Introduction
Lung cancer is one of the most common form of cancer
and has a poor prognosis (1).
Surgical treatment is the best option currently available to manage
advanced refractory lung tumors (2).
Chemotherapy may be useful, but cannot be administered to the
majority of patients with lung cancer in advanced stages due to
poor tolerance (3). Recent advances
in the novel approach of adoptive immunotherapy for the treatment
of advance lung cancer are promising for the management of these
patients (4).
Cytokine-induced killer (CIK) cells are a
heterogeneous population of ex vivo expanded T lymphocytes
with diverse T cell receptor specificities, and are endowed with
non-major histocompatibility complex (MHC)-restricted cytotoxic
activities against tumor cells (5).
This antitumor activity is mainly associated with cluster of
differentiation (CD)3+CD56+ cells (6).
The antitumor effects of CIK cells against a number
of hematologic and solid malignancies have been described in murine
tumor models and clinical studies (6–8). In the
severe combined immunodeficiency (SCID) mouse model, infusion of
human CIK cells significantly prolonged survival of SCID mice,
compared with control animals or those infused with lymphokine
activated killer cells (9). In other
studies using the SCID model, CIK cells exhibited in vivo
antitumor activity against a number of hematopoietic and solid
tumors (10). The first clinical
study on CIK cells included 10 patients with metastatic renal
carcinoma, colorectal cancer and lymphoma (8). Of these, 1 patient with lymphoma
experienced complete remission, while 6 patients exhibited disease
progression, and 3 did not experience any alteration on their
condition (5,11). Other clinical studies subsequently
confirmed the safety and benefits of CIK cell-based therapy,
alongside initial clinical activity (12,13).
Adaptive and innate cellular immunity are important
factors that act against tumor growth and aid the clearance of
cancer (14). Adoptive immunotherapy
relies on the ability of the body to efficiently kill tumor cells
and promote immune responses (9). The
number of immune cells, particularly type 1 T helper (Th1) cells,
CD8+ T cells, natural killer (NK) and NKT cells is
associated with the survival of cancer patients (14). Such antitumor cellular immune
responses may be greatly enhanced by adoptive transfer of CIK cells
(14,15).
Several studies have reported that a combination of
chemotherapy, surgical operation and radiotherapy alongside CIK
cell therapy may control local tumors while promoting antitumor
activity and immune responses (12,16).
However, as a newly emerging treatment method, we hypothesize that
there are several challenges that remain to be addressed to
maximize the benefits of the treatment, including the course of CIK
cell immunotherapy, the percentage of
CD3+CD56+ cells among the CIK cells
administered to the patient and the effect of previous treatments
on immune function in cancer patients. Since CIK cell treatment has
a pivotal role in patients with lung cancer, the interpretation of
the aforementioned concerns is important when considering different
treatment options for these patients. In the present study, flow
cytometry data of peripheral blood from patients with lung cancer
was collected to retrospectively analyze whether the course of CIK
cell immunotherapy, previous treatments and percentage of
CD3+CD56+ CIK cells have affected the immune
function in these patients.
Materials and methods
Patients
Patients with lung cancer who attended Dalian
Municipal Central Hospital (Dalian, China) from November 2011 to
May 2014 and agreed to receive CIK treatment were included in the
present study. Following histological or imaging examination, all
patients were diagnosed with stage II–IV lung cancer, according to
the tumor-node-metastasis (TNM) staging system, published by the
International Union Against Cancer in 2009 (17). Exclusion criteria were as follows: i)
History of autoimmune disease or chronic wasting disease and
infectious diseases; ii) use of immunosuppressive agents or notable
psychiatric disease; iii) evidence of other malignancies; and iv)
reception of CIK treatment prior to the study. The present study
was approved by the Ethics Committee of Dalian Municipal Central
Hospital. All patients provided written informed consent prior to
treatment initiation.
Patients were divided into three groups according to
the treatment received prior to enrollment in the study: i) CIK
group, which included patients who had not received any treatment
prior to CIK treatment; ii) Che-Sur group, which included patients
who had received 1–2 courses of chemotherapy and surgical
procedures prior to CIK treatment; and iii) Che-Rad group, which
included patients who had received 1–2 courses of chemotherapy and
1–2 courses of radiotherapy prior to CIK treatment.
All patients were administered CIK treatment and
standardized chemotherapy upon enrolment, based on the patients'
decision to undergo the treatment. Patients were divided into three
groups, according to the percentage of
CD3+CD56+ cells received during each course
of CIK treatment. The percentage of CD3+CD56+
cells received by each group was as follows: <10% for group 1
(Low-CIK group); 10–20% for group 2 (Mid-CIK group); and >20%
for group 3, (High-CIK group). Among these groups, no significant
differences were observed in regards to patients' age, gender,
tumor size, tumor stage, histopathological type and TNM stage.
Preparation of CIK cells
In vitro expansion and reinfusion of
autologous immune cells of the patients was conducted as follows:
Peripheral blood mononuclear cells (PBMCs) were isolated from 50 ml
samples of peripheral blood from patients by Ficoll density
gradient centrifugation at 1,258 × g for 10 min (GT5-4; Beijing Era
Beili Centrifuge Co., Ltd., Beijing, China), as previously
described (18). The yield of PBMCs
isolated was ~3–4×107 cells. Next, PBMCs were cultured
in X–VIVO™ complete medium (Lonza Group Ltd., Basel, Switzerland)
supplemented with 1% interferon (IFN)-γ (eBioscience, Inc., San
Diego, CA, USA) and 1% interleukin (IL)-2 (3SBio Inc., Shenyang,
China). Cells were seeded in culture flasks, and purified mouse
anti-human monoclonal CD3 (clone, OKT3; cat. no. 16-0037-85;
dilution, 1:100; eBioscience, Inc.) and mouse anti-human monoclonal
CD28 (clone, CD28.2; cat. no. 14-0289-82; dilution, 1:100;
eBioscience, Inc.) antibodies were added. The cells were incubated
at 37°C with 5% CO2. Culture medium was added every 2–3 days. Cells
were harvested 2 weeks later. At the end of the culture, a
bacterial culture test was performed. Cells free of contamination
by microorganisms were collected, and ~7–9×109 cells
were harvested in 100 ml normal saline (Huaren Pharmaceutical Co.,
Ltd., Qingdao, China) with 1 ml human serum albumin (Grifols USA,
Los Angeles, CA, USA), and administered to the patients once daily
for 3 consecutive days via the superficial vein.
Chemotherapy
Following the collection of peripheral blood from
patients, chemotherapy was started on that day or 1 day later.
Following culture for 14 days, CIK cells were transfused back into
the patients. These transfusions were defined as one course. The
subsequent course was performed 30 days following the last
collection of peripheral blood. The number of courses performed was
decided by the patients.
Patients with non-small cell lung cancer were
treated with intravenous gemcitabine (1,000 mg/m2, days
1 and 8 in 30 day cycle; Jiangsu Hansoh Pharmaceutical Co., Ltd.,
Lianyungang, China) plus intravenous platinum (35–45
mg/m2, days 1–2 in 30 day cycle; Jiangsu Hansoh
Pharmaceutical Co., Ltd.), whereas patients with small cell lung
cancer were treated with intravenous etoposide (100
mg/m2, days 1–3 in 30 days cycle; Jiangsu Hengrui
Medicine Co., Ltd., Lianyungang, China) plus intravenous cisplatin
(40 mg/m2, days 1–3 in 30 day cycle; Jiangsu Hansoh
Pharmaceutical Co., Ltd.). If any of the patients presented a
contraindication to that protocol, an alternative protocol was then
selected.
Flow cytometry
A total of 2 ml blood was extracted from each
patient to detect the subsets of CD3+CD4+ T,
CD3+ T, NK and NKT cells 1 day prior to each CIK course.
Unless otherwise indicated, all antibodies were purchased from
Beckman Coulter, Inc. (Brea, CA, USA).
The following antibodies were used to analyze the
cells: Fluorescein isothiocyanate (FITC)-conjugated mouse
anti-human monoclonal CD4 (clone, 13B8.2; cat. no. IM1650;
dilution, ready to use), phycoerythrin-cyanine 5-conjugated mouse
anti-CD3 (clone, UCHT1; cat. no. IM1650; dilution, ready to use),
phycoerythrin-conjugated mouse anti-CD16/56 (clone, 3G8; cat. no.
A07735; dilution, ready to use) and mouse FITC-conjugated anti-CD3
(clone, UCHT1; cat. no. A07746; dilution, ready to use) (all
purchased from Beckman Coulter, Inc.). The above monoclonal
antibodies (20 µl) were added to 100 µl fresh
ethylenediaminetetraacetic acid (BD Biosciences, Franklin Lakes,
NJ, USA)-anticoagulated peripheral blood, mixed and stained in the
darkness at room temperature for 20 min. Next, 1.0 ml erythrocyte
lysis buffer (Beckman Coulter, Inc.) was added, mixed and incubated
in the darkness at room temperature for 10 min to lyse the
erythrocytes. The mixture was then centrifuged at 2,000 × g for 5
min [L-500; ZiHe International Trade (Shanghai) Co., Ltd.,
Shanghai, China], and the supernatant was discarded. Subsequently,
1 ml 1X phosphate-buffered saline (Shijiazhuang Tianshun
Biotechnology Co., Ltd., Shijiazhuang, China) was added into each
tube, mixed and centrifuged at 2,000 × g for 5 min [L-500; ZiHe
International Trade (Shanghai) Co., Ltd.] to remove the
supernatant. From each tube, 10,000 cells were obtained, which were
then tested using a FC 500 flow cytometer (CXP2.1; Beckman Coulter,
Inc.).
Detection of cytokines by
enzyme-linked immunosorbent assay (ELISA)
Levels of IFN-γ and IL-10 were measured using
commercial ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA)
according to the manufacturer's protocol. The optical density
values at 450 nm were measured in a microplate reader (VICTOR3 V
1420 Multilabel Counter; PerkinElmer, Inc., Waltham, MA, USA). The
concentration of cytokines in each sample was calculated using a
standard curve generated by Microsoft Excel 2007 software
(Microsoft Corporation, Redmond, WA, USA) using recombinant
cytokines (R&D Systems, Inc.).
Statistical analysis
Data are presented as the mean ± standard error
(SE). Statistical significance of the differences was analyzed by
t-test for data of two groups, and one-way analysis of variance for
data of three groups. Pairwise comparison was performed using
Student-Newman-Keuls test, and differences in the distribution of
selected clinical characteristics were evaluated by χ2
test, using SPSS version 17.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients profile
From November 2011 to May 2014, a total of 57
patients aged 40–86 years (mean, 63.5±11.3 years) were included in
the present study. The baseline characteristics of the patients are
shown in Table I. There were no
significant differences in the clinical characteristics of the
patients included in the CIK, Che-Sur and Che-Rad groups.
 | Table I.Clinical characteristics of patients
with lung cancer included in the present study prior to CIK
treatment (n=57). |
Table I.
Clinical characteristics of patients
with lung cancer included in the present study prior to CIK
treatment (n=57).
| Characteristic | Total (n=57) | CIK (n=17) | Che-Sur (n=21) | Che-Rad (n=19) |
|---|
| Patient |
|
|
|
|
| Gender
(M/F) | 40/17 | 13/4 | 13/8 | 14/5 |
| Age,
years | 63.5±11.3 | 64.6±12.9 | 63.8±12.2 | 62.1±8.0 |
| Tumor stage |
|
|
|
|
| II | 7 | 3 | 3 | 1 |
|
III | 7 | 2 | 1 | 4 |
| IV | 43 | 12 | 17 | 14 |
| Histopathological
type |
|
|
|
|
|
Adenocarcinoma | 17 | 6 | 4 | 5 |
|
Squamous cell carcinoma | 10 | 1 | 8 | 3 |
| Small
cell lung cancer | 11 | 2 | 3 | 7 |
|
Adenosquamous carcinoma | 5 | 1 | 3 | 1 |
| Not
determined | 14 | 7 | 3 | 3 |
Administration of CIK cells
The proliferation of PBMCs following CIK induction
varied between individuals. On average, the number of PBMCs
increased >100-fold following 14 days of incubation. The number
of CIK cells peaked at day 14. A minimum of 7–9×109 CIK
cells were harvested and transfused into the patients every day
since day 12 to day 14, for a total of three times. The average
total number of CIK cells was 8.2×109 cells for all
patients. The average number of courses of CIK infusion was 2.8
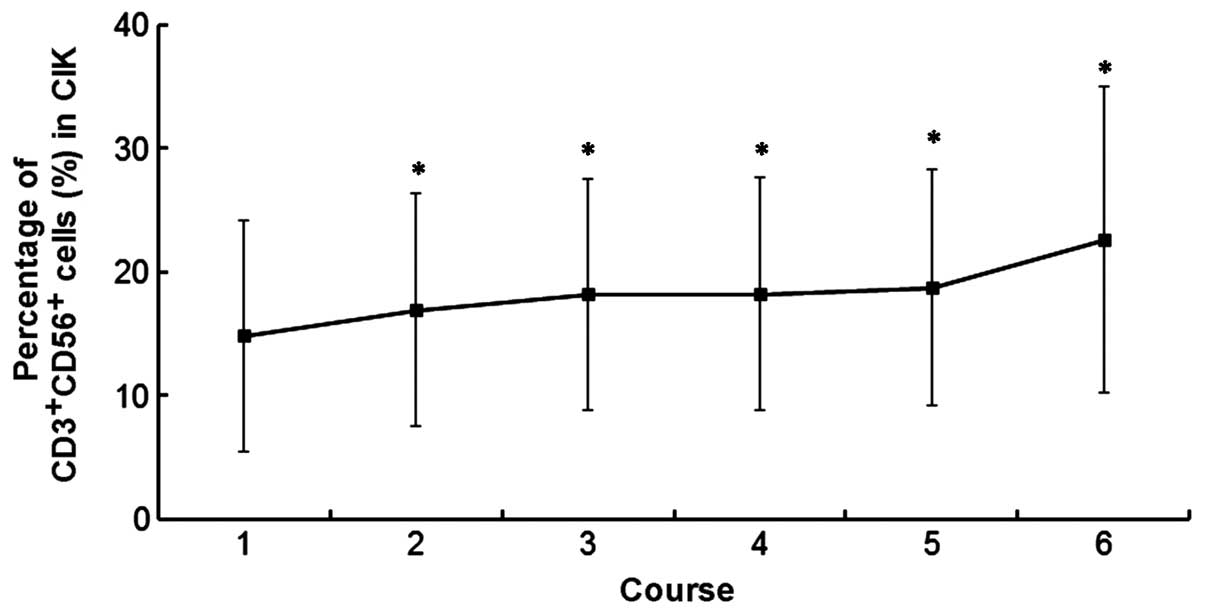
(range, 1–11 courses). The number of
CD3+CD56+ CIK cells in courses 1, 2, 3, 4, 5
and 6 was 14.8±9.3, 16.9±9.5, 18.2±9.3, 18.2±9.4, 18.7±9.6 and
22.6±12.4 cells, respectively (Fig.
1).
Immune status in patients with lung
cancer following CIK treatment
The number of immune cells present in the peripheral
blood of the patients was analyzed following each course of CIK
treatment in the three groups (CIK-group, Che-Sur group and Che-Rad
group). In the CIK-group, following 4–5 courses of treatment, the
average percentage of CD3+CD4+ and
CD3+ cells were significantly higher (P=0.043 and 0.033,
respectively) than those prior to CIK treatment (control). In the
Che-Sur group, following 3–4 courses of CIK treatment, the average
percentage of CD3+CD4+, CD3+ and
NKT cells was significantly higher than that prior to CIK treatment
(P=0.036, 0.040 and 0.047, respectively). In the Che-Rad group, the
average percentage of CD3+CD4+,
CD3+, NK and NKT cells was not altered following 5
courses of CIK treatment, compared with the values prior to the
treatment (P=0.542, 0.743, 0.533 and 0.360, respectively).
In addition, the differences in cell number across
the three treatment groups were also compared. The average
percentage of CD3+CD4+cells in the Che-Rad
group was significantly lower than that in CIK and Che-Sur groups
in each course (P=0.005, 0.008, 0.038 and 0.002, respectively),
whereas the average percentage of CD3+ cells did not
differ between the three groups (P=0.051, 0.173, 0.206 and 0.168,
respectively). The average percentage of NK cells in the Che-Rad
group was significantly higher than that observed in the CIK and
Che-Sur groups following the first course of CIK treatment
(P=0.027). The average percentage of NKT cells in CIK and Che-Rad
groups was significantly higher than that observed in the Che-Sur
group prior to the first course of CIK treatment (P=0.019). Data
are presented in Table II.
 | Table II.Number of immune cells in the
peripheral blood of patients following each course of CIK
treatment. |
Table II.
Number of immune cells in the
peripheral blood of patients following each course of CIK
treatment.
|
|
| Following CIK
treatment (course, n) |
|---|
|
|
|
|
|---|
| Cells | Prior to CIK
treatment | 1 | 2 | 3 | 4 |
|---|
|
CD3+CD4+ (%) |
|
|
|
|
|
| CIK
group | 34.4±9.3 | 28.7±8.8 | 31.7±10.7 | 36.7±4.9 |
41.3±9.3a |
| Che-Sur
group | 38.8±8.8 | 41.7±10.5 |
48.2±8.7a | 40.5±5.9 | 44.5±2.9 |
| Che-Rad
group | 26.4±7.6 | 26.1±7.4 | 26.0±9.4 | 24.6±5.9 | 24.5±5.0 |
|
P-valueb | 0.002 | 0.005 | 0.008 | 0.038 | 0.002 |
| CD3+
(%) |
|
|
|
|
|
| CIK
group | 62.7±9.3 | 55.4±9.0 | 63.0±13.1 |
77.4±7.3a |
79.8±6.6a |
| Che-Sur
group | 69.9±6.9 | 70.8±8.6 |
75.9±4.7a | 69.5±9.1 |
78.5±1.8a |
| Che-Rad
group | 68.0±11.8 | 65.4±12.6 | 68.3±15.3 | 64.6±12.2 | 66.2±12.7 |
|
P-valueb | 0.187 | 0.051 | 0.173 | 0.206 | 0.168 |
|
CD3−CD16+56+
(NK) (%) |
|
|
|
|
|
| CIK
group | 18.8±9.1 | 18.0±7.0 | 22.5±5.7 | 24.1±4.9 | 19.4±5.9 |
| Che-Sur
group | 19.7±9.4 | 19.4±9.2 | 18.1±10.1 | 18.3±12.5 | 19.5±14.0 |
| Che-Rad
group | 23.7±12.9 | 27.1±9.8 | 22.3±12.1 | 26.4±12.3 | 20.3±7.5 |
|
P-valueb | 0.514 | 0.027 | 0.643 | 0.381 | 0.993 |
|
CD3+CD16+56+
(NKT) (%) |
|
|
|
|
|
| CIK
group | 11.5±5.4 | 12.0±7.2 | 10.6±5.6 | 14.3±3.9 | 12.4±6.4 |
| Che-Sur
group | 4.9±2.4 | 6.6±4.7 | 5.6±3.2 | 6.3±3.0 |
8.4±4.0a |
| Che-Rad
group | 10.9±9.2 | 10.9±11.9 | 11.2±9.7 | 10.3±12.0 | 7.7±4.4 |
|
P-valueb | 0.019 | 0.385 | 0.222 | 0.513 | 0.437 |
The serum levels of INF-γ and IL-10 were also
detected in the three groups of patients (Table III). In the CIK and Che-Sur groups,
following 4–5 courses of CIK treatment, the levels of INF-γ were
significantly higher than those prior to the treatment (control)
(P=0.002 and 0.024, respectively). By contrast, in the Che-Sur
group the levels of IL-10 decreased following CIK treatment
(P=0.019).
 | Table III.Levels of INF-γ and IL-10 in serum of
patients with lung cancer prior and subsequent to CIK
treatment. |
Table III.
Levels of INF-γ and IL-10 in serum of
patients with lung cancer prior and subsequent to CIK
treatment.
|
| CIK | Che-Sur | Che-Rad |
|---|
|
|
|
|
|
|---|
|
| | | | | | |
| Group | Prior to CIK
treatment | Following 5
courses | Prior to CIK
treatment | Following 5
courses | Prior to CIK
treatment | Following 5
courses |
|---|
| IFN-γ (pg/ml) | 15.6±5.1 |
27.7±5.5a | 12.5±2.6 |
22.4±12.1a | 22.4±6.6 | 27.9±6.5 |
| IL-10 (pg/ml) | 20.4±5.7 | 16.2±1.3 | 29.1±9.1 |
16.0±3.2a | 49.8±13.5 | 46.3±10.3 |
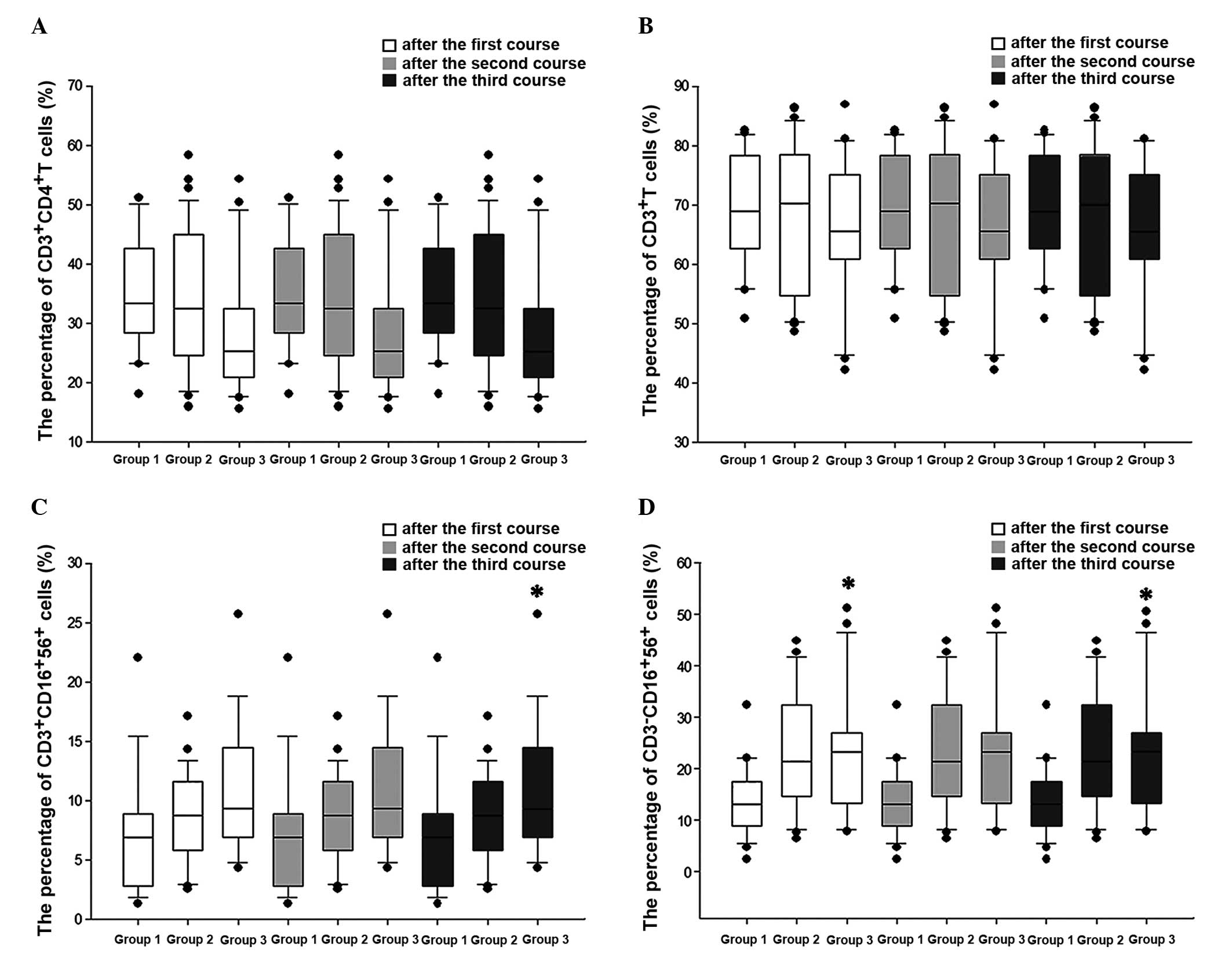
Effects of the percentage of
CD3+CD56+ CIK cells on the regulation of the immune
function in patients with lung cancer
The number of CD3+CD56+ CIK
cells that each patient received was different. Thus, to analyze
whether this was a factor that influenced the different immune
response observed across the patients, the differences in the
number of CD3+CD4+, CD3+, NK and
NKT cells that were present in the peripheral blood of the patients
the day prior to the following course of CIK treatment were
analyzed and compared across the three groups (Low-CIK, Mid-CIK and
High-CIK group). Due to limitations in the number of samples,
changes in the numbers of these cells were only analyzed following
the first three courses of CIK treatment. The results revealed that
the number of CD3+CD4+ and
CD3+cells did not vary across the three groups following
each course of treatment (Fig. 2). NK
cells increased with the increase in the percentage of
CD3+CD56+ cells following the first and third
courses, although there was no significant increase following the
second course (Fig. 2). NKT cells
exhibited a tendency to increase with the increase in the
percentage of CD3+CD56+ cells following the
second course of CIK treatment, although the increase was not
significant. Following the third course of treatment, the number of
NKT cells increased with the increase in the percentage of
CD3+CD56+ cells (Fig. 2).
Discussion
The incidence and mortality rates of lung cancer are
reported to be the highest ones among all the malignancies
worldwide (1). Patients with cancer
usually have a poor prognosis, despite being subjected to surgery,
chemotherapy or radiotherapy (19).
Chemotherapy or radiotherapy may be useful to a certain extent;
however, due to the high toxicity and other associated factors, the
implementation and benefit of these treatments are largely hampered
(20,21).
Besides surgery, chemotherapy and radiotherapy,
adoptive immunotherapy is a promising novel approach for the
treatment of solid tumors (5).
According to previous studies, cancer patients exhibit certain
dysfunctions in cellular immunity, including innate and adaptive
immune responses (22). Due to the
low immune function displayed by patients with lung cancer,
effective immune response cannot be achieved, and this is one of
the reasons why malignant tumors are incurable (23,24).
Autoimmune disorders in patients lung cancer are demonstrated by
reduced levels of CD4+ and
CD3+CD56+ cells, and increased levels of
CD8+cells (25,26). The response of the human immune system
against tumors mainly depends on cellular immunity (27). CD3+ T cells are mature T
cells, while CD4+ T cells are considered to have a
predefined role as Th cells (28). It
has been demonstrated that cytotoxicity against tumors is dependent
on an appropriate interaction between CD4+and
CD8+ T cells (29).
However, the ratios of T lymphocyte subsets in peripheral blood are
usually disordered in cancer patients (30,31). The
proportion of these cells in the human body must remain constant in
order to maintain its optimal state of balance and participate in
cellular immune surveillance (32).
NK and NKT cells are effector cells, which are involved in the
immune response against tumors during the early stages of tumor
development (33). These cells do not
require any specific antibodies or presensitized lymphocytes to
exert their function, and may be rapidly activated to suppress and
destroy a variety of tumor cells (34). In addition, NK and NKT cells are more
lethal upon being activated by lymphokines (34).
It has been reported that CIK cells regulate and
enhance cellular immune functions in patients with cancer (35,36). Thus,
the clinical translation of adoptive immunotherapy with CIK cells
as a potential treatment for patients with solid tumors has
recently gained attention, and is currently under investigation in
various clinical trials (19,37). At present, immunotherapy has become
the fourth treatment strategy for malignant tumors (38,39). The
use of CIK cells as adoptive immunotherapy against tumors has been
reported in several studies (36,39). CIK
cells are heterogeneous cell populations of T lymphocytes that
express CD3 and CD56, as well as NK group 2, member D, the
activating receptor of NK cells (9,40). CIK
cells possess MHC-unrestricted tumor-killing activity, but do not
display cytotoxicity toward normal cells (9,40). In
addition, CIK cells are considered to be antitumor effector cells,
which easily expand in vitro and exhibit stronger antitumor
activity compared with other antitumor effector cells (41,42).
Furthermore, CIK cells are able to regulate cellular immune
functions in patients with cancer (35,36). The
application of CIK cells as adoptive immunotherapy is important in
the treatment of cancer, since several clinical studies have
confirmed the safety of CIK therapy for patients (12,13).
Numerous clinical studies have been recently performed, whereby
adjuvant infusions of CIK cells following surgical resection
demonstrated a significant increase in survival time (43,44). In
addition, CIK infusions were also able to reduce the viral load of
hepatitis B virus (45).
In previous studies, the serum levels of tumor
markers were significantly reduced following CIK cell infusion, and
the short-term curative effect and quality of life improved in
patients treated with CIK cells (41,46). In
the present study, the average percentage of
CD3+CD4+, CD3+ and NKT cells, as
well as the levels of IFN-γ following several treatment courses,
were significantly higher than the values observed prior to CIK
treatment in the CIK and Che-Sur groups. Shi et al (47) observed that the percentage of
CD3+ and CD4+ cells, and the ratio of
CD4+/CD8+ cells were significantly higher
following the first course of CIK therapy, compared with the values
prior to treatment. However, in the present study, the percentages
of CD3+ and CD3+CD4+ cells were
observed to be significantly higher following several courses of
CIK therapy, which may be due to the difference in the detection
time point (1 month in the current study vs. 2 weeks in the study
by Shi et al subsequent to each course). Jin et al
(48) reported that only one
treatment course of CIK was unable to improve the immune function
in patients with lung cancer. Several researches have reported that
CD3+, CD3+CD4+ and other immune
cells peaked at 4 weeks following CIK treatment, while circulating
CIK cells persisted for ≤2 weeks following infusion (11). Those studies suggested that several
courses of CIK treatment would be required to achieve a stable
effect (47,48). The present findings indicate that CIK
cell therapy aids to improve the immune status of cancer patients
following several courses. Therefore, to gain therapeutic efficacy,
multiple courses of therapy should be administered to the patients.
Noticeably, in the present study, no changes were observed in the
Che-Rad group during any of the courses. Thus, the treatments
administered to the patients prior to CIK cell therapy seemed to
affect the outcome, since more courses were required to achieve
effective antitumor immune responses in the Che-Rad group, compared
with patients who had not been exposed to radiotherapy prior to CIK
treatment. It is interesting to note that the number of
CD3+CD4+ cells in the Che-Rad group was
already lower than that in the CIK and Che-Sur groups prior to CIK
treatment, whereas the number of NKT cells was higher in the
Che-Rad group prior to CIK treatment, compared with the Che-Sur
group. The reason for this discrepancy is unclear; however it may
be caused by the treatment administered prior to CIK treatment,
since the various treatments administered to the patients have
different affects on immune cells.
At present, there is controversy regarding the
number of cells required to be infused in CIK cell therapy, since
the antitumor activity of CIK cells is mainly associated with the
CD3+CD56+ fraction, rather than the
CD8+ fraction, which constitutes the highest percentage
of CIK cells (6). A previous study
suggested that the improvements in immune function exerted by CIK
cells were affected by the number of CIK cells (48). In the present study, the percentage of
CD3+CD56+ cells was ~18.9%. Patients were
divided into three groups according to the percentage of
CD3+CD56+ cells that received in each course.
It was observed that the number of CD3+CD4+
and CD3+ T lymphocytes did not change in any of the
three groups following each course of CIK treatment. These
demonstrated that the number of CIK cells did not affect
lymphocyte-associated functions. By contrast, the number of NK and
NKT cells increased with the increase in the percentage of
CD3+CD56+ cells. Several studies have
observed a markedly increased ratio of
CD3+CD56+ and NK cells following CIK
treatment, compared with the levels observed prior to CIK
treatment. In the present study, the number of NK and NKT cells
increased following CIK treatment. When the number of
CD3+CD56+ cells present in the CIK cells
infused back into the patients increased, the number of NK and NKT
cells increased in the peripheral blood of the patients, which
enabled the patients to effectively kill tumor cells.
The present study was a hospital-based study, which
was a limitation, since only a small number of patients were
enrolled in each group. In addition, only changes in the number of
immune cells were analyzed in different cases. Therefore, future
studies are required to confirm the present results and to evaluate
the functions of these cells, such as cytokine secretion and
cytotoxic activity.
In conclusion, the present study demonstrated that
CIK cells enhanced the immune status of patients with lung cancer,
although several courses of CIK treatment were required to observe
the aforementioned effect. Patients who had not received any
treatment and those who had been subjected to chemotherapy and
surgical resection prior to CIK treatment, but not those who had
received chemotherapy and radiotherapy prior to CIK treatment, were
the most benefited ones from the CIK treatment, since additional
courses of CIK therapy were required to achieve effective antitumor
immune responses in these patients. The increase in the number of
CD3+CD56+ cells among the infused CIK cells
notably enhanced the number of NK and NKT cells available to
effectively exert an antitumor effect. The present results provide
experimental evidence for the clinicians to select CIK therapy as a
treatment for patients with lung cancer.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahn SH, Han MS, Yoon JH, Jeon SY, Kim CH,
Yoo HJ and Lee JC: Treatment of stage I non-small cell lung cancer
with CyberKnife, image-guided robotic stereotactic radiosurgery.
Oncol Rep. 21:693–696. 2009.PubMed/NCBI
|
|
3
|
Park S, Kim IR, Baek KK, Lee SJ, Chang WJ,
Maeng CH, Hong JY, Choi MK, Kim YS, Sun JM, et al: Prospective
analysis of quality of life in elderly patients treated with
adjuvant chemotherapy for non-small-cell lung cancer. Ann Oncol.
24:1630–1639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rice SJ, Miller B, Wagman M, Jamorabo DS,
Liu X and Belani CP: Clinical approaches to immunotherapy in
non-small cell lung cancer: Current and future perspectives. Curr
Mol Pharmacol. Jul 16–2015.(Epub ahead of print). PubMed/NCBI
|
|
5
|
Sangiolo D, Martinuzzi E, Todorovic M,
Vitaggio K, Vallario A, Jordaney N, Carnevale-Schianca F, Capaldi
A, Geuna M, Casorzo L, et al: Alloreactivity and anti-tumor
activity segregate within two distinct subsets of cytokine-induced
killer (CIK) cells: Implications for their infusion across major
HLA barriers. Int Immunol. 20:841–848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuçi S, Rettinger E, Voss B, Weber G,
Stais M, Kreyenberg H, Willasch A, Kuçi Z, Koscielniak E, Klöss S,
et al: Efficient lysis of rhabdomyosarcoma cells by
cytokine-induced killer cells: Implications for adoptive
immunotherapy after allogeneic stem cell transplantation.
Haematologica. 95:1579–1586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hontscha C, Borck Y, Zhou H, Messmer D and
Schmidt-Wolf IG: Clinical trials on CIK cells: First report of the
international registry on CIK cells (IRCC). J Cancer Res Clin
Oncol. 137:305–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume
KG and Weissman IL: Use of a SCID mouse/human lymphoma model to
evaluate cytokine-induced killer cells with potent antitumor cell
activity. J Exp Med. 174:139–149. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thanendrarajan S, Nowak M, Abken H and
Schmidt-Wolf IG: Combining cytokine-induced killer cells with
vaccination in cancer immunotherapy: More than one plus one? Leuk
Res. 35:1136–1142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmidt-Wolf IG, Finke S, Trojaneck B,
Denkena A, Lefterova P, Schwella N, Heuft HG, Prange G, Korte M,
Takeya M, et al: Phase I clinical study applying autologous
immunological effector cells transfected with the interleukin-2
gene in patients with metastatic renal cancer, colorectal cancer
and lymphoma. Br J Cancer. 81:1009–1016. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sangiolo D: Cytokine induced killer cells
as promising immunotherapy for solid tumors. J Cancer. 2:363–368.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu Y, Zhang H, Li Y, Bai J, Liu L, Liu Y,
Qu Y and Qu X: Efficacy of postoperative adjuvant transfusion of
cytokine-induced killer cells combined with chemotherapy in
patients with colorectal cancer. Cancer Immunol Immunother.
62:1629–1635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang ZM, Li W, Li S, Gao F, Zhou QM, Wu
FM, He N, Pan CC, Xia JC, Wu PH and Zhao M: Cytokine-induced killer
cells in combination with transcatheter arterial chemoembolization
and radiofrequency ablation for hepatocellular carcinoma patients.
J Immunother. 36:287–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang J, Wu C and Lu B: Cytokine-induced
killer cells promote antitumor immunity. J Transl Med. 11:832013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Zhu L, Zhang Q, He X, Yin Y, Gu
Y, Guo R, Lu K, Liu L, Liu P and Shu Y: Effects of cytokine-induced
killer cell treatment in colorectal cancer patients: A
retrospective study. Biomed Pharmacother. 68:715–720. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang XP, Xu M, Gao HF, Zhao JF and Xu KC:
Intraperitoneal perfusion of cytokine-induced killer cells with
local hyperthermia for advanced hepatocellular carcinoma. World J
Gastroenterol. 19:2956–2962. 2013.PubMed/NCBI
|
|
17
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: TNM Classification of Malignant Tumours (7th). Wiley-Blackwell.
Hoboken, NY: 2009.
|
|
18
|
Schmidt-Wolf GD, Negrin RS and
Schmidt-Wolf IG: Activated T cells and cytokine-induced
CD3+ CD56+ killer cells. Ann Hematol.
74:51–56. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Zhu L, Wei J, Liu L, Yin Y, Gu Y
and Shu Y: The effects of cytokine-induced killer cells for the
treatment of patients with solid tumors: A clinical retrospective
study. J Cancer Res Clin Oncol. 138:1057–1062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baas P, Belderbos JS and Van den Heuvel M:
Chemoradiation therapy in nonsmall cell lung cancer. Curr Opin
Oncol. 23:140–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsu HS, Huang PI, Chang YL, Tzao C, Chen
YW, Shih HC, Hung SC, Chen YC, Tseng LM and Chiou SH: Cucurbitacin
I inhibits tumorigenic ability and enhances radiochemosensitivity
in nonsmall cell lung cancer-derived CD133-positive cells. Cancer.
117:2970–2985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun K, Wang L and Zhang Y: Dendritic cell
as therapeutic vaccines against tumors and its role in therapy for
hepatocellular carcinoma. Cell Mol Immunol. 3:197–203.
2006.PubMed/NCBI
|
|
23
|
Méndez R, Ruiz-Cabello F, Rodríguez T, Del
Campo A, Paschen A, Schadendorf D and Garrido F: Identification of
different tumor escape mechanisms in several metastases from a
melanoma patient undergoing immunotherapy. Cancer Immunol
Immunother. 56:88–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wongkajornsilp A, Somchitprasert T,
Butraporn R, Wamanuttajinda V, Kasetsinsombat K, Huabprasert S,
Maneechotesuwan K and Hongeng S: Human cytokine-induced killer
cells specifically infiltrated and retarded the growth of the
inoculated human cholangiocarcinoma cells in SCID mice. Cancer
Invest. 27:140–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee WC, Wu TJ, Chou HS, Yu MC, Hsu PY, Hsu
HY and Wang CC: The impact of CD4+ CD25+ T
cells in the tumor microenvironment of hepatocellular carcinoma.
Surgery. 151:213–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW
and Zheng SS: Intratumoral regulatory T cells alone or in
combination with cytotoxic T cells predict prognosis of
hepatocellular carcinoma after resection. Med Oncol. 29:1817–1826.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perdicchio M, Cornelissen LA,
Streng-Ouwehand I, Engels S, Verstege MI, Boon L, Geerts D, van
Kooyk Y and Unger WW: Tumor sialylation impedes T cell mediated
anti-tumor responses while promoting tumor associated-regulatory T
cells. Oncotarget. Jan 5–2016.(Epub ahead of print). PubMed/NCBI
|
|
28
|
Attallah AM, Tabll AA, El-Sadany M,
Ibrahim TA and El-Dosoky I: Dysregulation of blood lymphocyte
subsets and natural killer cells in schistosomal liver cirrhosis
and hepatocellular carcinoma. Clin Exp Med. 3:181–185. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Motz GT and Coukos G: Deciphering and
reversing tumor immune suppression immunity. Immunity. 39:61–73.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kiessling R, Wasserman K, Horiguchi S, et
al: Tumor-induced immune dysfunction. Cancer Immunol Immunother.
48:353–362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rayman P, Uzzo RG, Kolenko V, Bloom T,
Cathcart MK, Molto L, Novick AC, Bukowski RM, Hamilton T and Finke
JH: Tumor-induced dysfunction in interleukin-2 production and
interleukin-2 receptor signaling: A mechanism of immune escape.
Cancer J Sci Am. 6(Suppl 1): S81–S87. 2000.PubMed/NCBI
|
|
32
|
Shepherd FA, Douillard JY and Blumenschein
GR Jr: Immunotherapy for non-small cell lung cancer: Novel
approaches to improve patient outcome. J Thorac Oncol. 6:1763–1773.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Subleski JJ, Wiltrout RH and Weiss JM:
Application of tissue-specific NK and NKT cell activity for tumor
immunotherapy. J Autoimmun. 33:275–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuss I, Hathaway B, Ferris RL, Gooding W
and Whiteside TL: Decreased absolute counts of T lymphocyte subsets
and their relation to disease in squamous cell carcinoma of the
head and neck. Clin Cancer Res. 10:3755–3762. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmidt-Wolf IG, Lefterova P, Mehta BA,
Fernandez LP, Huhn D, Blume KG, Weissman IL and Negrin RS:
Phenotypic characterization and identification of effector cells
involved in tumor cell recognition of cytokine-induced killer
cells. Exp Hematol. 21:1673–1679. 1993.PubMed/NCBI
|
|
36
|
Joshi PS, Liu JQ, Wang Y, Chang X,
Richards J, Assarsson E, Shi FD, Ljunggren HG and Bai XF:
Cytokine-induced killer T cells kill immature dendritic cells by
TCR-independent and perforin-dependent mechanisms. J Leukoc Biol.
80:1345–1353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen Y, Lin WS, Zhu WF, Lin J, Zhou ZF,
Huang CZ, Chen G, Shi Y, Guo ZQ and Ye YB: Tumor MICA status
predicts the efficacy of immunotherapy with cytokine-induced killer
cells for patients with gastric cancer. Immunol Res. 64:251–259.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blattman JN and Greenberg PD: Cancer
immunotherapy: A treatment for the masses. Science. 305:200–205.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu L, Zhang W, Qi X, Li H, Yu J, Wei S,
Hao X and Ren X: Randomized study of autologous cytokine-induced
killer cell immunotherapy in metastatic renal carcinoma. Clin
Cancer Res. 18:1751–1759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Verneris MR, Karimi M, Baker J, Jayaswal A
and Negrin RS: Role of NKG2D signaling in the cytotoxicity of
activated and expanded CD8+ T cells. Blood.
103:3065–3072. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schwaab T, Schwarzer A, Wolf B, Crocenzi
TS, Seigne JD, Crosby NA, Cole BF, Fisher JL, Uhlenhake JC,
Mellinger D, et al: Clinical and immunologic effects of intranodal
autologous tumor lysate-dendritic cell vaccine with Aldesleukin
(Interleukin 2) and IFN-{alpha}2a therapy in metastatic renal cell
carcinoma patients. Clin Cancer Res. 15:4986–4992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li R, Wang C, Liu L, Du C, Cao S, Yu J,
Wang SE, Hao X, Ren X and Li H: Autologous cytokine-induced killer
cell immunotherapy in lung cancer: A phase II clinical study.
Cancer Immunol Immunother. 61:2125–2133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang JT, Shen YP, Wu CP, Zhu YB, Wei WX,
Chen LJ, Zheng X, Sun J, Lu BF and Zhang XG: Increasing the
frequency of CIK cells adoptive immunotherapy may decrease risk of
death in gastric cancer patients. World J Gastroenterol.
16:6155–6162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Niu Q, Wang W, Li Y, Qin S, Wang Y, Wan G,
Guan J and Zhu W: Cord blood-derived cytokine-induced killer cells
biotherapy combined with second-line chemotherapy in the treatment
of advanced solid malignancies. Int Immunopharmacol. 11:449–456.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shi M, Zhang B, Tang ZR, Lei ZY, Wang HF,
Feng YY, Fan ZP, Xu DP and Wang FS: Autologous cytokine-induced
killer cell therapy in clinical trial phase I is safe in patients
with primary hepatocellular carcinoma. World J Gastroenterol.
10:1146–1151. 2004.PubMed/NCBI
|
|
46
|
Jiang J, Xu N, Wu C, Deng H, Lu M, Li M,
Xu B, Wu J, Wang R, Xu J and Nilsson-Ehle P: Treatment of advanced
gastric cancer by chemotherapy combined with autologous
cytokine-induced killer cells. Anticancer Res. 26:2237–2242.
2006.PubMed/NCBI
|
|
47
|
Shi L, Zhou Q, Wu J, Ji M, Li G, Jiang J
and Wu C: Efficacy of adjuvant immunotherapy with cytokine-induced
killer cells in patients with locally advanced gastric cancer.
Cancer Immunol Immunother. 61:2251–2259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jin CG, Chen XQ, Li J, Wu ZP, Liu X and
Wang XC: Moderating effects and maintenance of lung cancer cellular
immune functions by CIK cell therapy. Asian Pac J Cancer Prev.
14:3587–3592. 2013. View Article : Google Scholar : PubMed/NCBI
|