Introduction
Metformin (1,1-dimethylbiguanide hydrochloride), a
widely-prescribed antihyperglycemic drug and first-line therapy for
the treatment of diabetes mellitus type 2 (DM-II), has demonstrated
notable antineoplastic effects in vivo and in vitro
(1). A growing body of evidence has
suggested that metformin may potentially reduce the risk of
developing a number of types of cancer, including breast (2), colorectal (3), prostate (4) and lung cancer (5). However, Smiechowski et al
(6) investigated 8572 patients with
DM-II, including 808 lung cancer cases and 7764 controls, and did
not identify a significant role of metformin in the risk of lung
cancer [odds ratio (OR), 0·94; 95% confidence interval (CI),
0·76-1·17]. A recent meta-analysis incorporating 11 studies also
did not observe a significant association between metformin and
lung cancer risk (OR, 0.99; 95% CI, 0.87–1.12) (7). A common caveat of the aforementioned
studies was a lack of consideration of the age and subtypes of lung
cancer.
Compared with old patients, young age lung cancer
(YALC) cases had a higher proportion of women and were prone to
adenocarcinoma development and distant metastases (8). Furthermore, a cohort demonstrated that
younger patients had a higher proportion of adenocarcinoma, a
higher proportion of stage I disease and a lower proportion of
stage III disease (9). The
aforementioned results demonstrate the varying clinical
characteristics of YALC (8,9).
With regard to young age DM, a previous study
reported that the majority of patients were diagnosed during
puberty as obese or at risk for obesity (10); this was considered to occur as a
result of a variety of genetic conditions (for example
maturity-onset diabetes) (11). Such
findings support the notion that young age DM presents with
specific characteristics that differ from those observed in older
cases (10,11).
To the best of our knowledge, there are a limited
number of available case reports that focus on the high risk of
neuroendocrine tumors (NETs), including carcinoid tumors and small
cell lung cancers (SCLC), in YALC and DM treated with oral
metformin (6,12). In the present study, patients with
YALC and DM were investigated. When compared with patients with
typical lung cancer (in the sixth to eighth decade of life), it was
hypothesized as unlikely that the YALC and DM patients had been
differentially impacted by environmental carcinogen exposure
(13). If proved to be correct, this
may indicate that DM or the treatment of DM with metformin may be
associated with increased risk of a specific subtype of lung
cancer, (e.g. NETs), which have distinct features of clinical
behavior, epidemiology, treatment and prognosis (14).
Case series report
In the present study, the Mayo Clinic Lung Cancer
Cohort database, established in the Epidemiology and Genetics of
Lung Cancer research program (15–17), was
used to identify 571 consecutive patients with pathologically
diagnosed YALC treated at the Mayo Clinic College of Medicine
(Rochester, MN, USA) between 1997 and 2011, who were <45 years
old at the time of primary lung cancer diagnosis (Table I). Written informed consent was
obtained from all patients. Samples were obtained at the time of
tumor diagnosis. Formalin-fixed paraffin-embedded samples (if
available after diagnosis), slides (for diagnosis) or both were
stored at the Department of Laboratory Medicine and Pathology, Mayo
Clinic College of Medicine and patients were followed up for 10
years after diagnosis (18). Among
the 571 patients selected, 278 (48.7%) exhibited adenocarcinoma, 76
(13.4%) carcinoid tumors, 52 (9.1%) squamous carcinoma, 34 (6.0%)
SCLC and 131 (22.9%) possessed other or unspecified cell type
tumors. A review of patient medical history revealed that 10/571
patients had exhibited primary DM at least one year prior to lung
cancer diagnosis (Table I);
specifically, there were 2 cases of DM type 1 (DM-I) and eight of
DM-II. Three notable observations were made regarding these
patients: i) 8/10 patients were overweight or obese, as determined
by their body mass index (BMI; BMI, >24.99; Table II); ii) 5/8 patients with DM-II
(62.5%) exhibited pulmonary NETs, including carcinoid tumors and
SCLC, which was a higher proportion than that observed in the
non-diabetic patients (19.4%; 5/8 vs. 104/561; Fisher's test,
P<0.05); and iii) most notably, 4 patients exhibiting NETs and
one with lymphoma (Table II) had
received metformin for the treatment of DM-II. By contrast, the two
patients exhibiting adenocarcinoma and DM-II had not been
administered metformin. Although 5/8 patients with DM-II treated
with metformin were current or former smokers, these patients
developed lung cancer 20–30 years earlier in life than the majority
of patients, who are lifelong heavy smokers. The proportion of
pulmonary NETs in metformin treated patients was significantly
higher than those who did not receive metformin treatment (4/5 vs.
106/566; Fisher's test, P<0.05).
 | Table I.Clinicopathological characteristics of
571 consecutively diagnosed young age lung cancer patients. |
Table I.
Clinicopathological characteristics of
571 consecutively diagnosed young age lung cancer patients.
| Parameter | Patients, n (%) |
|---|
| Age at diagnosis,
years |
|
| Mean
(SD) | 39.1
(5.1) |
|
Range |
17.0–44.0 |
| Gender |
|
|
Female | 309 (54.1) |
| Male | 262 (45.9) |
| Pathological cell
type |
|
|
Adenocarcinoma | 278 (48.7) |
|
Squmaous | 52
(9.1) |
| Small
cell | 34
(6.0) |
|
Carcinoid | 76
(13.3) |
|
Other | 131 (22.9) |
| Tumor stage |
|
| I | 85
(14.9) |
| II | 35
(6.1) |
| III | 147 (25.7) |
| IV | 251 (44.0) |
| Limited
(small cell)a | 16
(2.8) |
| Extensive
(small cell)b | 17
(3.0) |
|
Unknown | 20
(3.5) |
| Tumor grade |
|
| Well
differentiated | 65
(11.4) |
| Moderate
differentiated | 141 (24.7) |
|
Poor/undifferentiated | 191 (33.4) |
|
Ungradeablec | 174 (30.5) |
| Smoking status |
|
|
Never-smoker | 197 (34.4) |
|
Former-smoker | 118 (20.7) |
|
Current-smoker | 251 (44.0) |
|
Unknown | 5
(0.9) |
 | Table II.Clinical characteristics of patients
with young age lung cancer exhibiting primary diabetes
mellitus. |
Table II.
Clinical characteristics of patients
with young age lung cancer exhibiting primary diabetes
mellitus.
| Age, years | Gender | Tumor type | DM Type | Treatment of DM | Duration of
treatment, months | Smoking status | BMI,
kg/m2 |
|---|
| 42 | Male |
Neuroendocrinea | 2 | Insulin therapy | 6 | Never | 33.92 |
| 41 | Male |
Neuroendocrinea | 2 | Metformin | 20 | Former | 33.67 |
| 42 | Male |
Neuroendocrineb | 1 | Insulin therapy | Unknown | Current | 19.56 |
| 42 | Male |
Neuroendocrineb | 2 | Metformin | 24 | Former | 39.48 |
| 43 | Male |
Neuroendocrineb | 2 | Metformin | >12 | Current | 25.94 |
| 38 | Male |
Neuroendocrineb | 2 | Metformin | >12 | Current | 36.66 |
| 44 | Female | Adenocarcinoma | 2 |
Glucotrold | 36 | Former | 29.27 |
| 44 | Female | Adenocarcinoma | 2 | None recorded | N/A | Current | 28.2 |
| 43 | Male | Pulmonary
lymphomac | 2 | Metformin | >12 | Current | 42.62 |
| 24 | Female | Unclassified
carcinoma | 1 | None recorded | N/A | Never | 19.41 |
Discussion
The incidence of pulmonary NETs has been reported to
be ~21/100,000 per year in the Caucasian population of Denmark
(14). This figure includes all
Caucasian adults; however, the incidence in the young adult
population may be lower. To the best of our knowledge, no such
published data is currently available. The high proportion (4/5;
80%) of pulmonary NETs observed in patients with metformin-treated
DM-II identified in the present study was suggested to be unlikely
to be a random event, based on logical suppositions. Regarding the
proposed favorable effects of metformin in a number of types of
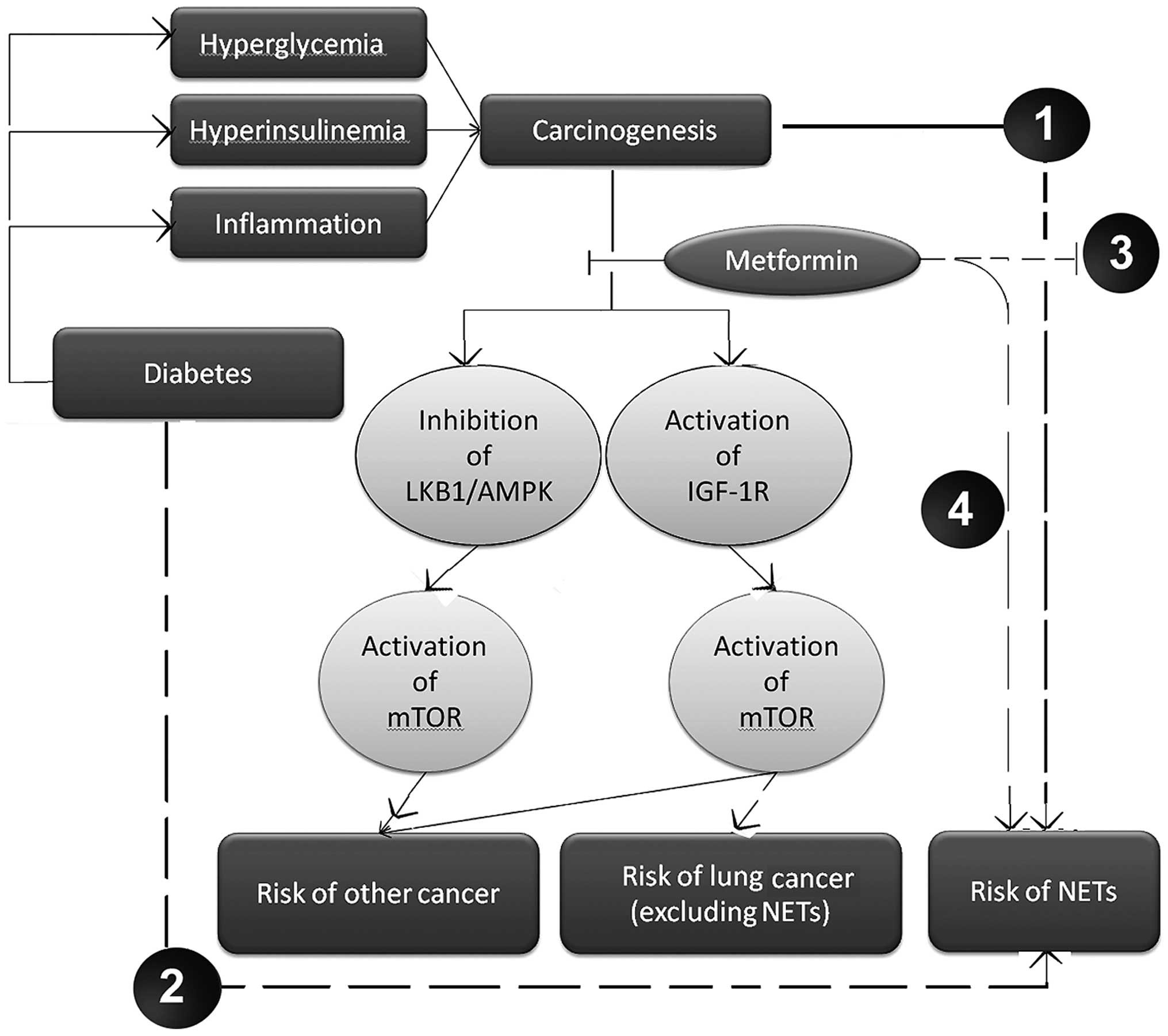
cancer, a suggested potential underlying mechanism is the
activation of the liver kinase B1 (LKB1) 5′ adenosine
monophosphate-activated protein kinase (AMPK) pathway, and
inhibition of the mammalian target of rapamycin (mTOR) downstream
signaling pathway by ribosomal protein S6 kinase β-1 (20,21), which
possesses a significant role in carcinogenesis and cancer
progression (21). In addition,
metformin may indirectly inhibit mTOR in lung tissue by decreasing
the activation of insulin-like growth factor-1 receptor
(IGF-1R)/insulin receptor, rather than through activation of the
LKB1/AMPK pathway (22). Furthermore,
IGF-1R has been demonstrated not to be expressed in the majority of
NET tissues (22), suggesting that
the attenuation of IGF-1R activity may be ineffective in the
treatment of NETs. Finally, mTOR inhibition has been demonstrated
to be capable of inducing upstream receptor tyrosine kinase
signaling and activating protein kinase B (Akt) kinase, therefore
reducing the antitumor effects of mTOR inhibitors (21). Consequently, the present study
hypothesized that, in NETs, the inhibition of mTOR due to the
activity of metformin may also activate additional unknown upstream
signaling pathways and factors, for example Akt kinase, which
subsequently decreases the antitumor effects of metformin.
Although hyperinsulinemia, hyperglycemia and chronic
inflammation may be significant mechanisms in the neoplastic
process of a number of types of cancer, the carcinogenic mechanisms
induced by DM in NETs remain to be elucidated (23). A Taiwanese population-based study
revealed that diabetes was associated with a significantly higher
risk of lung cancer (24). In
addition, a correlation between DM and pancreatic NETs has been
reported (25). Therefore, we
hypothesize that DM may present a major risk factor for the
development of pulmonary NETs, however, further investigation is
required. To the best of our knowledge, summarizing all evidence
available to date, and as depicted in Fig. 1, the present study hypothesized that
metformin was able to decrease IGF-1R expression in lung tissues,
consequently inhibiting the downstream mTOR signaling pathway and
decreasing the risk of lung cancer (excluding the subgroup of
NETs). However, the blockage of IGF-1R activity by metformin is
ineffective in NETs. Furthermore, the current study hypothesized
that mTOR inhibition, following administration of metformin, may
induce the activation of additional upstream oncogenic
cascades.
The occurrence of NETs involves unknown signaling
pathways (Fig. 1). Previous studies
have demonstrated that the use of metformin is not associated with
overall lung cancer risk (6,12). However, after stratifying the results
by lung cancer subtype, it was found that a longer duration of
metformin treatment may be associated with higher risk of small
cell carcinoma (12). However, in
this study, a high proportion of NETs were observed in the cases of
metformin-treated DM-II. Thus, if the results of the present study
are able to be verified, they may be useful as a novel alert for
the significance of monitoring young patients with diabetes,
particularly those who are overweight and receiving metformin, who
may possess an increased risk of developing pulmonary NETs.
Acknowledgements
The present study was supported by the USA National
Institute of Health (grant nos. R03 CA77118, R01 CA80127 and R01
CA84354), as well as the Mayo Clinic Foundation (grant no.
300-92021) and Third Military Medical University (MiaoPu project;
grant no. MP2012-6-20). The authors would like to thank Ms. Susan
Ernst, M.A., for her technical assistance with the manuscript.
References
|
1
|
Wang Y, Dai W, Chu X, Yang B, Zhao M and
Sun Y: Metformin inhibits lung cancer cells proliferation through
repressing microRNA-222. Biotechnol Lett. 35:2013–2019. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Col NF, Ochs L, Springmann V, Aragaki AK
and Chlebowski RT: Metformin and breast cancer risk: A
meta-analysis and critical literature review. Breast Cancer Res
Treat. 135:639–646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Currie CJ, Poole CD and Gale EA: The
influence of glucose-lowering therapies on cancer risk in type 2
diabetes. Diabetologia. 52:1766–1777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wright JL and Stanford JL: Metformin use
and prostate cancer in Caucasian men: Results from a
population-based case-control study. Cancer Causes Control.
20:1617–1622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Memmott RM, Mercado JR, Maier CR, Kawabata
S, Fox SD and Dennis PA: Metformin prevents tobacco
carcinogen-induced lung tumorigenesis. Cancer Prev Res (Phila).
3:1066–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smiechowski BB, Azoulay L, Yin H, Pollak
MN and Suissa S: The use of metformin and the incidence of lung
cancer in patients with type 2 diabetes. Diabetes Care. 36:124–129.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nie SP, Chen H, Zhuang MQ and Lu M:
Anti-diabetic medications do not influence risk of lung cancer in
patients with diabetes mellitus: A systematic review and
meta-analysis. Asian Pac J Cancer Prev. 15:6863–6869. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomas A, Chen Y, Yu T, Jakopovic M and
Giaccone G: Trends and characteristics of young non-small cell lung
cancer patients in the United States. Front Oncol. 5:1132015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu M, Cai X, Yu W, Lv C and Fu X:
Clinical significance of age at diagnosis among young non-small
cell lung cancer patients under 40 years old: A population-based
study. Oncotarget. Oct 26–2015.(Epub ahead of print).
|
|
10
|
Pinhas-Hamiel O and Zeitler P: Clinical
presentation and treatment of type 2 diabetes in children. Pediatr
Diabetes. 8(Suppl 9): 16–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Institute for Health and Care
Excellence: Diabetes (type 1 and type 2) in children and young
people: Diagnosis and management. London, UK: 2015.http://www.nice.org.uk/guidance/ng18/resources/diabetes-type-1-and-type-2-in-children-and-young-people-diagnosis-and-management-1837278149317Accessed.
February 05–2015
|
|
12
|
Sakoda LC, Ferrara A, Achacoso NS, Peng T,
Ehrlich SF, Quesenberry CP Jr and Habel LA: Metformin use and lung
cancer risk in patients with diabetes. Cancer Prev Res (Phila).
8:174–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mack JW, Cronin A, Fasciano K, Block SD
and Keating NL: Cancer treatment decision-making among young adults
with lung and colorectal cancer: A comparison with adults in middle
age. Psychooncology. Sep 2–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Skuladottir H, Hirsch FR, Hansen HH and
Olsen JH: Pulmonary neuroendocrine tumors: Incidence and prognosis
of histological subtypes. A population-based study in Denmark. Lung
Cancer. 37:127–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Z, Aubry MC, Deschamps C, et al:
Histologic grade is an independent prognostic factor for survival
in non-small cell lung cancer: An analysis of 5018 hospital- and
712 population-based cases. J Thorac Cardiovasc Surg.
131:1014–1020. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jatoi A, Qi Y, Wampfler JA, Busta AJ, Yang
P and Mandrekar S: The purported effects of alcohol on appetite and
weight in lung cancer patients. Nutr Cancer. 63:1251–1255. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng B, Cassivi SD, de Andrade M, et al:
Clinical outcomes and changes in lung function after segmentectomy
versus lobectomy for lung cancer cases. J Thorac Cardiovasc Surg.
148:1186–1192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang P, Cheville AL, Wampfler JA, Garces
YI, Jatoi A, Clark MM, Cassivi SD, Midthun DE, Marks RS, Aubry MC,
et al: Quality of life and symptom burden among long-term lung
cancer survivors. J Thorac Oncol. 7:64–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Reilly KE, Rojo F, She QB, Solit D,
Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al:
mTOR inhibition induces upstream receptor tyrosine kinase signaling
and activates Akt. Cancer Res. 66:1500–1508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: American Joint Committee on Cancer: AJCC
Cancer Staging Manual. Springer. New York: 2010.
|
|
21
|
Libutti SK: Therapy: Blockade of IGF-1R -
not effective in neuroendocrine tumours. Nat Rev Endocrinol.
9:389–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vlotides G, Tanyeri A, Spampatti M,
Zitzmann K, Chourdakis M, Spttl C, Maurer J, Nölting S, Göke B and
Auernhammer CJ: Anticancer effects of metformin on neuroendocrine
tumor cells in vitro. Hormones. 13:498–508. 2014.PubMed/NCBI
|
|
23
|
Gallagher EJ and LeRoith D: Obesity and
diabetes: The increased risk of cancer and cancer-related
mortality. Physiol Rev. 95:727–748. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tseng CH: Diabetes but not insulin
increases the risk of lung cancer: A Taiwanese population-based
study. PLoS One. 9:e1015532014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maxwell JE, O'Dorisio TM, Bellizzi AM and
Howe JR: Elevated pancreatic polypeptide levels in pancreatic
neuroendocrine tumors and diabetes mellitus: Causation or
association? Pancreas. 43:651–656. 2014. View Article : Google Scholar : PubMed/NCBI
|