Introduction
Lung cancer remains the leading cause of
cancer-related mortality globally, with non-small-cell lung cancer
(NSCLC) accounting for ~85% of total lung malignancies (1). At present, chemotherapy plays a critical
role in the treatment of lung cancer (2). While the prevailing chemotherapy
regimens can significantly suppress symptoms and improve quality of
life for lung cancer patients, there is little effect on prolonging
their overall survival (3,4). Therefore, improved therapeutic options
for lung cancer are urgently needed.
There is increasing evidence that phytochemicals
from Chinese medicinal herbs may show promise as alternative
therapeutic resources for treating malignancies (5–7).
Sesquiterpene lactones are predominantly isolated from members of
the Compositae and Magnoliaceae plant families, and
have attracted widespread attention due to their anti-tumor and
anti-inflammatory activity, (8,9).
Costunolide, a sesquiterpene lactone, which is isolated from
Chinese herb Saussurea lappa, is a popular herbal remedy,
with anti-ulcer (10),
anti-inflammatory (11), anti-fungal
(12) and anti-viral properties
(13). Previous research has reported
that costunolide can inhibit the expression of inducible nitric
oxide synthase and the DNA-binding activity of NF-κB (14,15).
Furthermore, costunolide potentiates 1,25-(OH)2D3-induced
differentiation in HL-60 promyelocytic leukemia cells by modulating
NF-κB activation (16,17). Previous studies have demonstrated that
costunolide has anti-tumor potential by inhibiting proliferation,
inducing apoptosis and reducing invasion and metastasis in a number
of tumor cells including intestinal neoplasia and melanoma,
leukemia and hepatocellular carcinoma cells, and cervical,
prostate, bladder, colon and breast cancer cells (6,18–25). The effects of costunolide on human
lung squamous carcinoma cells are still unknown. The present study
aimed to elucidate the effects of costunolide on the proliferation
of SK-MES-1 cells, and to explore the possible mechanism of
costunolide-induced apoptosis in this lung cancer cell line, in
order to assess its potential as a future therapeutic option for
lung cancer patients.
Materials and methods
Materials
The SK-MES-1 human lung squamous carcinoma cell line
was a gift from Dr. T.H. Ma (Jilin University Bethune Second
Hospital, Changchun, China). Costunolide was purchased from the
Tongtian (Shanghai, China), and was dissolved in dimethyl sulfoxide
(DMSO), purchased from Shenggong (Shanghai, China) to make a stock
solution. Fetal bovine serum (FBS) was purchased (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
Hoechst 33342, Dulbecco's Modified Eagle's Medium (DMEM) and
rhodamine-123 mitochondrial specific fluorescent dye were purchased
from Sigma-Aldrich, St. Louis, MO, USA. The cell cycle analysis and
reactive oxygen species assay kits were purchased from Beyotime
Institute of Biotechnology (Shanghai, China). BCA protein assay kit
and Annexin V-FITC apoptosis detection kit were purchased from
Nanjing KeyGen Biotech Co., Ltd., (Nanjing, China). Polyclonal
anti-mouse antibodies raised against β-actin (catalog no., AA128;
dilution, 1:2,000), p21 (catalog no., AP021; dilution, 1:500), p27
(catalog no., AP027; dilution, 1:500) and phospho-Rb (catalog no.,
AR092); dilution, 1:1,000), as well as horseradish peroxidase
(HRP)-conjugated secondary antibodies (goat anti-mouse IgG; catalog
no., A0216; dilution, 1:1,000) were purchased from Beyotime
Institute of Biotechnology. Polyclonal antibodies raised against
Bax (catalog no., 2772S; dilution, 1:1,000), Bcl-2 (catalog no.,
2876S; dilution, 1:1,000), pro-caspase-3 (catalog no., 9662P;
dilution, 1:1,000), p53 (catalog no., 9282S; dilution, 1:1,000),
poly-ADP-ribose polymerase (PARP; catalog no., 9542S; dilution,
1:1,000) and horseradish peroxidase-conjugated secondary antibodies
(goat anti-rabbit IgG; catalog no., 7074P2; dilution, 1:2,000) were
purchased from Cell Signaling Technology, Inc., Shanghai, China.
Western Blotting detection kit was purchased from EMD Millipore
(Billerica, MA, USA).
Cell culture
Human lung squamous carcinoma SK-MES-1 cells were
cultured in DMEM nutrients mixture supplemented with 10% FBS at
37°C in a humidified atmosphere with 5% CO2.Cells were
cultured in 10 cm culture dishes and allowed to reach ~70%
confluence before being used in experiments.
Cell growth inhibition assay
The cytotoxic effects of the costunolide on the cell
were determined using the MTT assay. Briefly, SK-MES-1 cells were
seeded at a density of 1×104 cells/well in 96-well plates and
incubated overnight. Cells were treated with drug monomer with
different concentrations. Each of the total incubation volume well
was 100 µl. Following 24 h of incubation, 10 µl MTT was added to
each well and incubated for an additional 4 h. The supernatants
were then removed and 150 µl DMSO was added to dissolve the
formazan crystals. In this assay, viable cell number is directly
proportional to the production of formazan. Absorbance was then
read in a Varioskan™ Flash Multimode Reader (Thermo Scientific) at
a wavelength of 570 nm. The assay was repeated three times. The
half maximal inhibitory concentration (IC50) values were
calculated using GraphPad Prism 5 (GraphPad Software, Inc., La
Jolla, CA, USA)
The percentage of inhibition, the inhibitory ratio
(IR), was calculated in the present study using the following
formula: IR (%) = (A570 [control] - A570
[sample]) / A570 [control] x100%.
Flow cytometric cell cycle
analysis
Cell cycle analysis was detected by flow cytometry
using a propidium iodide (PI) cell cycle detection kit (catalog
no., C1052; Beyotime Institute of Biotechnology). Briefly, SK-MES-1
cells were seeded into 6-well plates and incubated overnight.
Costunolide at concentrations of 0, 40 and 80 µM was added to the
wells and incubated for a further 24 h. Cells were then harvested
and fixed in 500 µl 70% ice-cold ethanol at 4°C for 2 h. Then,
samples were washed with phosphate-buffered saline (PBS) and
incubated with RNase A and PI staining solution, according to the
manufacturer's instructions.
Nuclei fragmentation analysis by
Hoechst 33342 staining
SK-MES-1 cells were treated with 0, 40 and 80 µM
costunolide for 24 h. The cells were fixed with 4% paraformaldehyde
for 30 min at room temperature. After washing with PBS, the cells
were stained with Hoechst 33342 (10 µg/ml) at 37°C for 20 min in
the dark. Finally, the cells were washed and resuspended in PBS for
the observation of nuclear morphology using an Olympus 1×71
fluorescence microscope (Olympus Corp., Tokyo, Japan).
Apoptosis analysis by flow cytometry
using annexin-PI staining
The apoptosis of SK-MES-1 cells was assessed by flow
cytometry using annexin V-FITC/PI staining. Briefly, the SK-MES-1
cells were seeded into 6-well plates and incubated overnight.
Costunolide at concentrations of 0, 40 and 80 µM was added to the
cells and incubated for 24 h. The cells were then collected,
washed, and resuspended in PBS. The apoptotic cell death rate was
examined by Annexin V-FITC and PI double staining using the Annexin
V-FITC apoptosis detection kit (catalog no., KGA105; Nanjing KeyGen
Biotech Co., Ltd.), according to the manufacturer's instructions.
Following the Annexin V and PI staining, the cells were subjected
to flow cytometric analysis and data were analyzed using Cell Quest
software.
Flow cytometric determination of
mitochondrial membrane potential (ΔΨm)
Rhodamine 123 was used to evaluate perturbations in
mitochondrial transmembrane potential in SK-MES-1 cells by flow
cytometry. Briefly, SK-MES-1 cells were plated in 6-well dishes and
cells were treated with 0, 40 and 80 µM of costunolide for 24 h.
Cells were collected in centrifuge tube and resuspended in 500 µl
PBS, and then incubated with the rhodamine 123 (10 µM;
Sigma-Aldrich) at 37°C for 20 min. Cells were centrifuged at 1500
rpm for 5 min, the supernatant was removed, and cell pellets were
gently rinsed once with PBS, then resuspended in 200 µl PBS.
Following filtration, the suspension was analyzed by flow
cytometry.
Western blot analysis of protein
expression
To evaluate the effect of costunolide and reveal the
mechanism of apoptosis induction, western blotting was used to
measure the expression levels of apoptosis-related proteins.
Firstly, SK-MES-1 cells were treated with 0, 40 and 80 µM of
costunolide for 24 h. Both adherent and floating cells were
collected in 15 ml centrifuge tubes and washed with PBS. The cell
pellets were resuspended in RIPA lysis buffer and were then
ultrasound lysed on ice. After centrifugation for 5 min, the
supernatant was collected and the protein content of the
supernatant was determined using a bicinchoninic acid assay (BCA)
protein assay kit, and the protein samples were stored at −80°C.
Protein lysates were separated by electrophoresis on a 10% sodium
dodecyl sulfate (SDS)-polyacrylamide gel and transferred to a
polyvinylidene fluoride membrane. The membranes were then soaked in
blocking buffer (5% skimmed milk) for 1 h. To probe for the
proteins of interest, membranes were incubated overnight at 4°C
with the relevant aforementioned antibodies, followed by
appropriate HRP-conjugated secondary antibodies and enhanced
chemiluminescence reagents (catalog no., WBKLS0100; EMD Millipore).
A Gel-Pro Analyzer (Gel-Pro 32, version 4.0; Media Cybernetics,
Inc., Rockville, MD, USA) was used to extract qualitative and
quantitative information from the electrophoretic gels to document
and store the western blot data.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Comparisons were made using a one-way ANOVA, followed by
Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of costunolide on SK-MES-1 cell
proliferation
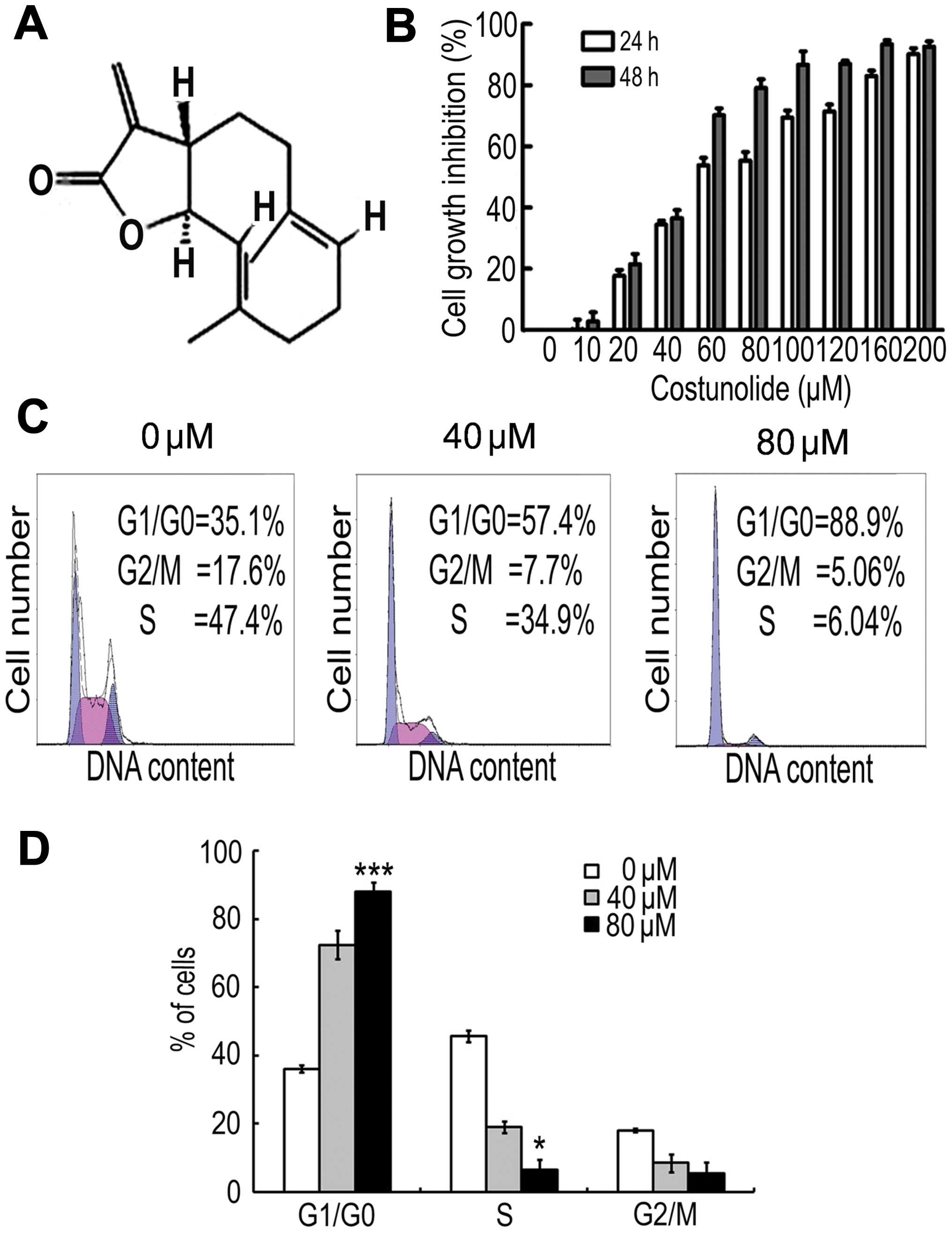
To detect the effect of costunolide (structure shown
in Fig. 1A) on the proliferation of
SK-MES-1 cells, an MTT assay was performed. The results demonstrate
that costunolide reduced cell viability in a time- and
dose-dependent manner (Fig. 1B). The
IC50 values were ~60 µM after 24 h of treatment, and ~50
µM following 48 h of costunolide treatment.
Costunolide induces cell cycle arrest
in SK-MES-1 cells
Cell cycle arrest is one of the major causes of cell
growth inhibition. In order to find out whether cell growth
inhibition was due to cell cycle arrest at a specific phase of cell
cycle, the cell cycle profile was determined by PI staining and
flow cytometry analysis. The results demonstrate that costunolide
arrested the cell cycle at G1/S phase in a dose-dependent manner
(Fig. 1C).
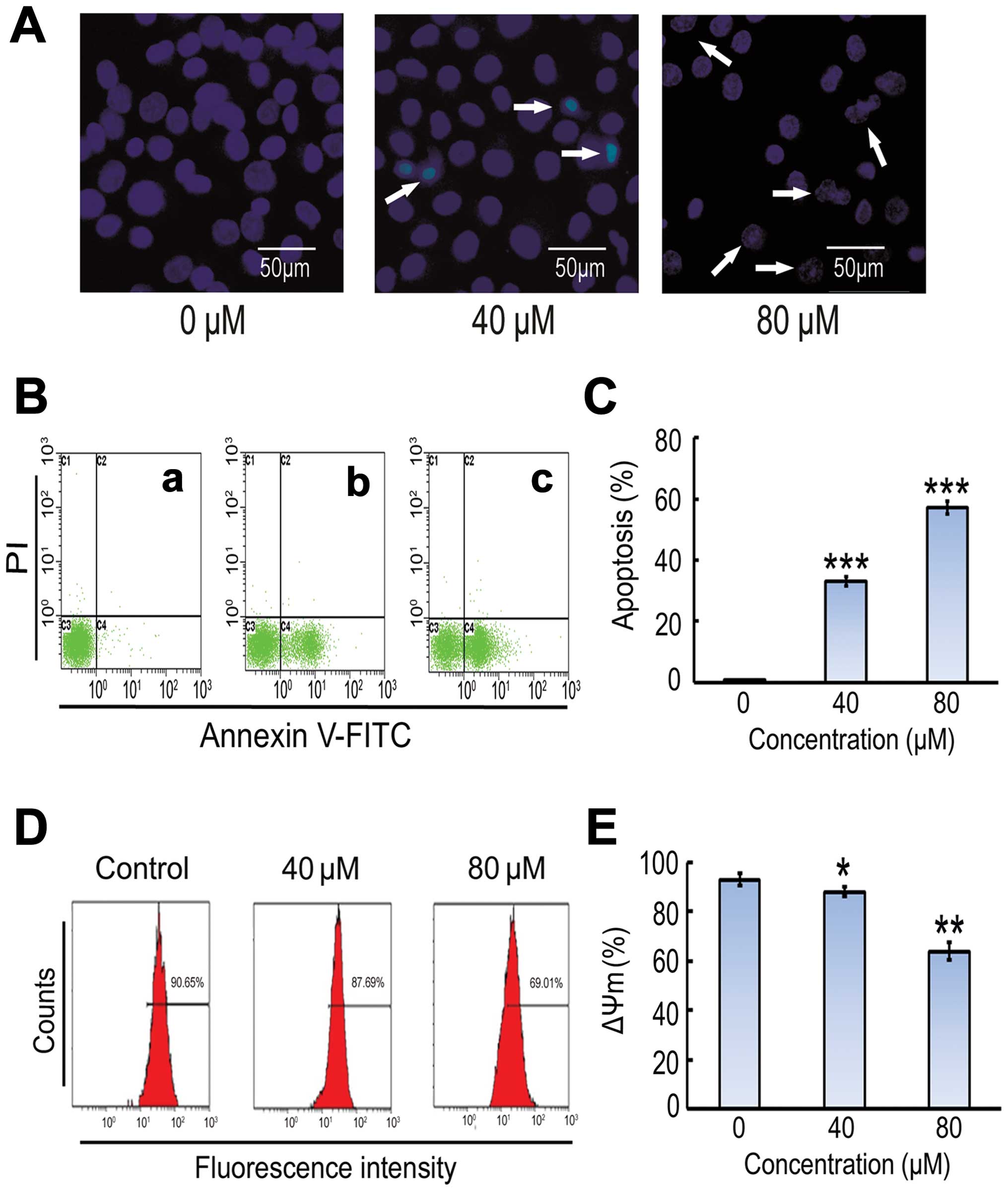
Costunolide induces apoptotic cell
death in SK-MES-1 cells
DNA fragmentation and loss of plasma membrane
asymmetry are the major features of apoptotic cell death. The
effect of costunolide on cell death was analyzed by observing the
nuclear morphological changes using Hoechst 33342 staining and
fluorescent microscopy. As shown in Fig.
2A, costunolide induced obvious nuclear morphological changes,
including nuclear shrinkage and DNA fragmentation in SK-MES-1 cells
in a dose-dependent manner. Induction of apoptosis was further
confirmed by Annexin V-FITC and PI staining (Fig. 2B).
Costunolide induces apoptosis in
SK-MES-1 cells and mitochondrial membrane potential
To further investigate costunolide-induced
inhibitory effect, SK-MES-1 cells were treated with costunolide as
described in the methods section, and the percentage of cells
undergoing apoptosis or necrosis was determined using flow
cytometric analysis with annexin V-FITC and PI staining. The
results demonstrate that costunolide treatment induced apoptosis in
a dose-dependent manner (Fig. 2B). A
significant increase was observed in early apoptosis in
experimental group compared to control group (Fig. 2B and C). The effects of costunolide on
the mitochondrial membrane potential of SK-MES-1 cells were
determined by flow cytometry using rhodamine 123 staining. The
results demonstrated that rates of depletion of mitochondrial
membrane potential were reduced following costunolide treatment
(Fig. 2D and E).
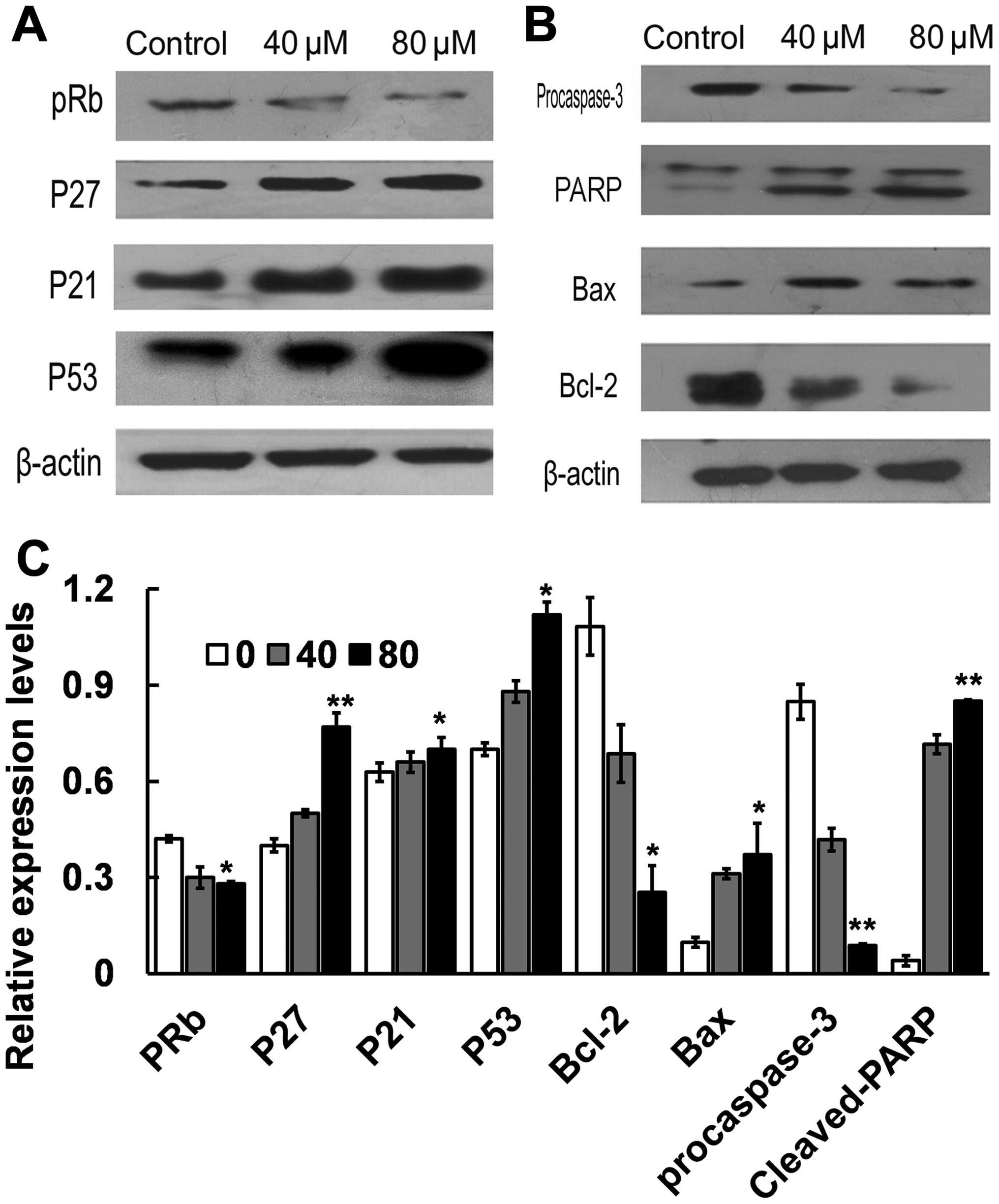
Effect of costunolide on the
expression of cell cycle regulators
To elucidate the molecular mechanism underlying G1/S
phase arrest induced by costunolide, the key proteins involved in
the regulation of G1/S transition in SK-MES-1 cells were
investigated. Cells were treated with different doses of
costunolide (40, 80 µM) for 24 h. The expression of p53, p21, p27
and pRb proteins were analyzed by western blotting. Treatment with
costunolide led to upregulation the expression of p53, p27 and p21,
decreased the expression of pRb in a dose-dependent manner in
SK-MES-1 cells (Fig. 3A, C).
Effect of costunolide on the
expression of apoptosis regulators
The results of the present study demonstrated that
costunolide increases the protein expression levels of p53, which
is related to Bcl-2 protein family. The expression of Bax and Bcl-2
was examined by western blot in SK-MES-1 cells treated with 0, 40
and 80 µM of costunolide for 24 h. The results demonstrated that
the expression of Bax was markedly increased following treatment
with costunolide, accompanied with decreased expression of Bcl-2 in
a dose-dependent manner. Next, we examined the effect of
costunolide on caspase-3 activation by western blot analysis. The
results demonstrated that expression of cleaved PARP (85 kDa
fragment) and procaspase 3 were decreased following costunolide
treatment (Figs. 3B and C).
Discussion
The aim of the present study was to identify a novel
therapeutic agent to target lung cancer. Sesquiterpene lactones are
phytochemicals derived from Chinese medicinal herbs, and have been
demonstrated previously to have anti-neoplastic and
anti-inflammatory activities (8).
Costunolide is a well-known sesquiterpene lactone and a number of
previous studies have shown that it has a broad spectrum of
cytotoxicity against human cancer cell lines of different origins
(6,18,20–22,26,27).
In the present study, the inhibitory effect of
costunolide on the proliferation of lung cancer cells SK-MES-1 was
investigated in vitro. The results of the MTT assay
demonstrated that costunolide reduced cell viability in a time- and
dose-dependent manner (Fig. 1B).
Previous studies have revealed that cell cycle arrest and apoptosis
are two mechanisms involved in the induction of cell death
(28). Studies on cell cycle
regulation have shown that cell cycle progression is tightly
controlled by various checkpoints in normal cells while alterations
in the checkpoints of cell cycle progression lead to aberrant cell
proliferation and development of cancer (29). Tumor cells frequently acquiring
defects in the checkpoints leads to unrestrained proliferation
(30). Many anti-tumor drugs induce
cell cycle arrest at a specific checkpoint and thereby induce
apoptosis (31,32). The present study identified that
costunolide-induced cell cycle arrest at G1/S phase is accompanied
by a reduction in G2/M and S phase in a dose-dependent manner by
using flow cytometric analysis. These findings are in line with
other reports (21). Furthermore, the
current study provided evidence that G1/S phase cell cycle arrest
is one of the mechanisms in the growth inhibitory effect of
costunolide in SK-MES-1 cells. In addition to cell cycle arrest,
costunolide exerts its cytotoxic effects via the induction of
apoptosis in SK-MES-1 cells. These data strongly suggested that the
cytotoxic effect of costunolide in SK-MES-1 cells via induction of
apoptosis, and in agreement with previous studies, induction of
other cancer cells including leukemia (16), prostate cancer cells (21), ovarian cancer cells (27) and bladder cancer cells (6).
P53, a tumor suppressor protein, plays a key role in
the regulation of cell cycle progression, checkpoint activation and
apoptosis (33,34). The data presented herein suggest that
costunolide treatment upregulates the expression of p53 protein and
increases the expression of p21, a downstream target of p53. The
cell cycle dependent kinase inhibitor p21, one of the Clp family
members, is located downstream of the p53 gene. Cell cycle proteins
p21 and protein kinase 2/E, lead to inhibiting the activity of the
complexes and retinoblastoma protein (Rb) phosphorylation. Rb
cannot release the E2F subunit, which participate in DNA synthesis
(35). As a result, cell cycle
arrested in G1 phase. In addition, p27 is a cyclin-dependent kinase
inhibitor which controls G1/S transition by inhibiting the activity
of a wide variety of cyclin/cyclin dependent kinase (CDK) complex
(36). In the present study,
costunolide-treated SK-MES-1 cells decreased in the expression of
p27. Our data showed that the costunolide-mediated G1/S phase cell
cycle arrest in SK-MES-1 cells was associated with the increase
expression of p27. These findings may explain in part the
mechanisms underlying G1/S phase arrest, and further studies are
required to fully elucidate these molecular mechanisms.
Many reports have shown that p53 is a tumor
suppressor protein which triggers apoptosis by inducing
mitochondrial membrane permeabilization through regulating the
expression of apoptosis mediated proteins (37,38). The
Bcl-2 protein family is a large family of apoptosis regulating
proteins that modulate the mitochondrial pathway and includes
anti-apoptotic proteins and pro-apoptotic proteins such as Bcl-2
and Bax (39). To explore the further
molecular mechanisms underpinning costunolide-induced apoptosis in
SK-MES-1 cells, the expression of Bax and Bcl-2 protein in SK-MES-1
cells of each group was examined. The results demonstrated that the
expression of Bax gradually increased and Bcl-2 decreased in
treatment groups in a dose-dependent manner. Taken together, these
data demonstrate that p53 plays a critical role in
costunolide-mediated apoptosis in SK-MES-1 cells.≥
As the expression of Bax increases, a significant
reduction in mitochondrial transmembrane potential is observed in
the cells of treatment groups. Mitochondrial permeability
transition pores are opened, which lead to the release of
cytochrome C and other pro-apoptotic molecules from intermembranous
space to cytosol, activating downstream caspases and ultimately
caspase 3 (40). Caspase 3 is a
frequently activated death protease which cleave PARP, a DNA repair
enzyme (41). The present study
demonstrated the cleavage of PARP into its 85 kDa fragment and the
decreased expression of procaspase 3. Our results clearly
demonstrate that the mitochondrial-mediated caspase activation
pathway is involved in costunolide-mediated apoptosis in
SK-MES-1cells.
While the mechanisms of the growth inhibitory effect
of costunolide in some cancer cells have previously been
demonstrated (6,19,25,27), our
study is the first time to describe this in human lung squamous
carcinoma cells. In conclusion, costunolide induced apoptosis in
SK-MES-1 cells accompany with a marked loss of G1/S phase cells.
Costunolide-induced apoptosis marked with upregulation of Bax and
p53, downregulation of Bcl-2 and caspase-3 with cleaved PARP in a
dose-dependent manner. These findings identify that costunolide may
become a potential therapeutic target for the future development of
anti-lung cancer agents.
Acknowledgements
The present study was supported by grants from the
Science and Technology Services of Jilin Province Scientific and
Technological Project (grant no. 20140521) and the Natural Science
Foundation of China (grant no. 81272472).
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saba NF and Khuri FR: Chemoprevention
strategies for patients with lung cancer in the context of
screening. Clin Lung Cancer. 7:92–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chou JY, Lai SY, Pan SL, Jow GM, Chern JW
and Guh JH: Investigation of anticancer mechanism of
thiadiazole-based compound in human non-small cell lung cancer A549
cells. Biochem Pharmacol. 66:115–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonomi P, Kim K, Fairclough D, Cella D,
Kugler J, Rowinsky E, Jiroutek M and Johnson D: Comparison of
survival and quality of life in advanced non-small-cell lung cancer
patients treated with two dose levels of paclitaxel combined with
cisplatin versus etoposide with cisplatin: Results of an Eastern
Cooperative Oncology Group trial. J Clin Oncol. 18:623–631.
2000.PubMed/NCBI
|
|
5
|
Lin Y, Xu J, Liao H, Li L and Pan L:
Piperine induces apoptosis of lung cancer A549 cells via
p53-dependent mitochondrial signaling pathway. Tumour Biol.
35:3305–3310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rasul A, Bao R, Malhi M, Zhao B, Tsuji I,
Li J and Li X: Induction of apoptosis by costunolide in bladder
cancer cells is mediated through ROS generation and mitochondrial
dysfunction. Molecules. 18:1418–1433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bonomi P: Review of paclitaxel/carboplatin
in advanced non-small cell lung cancer. Semin Oncol. 26(Suppl 2):
S55–S59. 1999.
|
|
8
|
Gu JQ, Gills JJ, Park EJ, Mata-Greenwood
E, Hawthorne ME, Axelrod F, Chavez PI, Fong HH, Mehta RG, Pezzuto
JM and Kinghorn AD: Sesquiterpenoids from Tithonia diversifolia
with potential cancer chemopreventive activity. J Natl Prod.
65:532–536. 2002. View Article : Google Scholar
|
|
9
|
Koch E, Klaas CA, Rüngeler P, Castro V,
Mora G, Vichnewski W and Merfort I: Inhibition of inflammatory
cytokine production and lymphocyte proliferation by structurally
different sesquiterpene lactones correlates with their effect on
activation of NF-kappaB. Biochem Pharmacol. 62:795–801. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robles M, Aregullin M, West J and
Rodriguez E: Recent studies on the zoopharmacognosy, pharmacology
and neurotoxicology of sesquiterpene lactones. Planta Med.
61:199–203. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park HJ, Jung WT, Basnet P, Kadota S and
Namba T: Syringin 4-O-beta-glucoside, a new phenylpropanoid
glycoside and costunolide, a nitric oxide synthase inhibitor, from
the stem bark of Magnolia sieboldii. J Nat Prod. 59:1128–1130.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wedge DE, Galindo JC and Macías FA:
Fungicidal activity of natural and synthetic sesquiterpene lactone
analogs. Phytochemistry. 53:747–757. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen HC, Chou CK, Lee SD, Wang JC and Yeh
SF: Active compounds from Saussurea lappa Clarks that suppress
hepatitis B virus surface antigen gene expression in human hepatoma
cells. Antiviral Res. 27:99–109. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koo TH, Lee JH, Park YJ, Hong YS, Kim HS,
Kim KW and Lee JJ: A sesquiterpene lactone, costunolide, from
Magnolia grandiflora inhibits NF-kappa B by targeting I kappa B
phosphorylation. Planta Med. 67:103–107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukuda K, Akao S, Ohno Y, Yamashita K and
Fujiwara H: Inhibition by costunolide of phorbol ester-induced
transcriptional activation of inducible nitric oxide synthase gene
in a human monocyte cell line THP-1. Cancer Lett. 164:7–13. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi JH, Ha J, Park JH, Lee JY, Lee YS,
Park HJ, Choi JW, Masuda Y, Nakaya K and Lee KT: Costunolide
triggers apoptosis in human leukemia U937 cells by depleting
intracellular thiols. Jpn J Cancer Res. 93:1327–1333. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SH, Kang SN, Kim HJ and Kim TS:
Potentiation of 1,25-dihydroxyvitamin D(3)-induced differentiation
of human promyelocytic leukemia cells into monocytes by
costunolide, a germacranolide sesquiterpene lactone. Biochem
Pharmacol. 64:1233–1242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi YK, Cho SG, Woo SM, Yun YJ, et al:
Saussurea lappa clarke-derived costunolide prevents TNF α-induced
breast cancer cell migration and invasion by inhibiting NF-κB
activity. Evid Based Complement Alternat Med. 2013:9362572013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi YK, Seo HS, Choi HS, Choi HS, Kim SR,
Shin YC and Ko SG: Induction of Fas-mediated extrinsic apoptosis,
p21WAF1-related G2/M cell cycle arrest and ROS generation by
costunolide in estrogen receptor-negative breast cancer cells,
MDA-MB-231. Mol Cell Biochem. 363:119–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu CY, Chang HS, Chen IS, Chen CJ, Hsu
ML, Fu SL and Chen YJ: Costunolide causes mitotic arrest and
enhances radiosensitivity in human hepatocellular carcinoma cells.
Radiat Oncol. 6:562011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsu JL, Pan SL, Ho YF, Hwang TL, Kung FL
and Guh JH: Costunolide induces apoptosis through nuclear calcium2+
overload and DNA damage response in human prostate cancer. J Urol.
185:1967–1974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi JH and Lee KT: Costunolide-induced
apoptosis in human leukemia cells: Involvement of c-jun N-terminal
kinase activation. Biol Pharm Bull. 32:1803–1808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi SH, Im E, Kang HK, Lee JH, Kwak HS,
Bae YT, Park HJ and Kim ND: Inhibitory effects of costunolide on
the telomerase activity in human breast carcinoma cells. Cancer
Lett. 227:153–162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Komiya T, Yamada Y, Moteki H, Katsuzaki H,
Imai K and Hibasami H: Hot water soluble sesquiterpenes
[anhydroperoxy-costunolide and 3-oxoeudesma-1,4 (15),11
(13)triene-12,6alpha-olide] isolated from laurel (Laurus
nobilis L.) induce cell death and morphological change
indicative of apoptotic chromatin condensation in leukemia cells.
Oncol Rep. 11:85–88. 2004.PubMed/NCBI
|
|
25
|
Hibasami H, Yamada Y, Moteki H, Katsuzaki
H, Imai K, Yoshioka K and Komiya T: Sesquiterpenes (costunolide and
zaluzanin D) isolated from laurel (Laurus nobilis L.) induce cell
death and morphological change indicative of apoptotic chromatin
condensation in leukemia HL-60 cells. Int J Mol Med. 12:147–151.
2003.PubMed/NCBI
|
|
26
|
Kretschmer N, Rinner B, Stuendl N,
Kaltenegger H, Wolf E, Kunert O, Boechzelt H, Leithner A, Bauer R
and Lohberger B: Effect of costunolide and dehydrocostus lactone on
cell cycle, apoptosis and ABC transporter expression in human soft
tissue sarcoma cells. Planta Med. 78:1749–1756. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang YI, Kim JH and Choi JH: Costunolide
induces apoptosis in platinum-resistant human ovarian cancer cells
by generating reactive oxygen species. Gynecol Oncol. 123:588–596.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
King KL and Cidlowski JA: Cell cycle
regulation and apoptosis. Ann Rev Physiol. 60:601–617. 1998.
View Article : Google Scholar
|
|
29
|
Collins K, Jacks T and Pavletich NP: The
cell cycle and cancer. Proc Natl Acad Sci USA. 94:2776–2778. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pan J, She M, Xu ZX, Sun L and Yeung SC:
Farnesyltransferase inhibitors induce DNA damage via reactive
oxygen species in human cancer cells. Cancer Res. 65:3671–3681.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bergamo A, Gagliardi R, Scarcia V, Furlani
A, Alessio E, Mestroni G and Sava G: In vitro cell cycle arrest, in
vivo action on solid metastasizing tumors and host toxicity of the
antimetastatic drug NAMI-A and cisplatin. J Pharmacol Exp Ther.
289:559–564. 1999.PubMed/NCBI
|
|
32
|
Horwitz SB: Taxol (paclitaxel): Mechanisms
of action. Ann Oncol. 5(Suppl 6): S3S61994.
|
|
33
|
Fridman JS and Lowe SW: Control of
apoptosis by p53. Oncogene. 22:9030–9040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Budram-Mahadeo V, Morris PJ and Latchman
DS: The Brn-3a transcription factor inhibits the pro-apoptotic
effect of p53 and enhances cell cycle arrest by differentially
regulating the activity of the p53 target genes encoding Bax and
p21(CIP1/Waf1). Oncogene. 21:6123–6131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tchernev G and Orfanos CE: Downregulation
of cell cycle modulators p21, p27, p53, Rb and proapoptotic
Bcl-2-related proteins Bax and Bak in cutaneous melanoma is
associated with worse patient prognosis: Preliminary findings. J
Cutan Pathol. 34:247–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Polyak K, Kato JY, Solomon MJ, Sherr CJ,
Massague J, Roberts JM and Koff A: p27Kip1, a cyclin-Cdk inhibitor,
links transforming growth factor-beta and contact inhibition to
cell cycle arrest. Genes Dev. 8:9–22. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rasul A, Ding C, Li X, Khan M, Yi F, Ali M
and Ma T: Dracorhodin perchlorate inhibits PI3K/Akt and NF-κB
activation, up-regulates the expression of p53 and enhances
apoptosis. Apoptosis. 17:1104–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sakuragi N, Salah-eldin AE, Watari H, Itoh
T, Inoue S, Moriuchi T and Fujimoto S: Bax, Bcl-2 and p53
expression in endometrial cancer. Gynecol Oncol. 86:288–296. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Frenzel A, Grespi F, Chmelewskij W and
Villunger A: Bcl2 family proteins in carcinogenesis and the
treatment of cancer. Apoptosis. 14:584–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Porter AG and Jünicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|