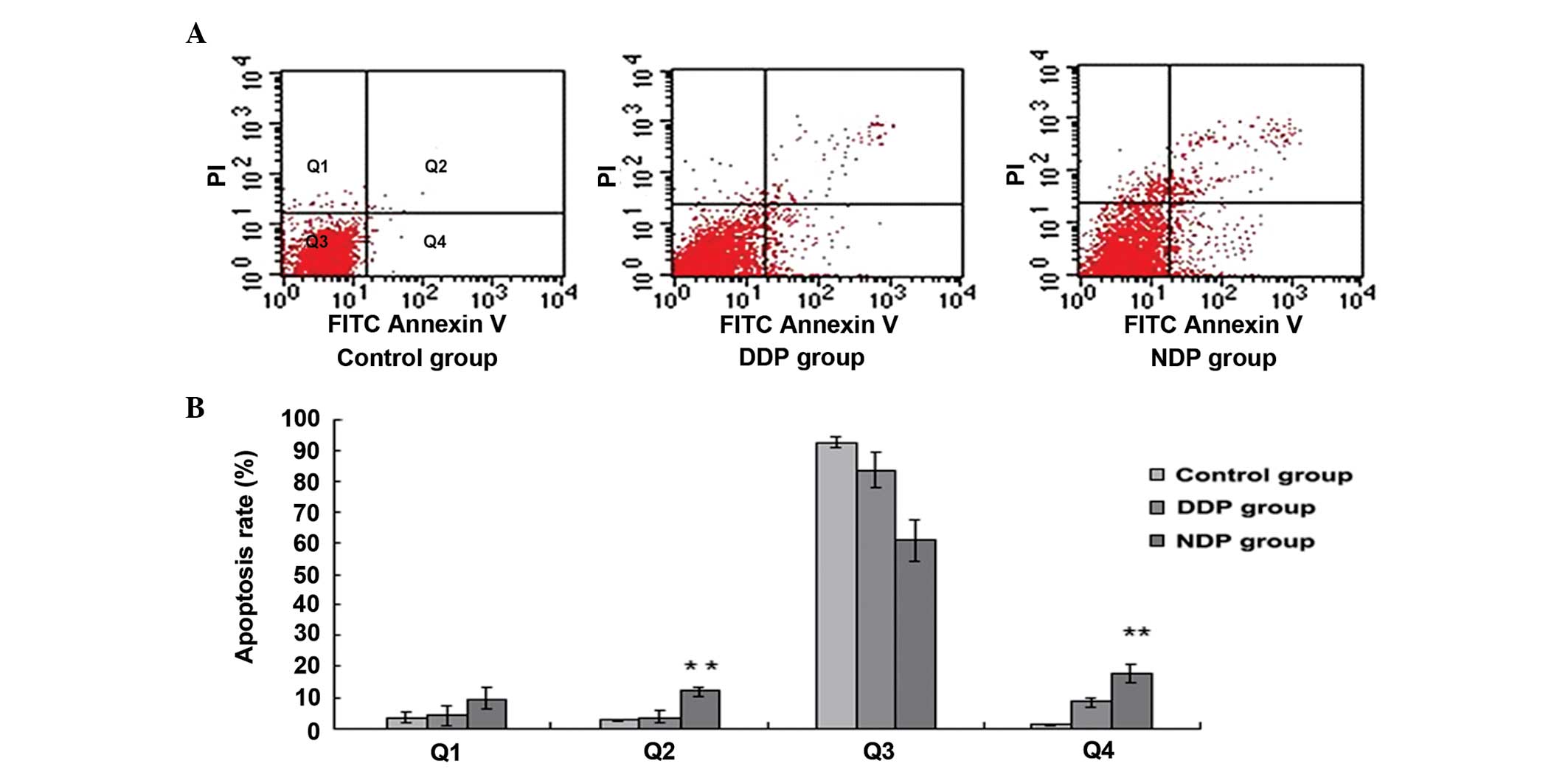
Cell apoptosis and the cell cycle, as
shown by flow cytometry
Flow cytometry was used to investigate the
differences in cell apoptosis and the cell cycle between the
A549DDP cells treated with DDP and NDP. After 48 h of intervention
with DDP (20 µg/ml) and NDP (20 µg/ml), the levels of cell
apoptosis in the NDP and DDP groups increased and were significant
when compared with the control group (each P<0.01). In
comparison with the DDP group, the degree of early and late
apoptosis in the NDP group increased, and this difference was
significant (P=0.010 and P=0.005, respectively) (Fig. 3; Table
III).
 | Table III.Cell apoptosis rate induced by DDP and
NDP, as detected by flow cytometry. |
Table III.
Cell apoptosis rate induced by DDP and
NDP, as detected by flow cytometry.
| Group | Q1 | Q2 | Q3 | Q4 |
|---|
| Control, % | 3.49±1.74 |
2.60±0.47 | 92.78±1.69 |
1.11±0.17 |
| DDP, % | 4.09±3.35 |
3.63±2.06 | 83.81±5.55 |
8.47±1.54 |
| NDP, % | 9.64±3.75 | 11.80±1.50 | 60.97±6.70 | 17.59±2.81 |
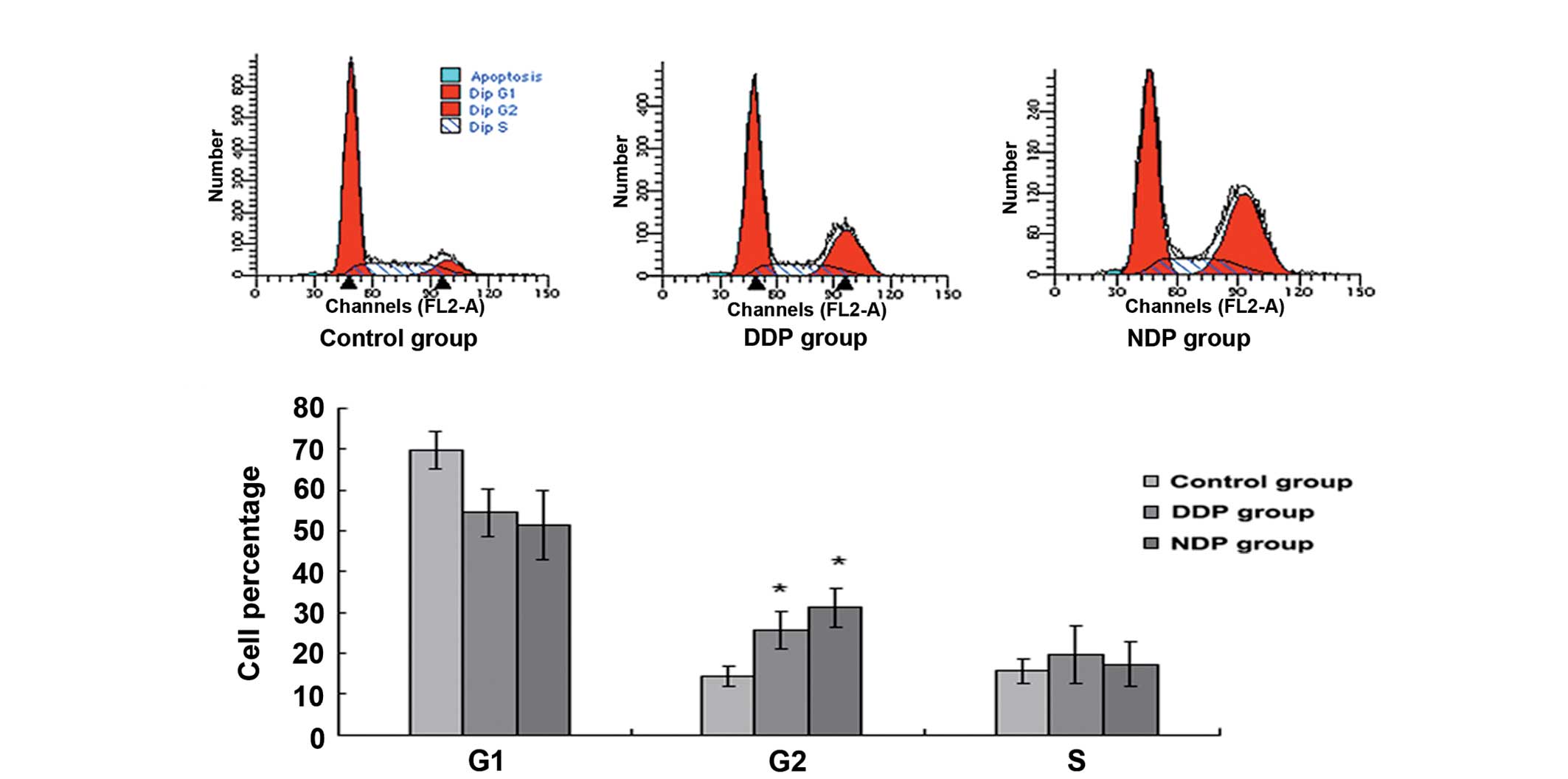
In comparison with the control group, the percentage
of cells in G2 increased (P=0.021 and P=0.005, respectively) and
the proportion in the G1 stage decreased (P=0.023 and P=0.031,
respectively) following intervention with DDP and NDP, and the
difference was significant (Fig. 4;
Table IV). Furthermore the
difference in the percentage of cells in the G2 phase between the
DDP and NDP groups was not significant (P=0.228).
 | Table IV.Detection of DDP- and NDP-induced
cell cycle arrest by flow cytometry. |
Table IV.
Detection of DDP- and NDP-induced
cell cycle arrest by flow cytometry.
| Group | G1 | S | G2 |
|---|
| Control, % | 68.04±3.50 | 15.81±3.04 | 14.44±2.59 |
| DDP, % | 54.53±5.84 | 19.73±6.93 | 25.73±5.84 |
| NDP, % | 51.52±8.59 | 17.34±5.39 | 31.47±4.76 |
Protein expression and western blot
analysis
Western blotting results revealed that the
expression levels of P-gp, p53, Bax and Bcl-2 in the NDP group were
different from the levels of expression in the control and DDP
groups.
P-gp relative expression in the NDP group was
0.50±0.03, which was significantly higher than that of the control
(0.80±0.12; P=0.015) and DDP (0.63±0.05; P=0.014) groups (Fig. 5). However, compared with the control
group, the expression of P-gp in the DDP group was not
significantly different (P=0.094).
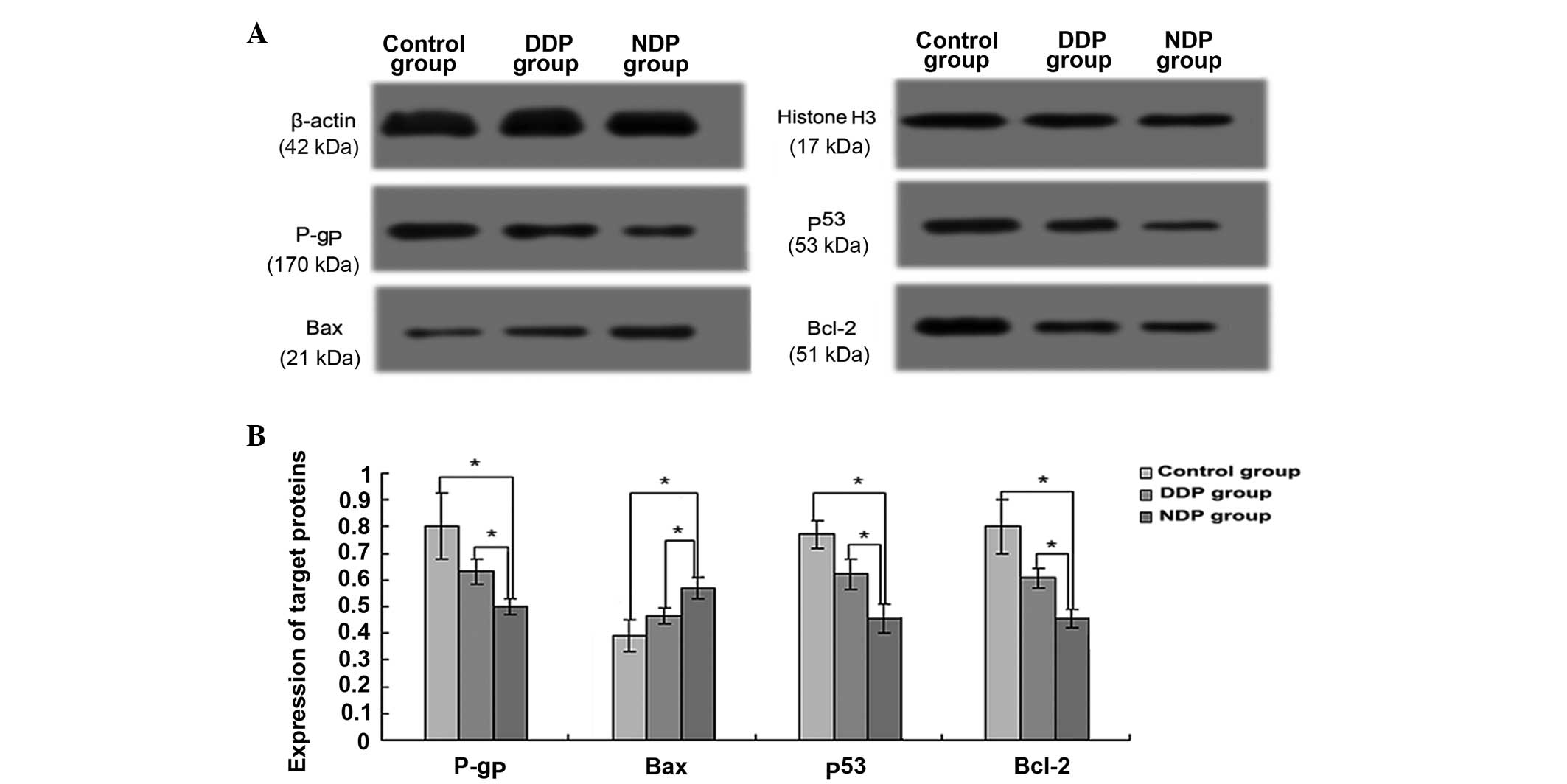 | Figure 5.Expression of P-gp, p53, Bcl-2 and Bax
in the three groups, as detected by western blot assay. (A) Protein
expression was examined using western blotting and recorded with a
scanner system. (B) The relative expression of target proteins
(compared with β-actin protein or histone H3 protein expression) in
the three groups. *Compared with the control or DDP groups, the NDP
group exhibited significantly lower or higher expression of the
target poteins (P<0.05). DDP, cisplatin; NDP, nedaplatin; P-gp,
P-glycoprotein; p53, tumor protein 53; Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X protein. |
The relative expression levels of p53 in the
control, DDP and NDP groups were 0.77±0.05, 0.62±0.06 and
0.45±0.05, respectively. p53 expression in the NDP and DDP groups
was significantly less than in the control group (P=0.002 and
P=0.020, respectively) (Fig. 5).
Compared with the DDP group, the NDP group exhibited a lower p53
expression level, and the difference was significant (P=0.020).
The relative expression of Bax in the NDP group was
0.57±0.04, which was significantly higher than that of the control
(0.39±0.06; P=0.012) and DDP (0.46±0.03; P=0.024) groups (Fig. 5). By contrast, the Bax expression in
the DDP group, when compared to the control group, did not exhibit
a significant difference (P=0.112).
However, the western blot analysis revealed that the
relative expression of Bcl-2 in the control, DDP and groups was
0.80±0.10, 0.60±0.04 and 0.45±0.04, respectively. Bcl-2 expression
in the NDP and DDP groups was significantly lower than that in the
control group (P=0.005 and P=0.005, respectively) (Fig. 5). Compared with the DDP group, the NDP
group exhibited lower Bcl-2 expression, and the difference was also
significant (P=0.008).
Discussion
The present study results showed that at the same
concentration, NDP had a higher cell inhibition rate than DDP in
A549DDP cells, particularly for concentrations of 20 and 25 µg/ml
(P=0.038 and P=0.009, respectively). It was also found that the
blockage of the cell cycle at the G2 phase in the A549DDP cells
increased significantly following intervention with DDP and NDP,
but that the difference between these two groups was not
significant. However, compared with the DDP group, the NDP group
exhibited significantly greater early and late apoptosis ratios.
Therefore, it was concluded that the use of NDP was more
advantageous than the use of DDP in A549DDP cells. In this study,
the results also showed that the expression levels of P-gp, p53 and
Bcl-2 in the NDP group were significantly less than those of the
other two groups, and that the expression of Bax in the NDP group
was significantly higher. Moreover it was found that the difference
in Bcl-2 expression between the NDP and DDP groups was more
pronounced (P=0.008).
P-gp is one of the major drug efflux transporters; it
increases the efflux of drugs out of cells against the concentration
gradient, thereby reducing the intracellular concentration of the
drug below the effective level, which finally leads to drug
resistance (8,15). It has been verified that a number of
anticancer drugs, including DDP, etoposide and vinblastine, are
P-gp substrates (16). The results of
the present study showed that the P-gp expression level of cells
exposed to NDP was significantly lower than that of cells exposed
to DDP. Thus, it was concluded that NDP could possibly inhibit the
P-gp expression level, thereby decreasing the efflux of drugs out of
the A549DDP cells. This would improve the NDP intracellular drug
concentration, which would suppress A549DDP cell proliferation.
Lung cancer cells have been shown to possess a
higher p53 mutation rate (70%); the mutation of the gene could
result in abnormal expression of the p53 protein (17). The wild-type p53 protein is able to
exert a range of anti-proliferative effects, including the
induction of apoptosis and causing a marked increase in the
sensitivity of these cells to DDP (18,19).
However malignancies with mutated p53 genes and aberrant p53
proteins in laboratory studies and one clinical study have been
observed to be less responsive to chemotherapy agents that induce
DNA damage, such as DDP (20,21). A number of studies have suggested that
the overexpression of the mutant p53 protein may directly enhance
tumor cell resistance to anticancer agents in a way that is
dependent on the particular mutation and the mechanism of action of
the drug (22–24). In a situation of cellular stress, such
as DNA damage, the mutated p53 genes and aberrant p53 proteins
participate in the process of inducing cell-cycle arrest, and can
enhance DNA repair or cell death and upregulate the expression of
P-gp (25,26). In the present study, compared with DDP
intervention, NDP intervention led to a significant downregulation
of p53 protein. Combined with the greater downregulation of P-gp
protein following NDP intervention, this results indicates that the
mutant p53 protein was likely detected in the A549DDP cells, and
that NDP could inhibit the expression of the mutant p53 protein,
thereby decreasing the upregulation of P-gp expression in order to
withstand DDP resistance.
There are numerous members in the Bcl-2 family, and
while certain members, such as Bcl-2, are anti-apoptotic, other,
such as Bax, are pro-apoptotic. The ratio between pro- and
anti-apoptotic Bcl-2 family members is a significant determinant of
cell survival and cell death. A number of cancer chemotherapeutic
agents ultimately act on these factors causing cells to undergo
apoptosis (27). The Bcl-2 family
plays a significant role in the cellular response to chemotherapy.
The overexpression of Bcl-2 increases the resistance to
drug-induced apoptosis, and the survival of Bcl-2-negative tumors
is less than that of Bcl-2-positive tumors (28,29). In
the present study, it was found that the Bcl-2 expression level of
NDP-exposed cells was significantly lower than that in cells
exposed to DDP, while the Bax expression level in the NDP-exposed
cells was significantly greater. It was concluded that NDP could
regulate Bcl-2 and Bax expression, thereby promoting the apoptosis
of A549DDP cells by allowing NDP to withstand DDP resistance.
Clinically, an association has been observed between
NDP and an improved response in DDP-resistant cancer (13,30,31).
Therefore, in the present study, the effect of NDP on A549DDP cells
was analyzed by in vitro experimentation, which has not yet
been verified in any previous findings. Eventually, this may assist
in providing important clues to guide clinicians towards better
therapy decisions. However, the present study has the certain
limitations. Firstly, the study failed to demonstrate the further
mechanism(s) of the effect of NDP on A549DDP cells. Secondly, these
findings should be extended to other resistant cell lines and
animal experiments. Finally, the collection and systematic
evaluation of extensive clinical data should be performed in order
to confirm the in vivo relevance of the findings.
In summary, the present study suggested that NDP
could have higher efficacy in DDP-resistant lung cancer cells and
that its effect may be multifactorial. Compared with DDP, NDP was
able to decrease the P-gp and p53 protein expression levels to
improve the NDP intracellular drug concentration, and could
regulate the expression of Bcl-2 family members to promote
apoptosis. Further studies applying more detailed analyses are
warranted to elucidate the mechanism(s) of the effect of NDP on
DDP-resistant lung cancer cells.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Azzoli CG, Temin S and Giaccone G: 2011
Focused update of 2009 American society of clinical oncology
clinical practice guideline update on chemotherapy for stage IV
non-small-cell lung cancer. J Oncol Pract. 8:63–66. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ettinger DS, Akerley W, Borqhaei H, Chang
AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Govindan R,
Grannis FW Jr, et al: Non-small cell lung cancer, version 2.2013. J
Natl Compr Canc Netw. 11:645–653. 2013.PubMed/NCBI
|
|
4
|
Rigas JR: Taxane-platinum combinations in
advanced non-small cell lung cancer: A review. Oncologist. 9(Suppl
2): S16–S23. 2004. View Article : Google Scholar
|
|
5
|
Davis A, Tinker AV and Friedlander M:
‘Platinum resistant’ ovarian cancer: What is it, who to treat and
how to measure benefit? Gynecol Oncol. 133:624–631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Torigoe T, Izumi H, Ishiguchi H, et al:
Cisplatin resistance and transcription factors. Curr Med Chem
Anticancer Agents. 5:15–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao L, Liu G, Ma J, Wang X, Wang F, Wang H
and Sun J: Paclitaxel nanosuspension coated with P-gp inhibitory
surfactants: II. Ability to reverse the drug-resistance of H460
human lung cancer cells. Colloids Surf B Biointerfaces.
117:122–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamagishi T, Sahni S, Sharp DM, Arvind A,
Jansson PJ and Richardson DR: P-glycoprotein mediates drug
resistance via a novel mechanism involving lysosomal sequestration.
J Biol Chem. 288:31761–31771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cavallo F, Feldman DR and Barchi M:
Revisiting DNA damage repair, p53-mediated apoptosis and cisplatin
sensitivity in germ cell tumors. Int J Dev Biol. 57:273–280. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang M, Shan X, Zhou X, Qiu T, Zhu W, Ding
Y, Shu Y and Liu P: miR-1271 regulates cisplatin resistance of
human gastric cancer cell lines by targeting IGF1R, IRS1, mTOR and
BCL2. Anticancer Agents Med Chem. 14:884–891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang G, Reed E and Li QQ: Molecular basis
of cellular response to cisplatin chemotherapy in non-small cell
lung cancer (Review). Oncol Rep. 12:955–965. 2004.PubMed/NCBI
|
|
12
|
Ota K: Nedaplatin. Gan To Kagaku Ryoho.
23:379–387. 1996.(In Japanese). PubMed/NCBI
|
|
13
|
Jin J, Xu X, Wang F, Yan G, Liu J, Lu W,
Li X, Tucker SJ, Zhong B, Cao Z and Wang D: Second-line combination
chemotherapy with docetaxel and nedaplatin for cisplatin-pretreated
refractory metastatic/recurrent esophageal squamous cell carcinoma.
J Thorac Oncol. 4:1017–1021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li CH, Liu MY, Liu W, Li DD and Cai L:
Randomized control study of nedaplatin or cisplatin concomitant
with other chemotherapy in the treatment of advanced non-small cell
lung cancer. Asian Pac J Cancer Prev. 15:731–736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharom FJ: Complex interplay between the
P-glycoprotein multidrug efflux pump and the membrane: Its role in
modulating protein function. Front Oncol. 4:412014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu D: Where is it and how does it get
There-intracellular localization and traffic of P-glycoprotein.
Front Oncol. 3:3212013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vaughan CA, Singh S, Windle B, Yeudall WA,
Frum R, Grossman SR, Deb SP and Deb S: Gain-of-function activity of
mutant p53 in lung cancer through up-regulation of receptor protein
tyrosine kinase axl. Genes Cancer. 3:491–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu ZZ, Sun NK and Chao CC: Knockdown of
CITED2 using short-hairpin RNA sensitizes cancer cells to cisplatin
through stabilization of p53 and enhancement of p53-dependent
apoptosis. J Cell Physiol. 226:2415–2428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bazzi H and Anderson KV: Acentriolar
mitosis activates a p53-dependent apoptosis pathway in the mouse
embryo. Proc Natl Acad Sci USA. 111:E1491–E1500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oren M and Rotter V: Mutant p53
gain-of-function in cancer. Cold Spring Harb Perspect Biol.
2:a0011072010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perrone F, Bossi P, Cortelazzi B, Locati
L, Quattrone P, Pierotti MA, Pilotti S and Licitra L: TP53
mutations and pathologic complete response to neoadjuvant cisplatin
and fluorouracil chemotherapy in resected oral cavity squamous cell
carcinoma. J Clin Oncol. 28:761–766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bossi G, Lapi E, Strano S, Rinaldo C,
Blandino G and Sacchi A: Mutant p53 gain of function: Reduction of
tumor malignancy of human cancer cell lines through abrogation of
mutant p53 expression. Oncogene. 25:304–309. 2006.PubMed/NCBI
|
|
23
|
Tian B, Liu J, Liu B, Dong Y, Liu J, Song
Y and Sun Z: p53 Suppresses lung resistance-related protein
expression through Y-box binding protein 1 in the MCF-7 breast
tumor cell line. J Cell Physiol. 226:3433–3441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cuddihy AR, Jalali F, Coackley C and
Bristow RG: WTp53 induction does not override MTp53 chemoresistance
and radioresistance due to gain-of-function in lung cancer cells.
Mol Cancer Ther. 7:980–992. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung SK, Zhu S, Xu Y and Fu X: Functional
analysis of the acetylation of human p53 in DNA damage responses.
Protein Cell. 5:544–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Podolski-Renić A, Jadranin M, Stanković T,
Banković J, Stojković S, Chiourea M, Aljančić I, Vajs V, Tešević V,
Ruždijić S, et al: Molecular and cytogenetic changes in multi-drug
resistant cancer cells and their influence on new compounds
testing. Cancer Chemother Pharmacol. 72:683–697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pore MM, Hiltermann TJ and Kruyt FA:
Targeting apoptosis pathways in lung cancer. Cancer Lett.
332:359–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar Biswas S, Huang J, Persaud S and
Basu A: Down-regulation of Bcl-2 is associated with cisplatin
resistance in human small cell lung cancer H69 cells. Mol Cancer
Ther. 3:327–334. 2004.PubMed/NCBI
|
|
29
|
Gumulec J, Balvan J, Sztalmachova M,
Raudenska M, Dvorakova V, Knopfova L, Polanska H, Hudcova K,
Ruttkay-Nedecky B, Babula P, et al: Cisplatin-resistant prostate
cancer model: Differences in antioxidant system, apoptosis and cell
cycle. Int J Oncol. 44:923–933. 2014.PubMed/NCBI
|
|
30
|
Akutsu Y, Shuto K, Kono T, Uesato M,
Hoshino I, Shiratori T, Miyazawa Y, Isozaki Y, Akanuma N and
Matsubara H: A phase 1/11 study of second-line chemotherapy with
fractionated docetaxel and nedaplatin for 5-FU/cisplatin-resistant
esophageal squamous cell carcinoma. Hepatogastroenterology.
59:2095–2098. 2012.PubMed/NCBI
|
|
31
|
Yoshioka T, Sakayori M, Kato S, Chiba N,
Miyazaki S, Nemoto K, Shibata H, Shimodaira H, Ohtsuka K, Kakudo Y,
et al: Dose escalation study of docetaxel and nedaplatin in
patients with relapsed or refractory squamous cell carcinoma of the
esophagus pretreated using cisplatin, 5-fluorouracil and radiation.
Int J Clin Oncol. 11:454–460. 2006. View Article : Google Scholar : PubMed/NCBI
|