Introduction
Lung cancer presents with a particularly high
mortality rate and is understood to be the leading cause of
worldwide cancer-associated mortalities. Non-small cell lung cancer
(NSCLC) accounts for 85% of all lung cancers (1). To date, the treatment options that are
available for patients with lung cancer consist of combinations of
chemotherapy and radiotherapy (RT) (2). Despite decades of research, alternative
NSCLC therapies remain insufficient, and the majority of lung
cancers demonstrate a high incidence of relapse due to the
development of resistance (3). RT is
a treatment that utilizes high-energy rays or particles to destroy
lung cancer cells. However, the efficacy of RT is limited due to
the survival of lung cancer cells following the treatment,
subsequently resulting in recurrence (4). Thus, the identification of novel
therapeutic drugs to mitigate possible chemo- or radioresistance is
critical to allow the development of successful lung cancer
treatments.
The majority of cancer cells exhibit elevated
glycolysis and depressed mitochondrial oxidative phosphorylation,
allowing the generation of ATP to function as their energy supply.
This phenomenon is known as the Warburg effect (5). Furthermore, this switch in energy
metabolism, and the associated increased expression of glycolytic
enzymes, provides a survival advantage for the cancer cells
(6). It is reported that dysregulated
glycolysis is closely associated with chemo- and radioresistance in
cancer (7), suggesting that the
inhibition of glycolysis may be an effective method to incorporate
during the development of optimal combination regimens for the
treatment of cancer.
MicroRNA (miRNA/miR) is non-coding, single-stranded
RNA of ~22 nucleotides in length. miRNA regulates gene expression
at a post-transcription level by binding onto specific mRNA
molecules at the 3′-untranslated region (3′-UTR), and performs
essential roles in a variety of biological processes (8). miRNAs are associated with proliferation,
differentiation, migration, the cell cycle and apoptosis (9). Furthermore, previous studies have
demonstrated that miRNAs have important functions within cancer
development, malignant transformation and drug resistance (10), and that they may serve as potential
oncogenes or tumor suppressors (9–11).
Recently, the role of miRNA-21 within NSCLC radioresistance has
been identified, indicating that miRNAs may be selected as a
possible therapeutic target (12).
Additionally, miR-133b in particular has been reported to function
as a tumor suppressor in multiple types of cancer (13–15).
However, the precise role of miR-133b within radiosensitivity is
not yet clear. In the present study, the role of miR-133b in NSCLC
radiosensitivity is investigated, with the association between
dysregulated glycolysis and radiosensitivity also examined. The
subsequent results demonstrate that miR-133b may function as a
therapeutic target for the treatment of radioresistant lung
cancer.
Materials and methods
Cell culture ionizing radiation
treatment
NSCLC A549 cells were purchased from the American
Type Culture Collection (Manassas, VA, USA) and were cultured in
Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal
bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin. Cell
cultures were incubated in a humidified atmosphere of 5%
CO2 at 37°C.
Ionizing radiation treatment of
cells
Cell cultures containing 5×105 cells were
exposed to different doses of radiation (0, 0.2, 0.4, 0.5, 0.6,
1.0, 2.0, 3.0, 4.0, 5.0, 6.0 Gy) at room temperature, with a Cs-137
irradiator (HWM D-2000; Siemens AG, Munich, Germany) at a dose rate
of 2 Gy/min. Following irradiation, the cells were subjected to the
following experiments, or were placed back in the incubator in a
humidified atmosphere of 5% CO2 at 37°C.
miRNA transfection
The cells were seeded in 6-well plates at
1×105 cells/well and cultured overnight. The cells were
subsequently transfected with 100 nM of the pre-miR-133b,
inhibitors [anti-miR-133b (Shanghai GenePharma Co., Ltd., Shanghai,
China)] or negative control mRNA (Shanghai GenePharma Co., Ltd.)
using Lipofectamine 2000® and Opti-MEM I reduced serum medium
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. The precursor and antisense of miR-133b
were chemically synthesized by Shanghai GenePharma Co., Ltd.
Following a total of 48 h post-transfection, the cells were
prepared for further analysis.
Plasmid DNA transfection
(Myc-DDK-tagged)-Human pyruvate kinase (PKM)
transcript variant 2 was purchased from OriGene Technologies, Inc.
(catalog no. RC219382; Rockville, MD, USA) and cloned into pCMV6
vector (catalog no. PS100001, OriGene Technologies, Inc.). pCMV6
empty vector was used as a control. Plasmid DNA transfection was
performed using Lipofectamine 2000® and Opti-MEM I reduced serum
medium (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocols. Following a total of 48 h
post-transfection, the cells were prepared for further
analysis.
Cell viability assay
A total of 1×105 cells for each well were
seeded in 12-well plates overnight. The medium was replaced daily.
Cell viability was measured using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
Absorbance was measured spectrophotometrically at 570 nm by the
EL800 Universal Microplate Reader (BioTek Instruments, Inc.,
Winooski, VT, USA).
RNA extraction and quantitative
polymerase chain reaction (qPCR)
miRNAs from the cultured cells were extracted and
purified using the mirVana miRNA Isolation kit (Ambion; Thermo
Fisher Scientific, Inc.), following the manufacturer's protocols.
Total RNA concentration was adjusted to 2 ng/µl using a
spectrophotometer. Total RNA (1 µg) was reverse transcribed with
the High Capacity cDNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The cDNA reaction was
diluted to 1:10 for use as a template for qPCR of mature miRNA.
miRNA expression patterns in the cultured cells and
tissues were evaluated using TaqMan® OpenArray® Human MicroRNA
Panels (Applied Biosystems; Thermo Fisher Scientific, Inc.).
miRNA from the samples was converted into cDNA using
156 specific stem-loop reverse transcription primers. Specific
TaqMan® qPCR primers and probes were then used to conduct qPCR and
determine the quantity of the miRNAs. qPCR was performed using the
GeneAmp® Fast PCR Master mix and the 7900HT Fast Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
qPCR conditions were as follows: 95°C for 10 min; 40 cycles of 95°C
for 15 sec; and 60°C for 60 sec. All reactions were performed in
duplicate. Expression levels of the mature miRNAs were evaluated
using the comparative quantification cycle (Cq) method of 2(−ΔΔCq).
The ΔCq for PKM2 mRNA expression was calculated relative to the Cq
of 18S ribosomal RNA. Relative mRNA expression was calculated using
the formula 2(−ΔΔCq). The primers used for qPCR were: PKM2 forward
primer, 5′-GAGGCCTCCTTCAAGTGCT-3′, and reverse primer,
5′-CCAGACTTGGTGAGGACGAT-3′. All reactions were performed at least
twice in duplicate.
Measurements of glucose
consumption
The glucose concentration in the diluted medium was
measured using the Glucose (GO) assay kit (Sigma-Aldrich, St.
Louis, MO, USA), according to the manufacturer's protocols. Glucose
consumption was calculated by subtracting the concentration of
glucose remaining in the medium at the indicated time from the
concentration of glucose present in fresh cell culture medium. The
results were normalized to the total amount of protein when
compared with the control cells.
Measurements of lactate
production
Lactate concentrations were determined using a
lactate assay kit (BioVision, Inc., Milpitas, CA, USA), according
to the manufacturer's protocols. Samples and lactate standard with
lactate assay buffer was prepared in a 96-well plate. A total of 50
µl of the reaction mix, containing the lactate enzyme mix, was
added to each well. The plate was then incubated for 30 min at room
temperature. Optical density values at 570 nm were measured with a
SpectraMax® M2e Multimode Microplate Reader (Molecular Devices,
LLC, Sunnyvale, CA, USA). The results were normalized to the total
amount of protein when compared with the control cells.
Western blot analysis
The cells were harvested and washed with ice-cold
phosphate-buffered saline. Cell lysates were obtained by
resuspending the cells in RIPA buffer [10 mM Tris (pH 7.4), 150 mM
NaCl, 1% Triton X-100 and 1% Na-deoxycholate (Kanto Chemical Co.,
Ltd., Tokyo, Japan)], and 5 mM ethylenediaminetetraacetic acid that
was supplemented with a protease inhibitor cocktail
(Sigma-Aldrich). The protein concentration of the cell lysates was
determined by using the Bradford protein assay kit (Beyotime
Institute of Biotechnology, Shanghai, China). Equal amounts of
protein were loaded and subsequently separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis prior to being
electrotransferred onto a nitrocellulose membrane (EMD Millipore,
Billerica, MA, USA). The membranes were blocked and incubated
overnight with primary antibodies at 1:1,000 dilution. The primary
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA) as follows: Rabbit anti-human PKM2 monoclonal
(dilution, 1:1,000; catalog no. 4053) and rabbit anti-human β-actin
monoclonal (dilution, 1:1,000; catalog no. 4967) antibodies. The
membranes were then washed and incubated at room temperature for 1
h with HRP-conjugated goat anti-rabbit immunoglobulin G (Nichirei
Bioscience Inc., Tokyo, Japan). Protein bands were visualized with
the Chemi-Lumi One L western blotting substrate (Nacalai Tesque,
Kyoto, Japan).
Statistical analysis
Prism version 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA) was used to calculate unpaired Student's t
test for data analysis. All data are presented as the mean ±
standard error. P<0.05 was considered to indicate a
statistically significant difference.
Results
Establishment of radioresistant NSCLC
cell line
To investigate the roles of miRNA-133b in the
radiosensitivity of human NSCLC, a radioresistant cell line was
established from A549 cells. A549 parental cells were exposed to
increased intensities of radiation (1 to 5 Gy) and the surviving
cells were selected. Following 1 month of consecutive selection,
the surviving cell clones were pooled and subjected to resistance
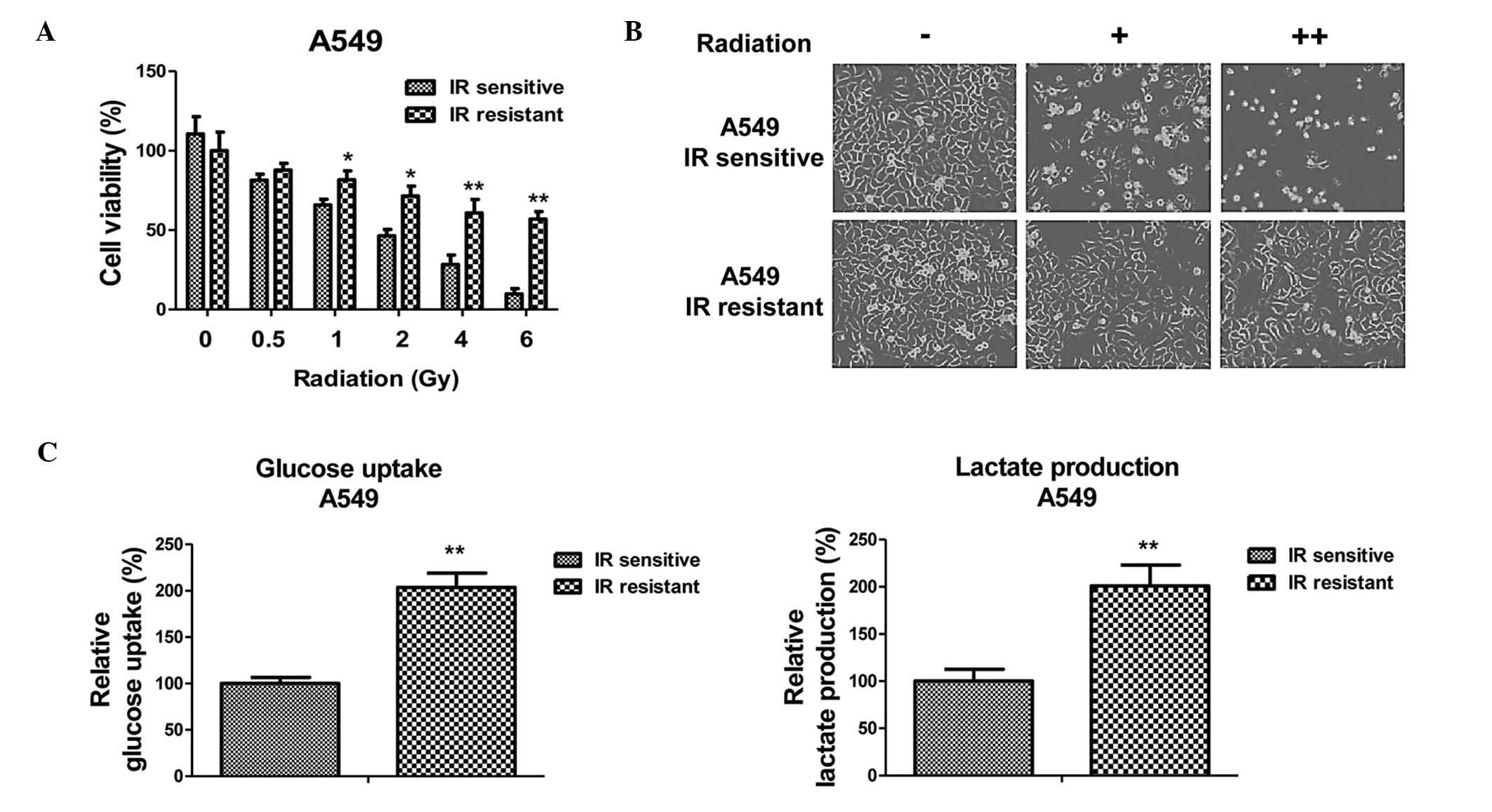
verification. Results of cell viability experiments are presented
in Fig. 1A and B. The A549
radiosensitive cells demonstrated a significant inhibition of
viability following irradiation with 0.5 to 6 Gy. By contrast, A549
resistant cells exhibited significantly increased viability
following radiation exposure. The irradiation dosage for 50% cell
viability inhibition in the radioresistant cells was 8 Gy, which
was greater than that of the radiosensitive cells.
Radioresistant A549 cells have
increased glucose metabolism
As aforementioned, dysregulated glucose metabolism
is associated with chemo- and radioresistance in cancer cells
(7). To investigate whether the
glucose metabolic profile was altered by radiation treatment, the
glucose uptake and lactate product of the A549 cells was measured
following different dosages of radiation treatment. Notably, the
results from the present study demonstrated that the glucose uptake
and lactate product were induced by radiation treatment (Fig. 1C), indicating that there may be an
association between glucose metabolism and radiosensitivity in
NSCLC cells. As expected, the radioresistant A549 cells exhibited
increased glucose metabolism when compared with the sensitive
cells, suggesting that the upregulated glucose metabolism may
contribute to radioresistance, and may be targeted to develop
anti-radioresistance drugs.
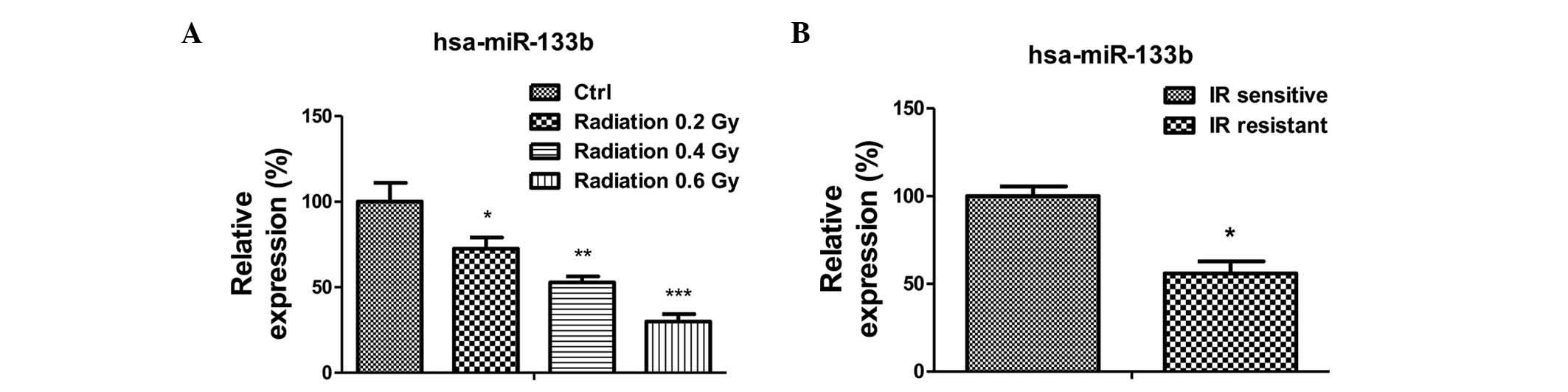
miR-133b is negatively correlated with
radioresistance
The present study subsequently investigated the
mechanism underlying the upregulated glucose metabolism in
radioresistant lung cancer cells. As it has been previously
reported that miR-133b acts as a tumor suppressor in lung cancer,
the expression levels of miR-133b in A549 cells were measured
following radiation treatment (13–18). The
expression of miR-133b was significantly reduced following
radiation treatments of 0.2 to 0.6 Gy (Fig. 2A). Additionally, the expression of
miR-133b in the radiation-resistant A549 cells was observed to be
lower than that in the radiation sensitive cells (Fig. 2B), suggesting miR-133b may be involved
in the regulation of radiation sensitivity.
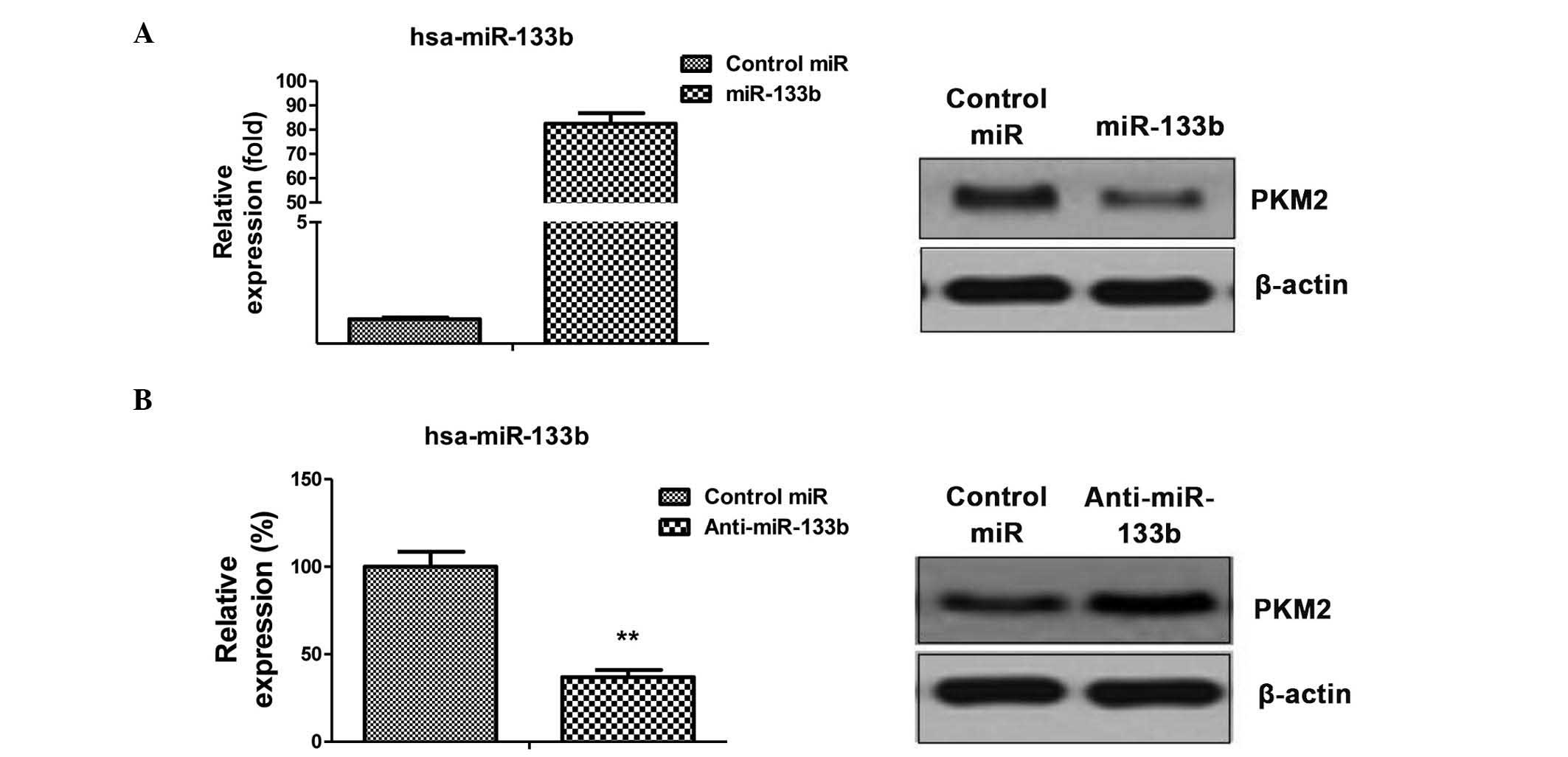
PKM2 is a target of miR-133b in NSCLC
cells
The aforementioned results identified the
correlation between dysregulated glycolysis, the expression of
miR-133b and radiation resistance. To investigate the possible
association between miR-133b and glycolysis, miRNA databases were
searched for potential miR-133b targets that may contribute to the
regulation of glycolysis. Results from miRBase (http://www.mirbase.org/) indicated that PKM2 may
function as a target for miR-133b, and that the 3′-UTR of PKM2
contains a highly-conserved binding site for miR-133b. To determine
whether PKM2 is the target gene of miR-133b, the protein expression
level of PKM2 in the A549 cell line was analyzed in response to the
overexpression or inhibition of miR-133b (Fig. 3A and B left). Reduced PKM2 expression
in cells transfected with miR-133b was observed (Fig. 3A), as was increased PKM2 levels
following the inhibition of miR-133b expression (Fig. 3B), indicating that miR-133b may
suppress the glycolysis of lung cancer cells by targeting PKM2.
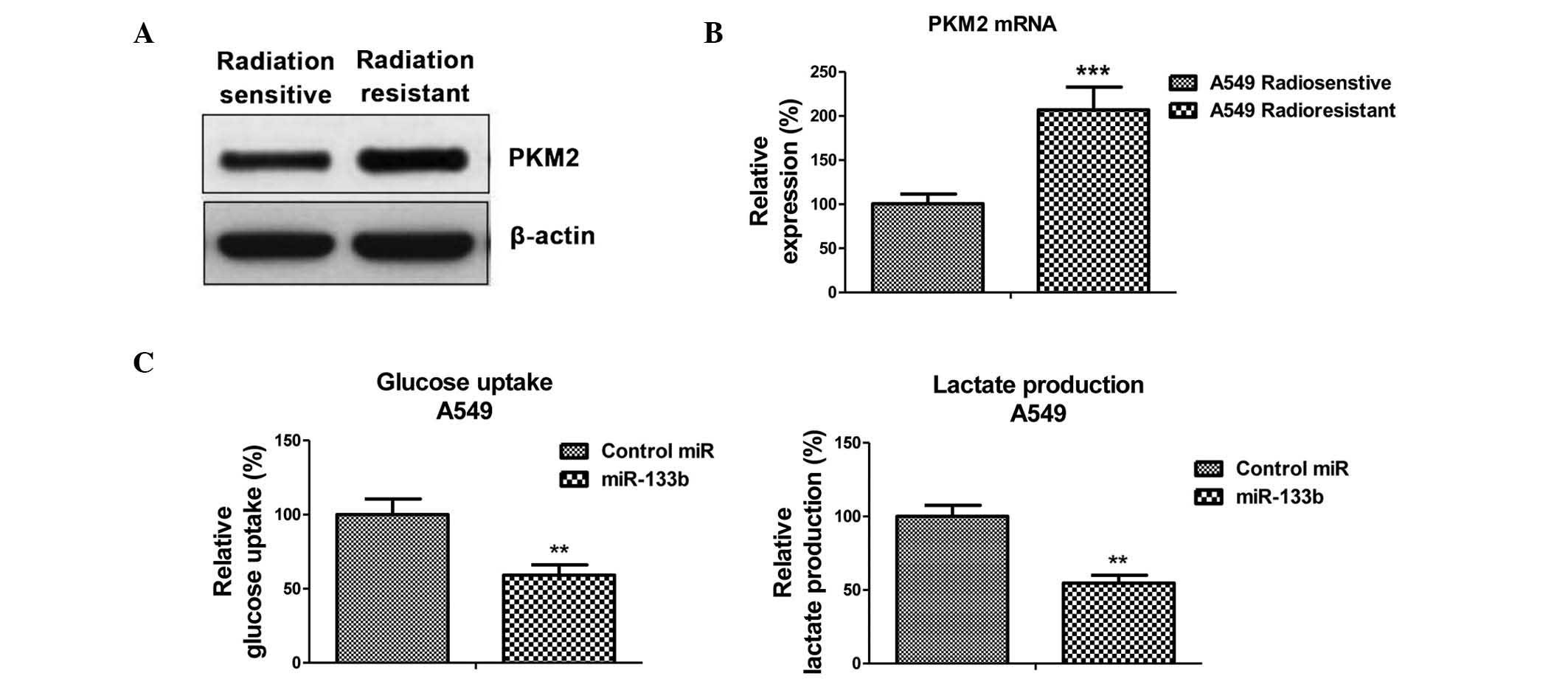
Radioresistant A549 cells exhibit
elevated PKM2 expression
To verify whether the inhibited PKM2 expression,
caused by miR-133b, is the mechanism for the radiosensitivity
observed in lung cancer cells, the expression of PKM2 at the
protein and mRNA levels in radiosensitive and -resistant cells was
measured. As expected, PKM2 was upregulated in radioresistant cells
when compared with sensitive cells (Fig.
4A and B), suggesting that the upregulation of glycolysis in
radioresistant cells is subsequent to the upregulation of PKM2.
Overexpression of miR-133b
resensitizes A549 radioresistant cells to irradiation through
targeting on PKM2
The present study examined the glycolysis rate
through the overexpression of miR-133b in the A549 cells. The
results, presented in Fig. 4C,
demonstrated that miR-133b negatively regulated glucose uptake and
lactate product, thus the overexpression of miR-133b significantly
suppressed glucose metabolism. To investigate whether the
overexpression of miR-133b in lung cancer cells could resensitize
radioresistant cells to irradiation through the inhibition of PKM2,
pre-miR-133b was transfected with the overexpression vector
containing wild-type PKM2 or a control vector and was added to the
A549 radioresistant cells. The exogenous overexpression of PKM2
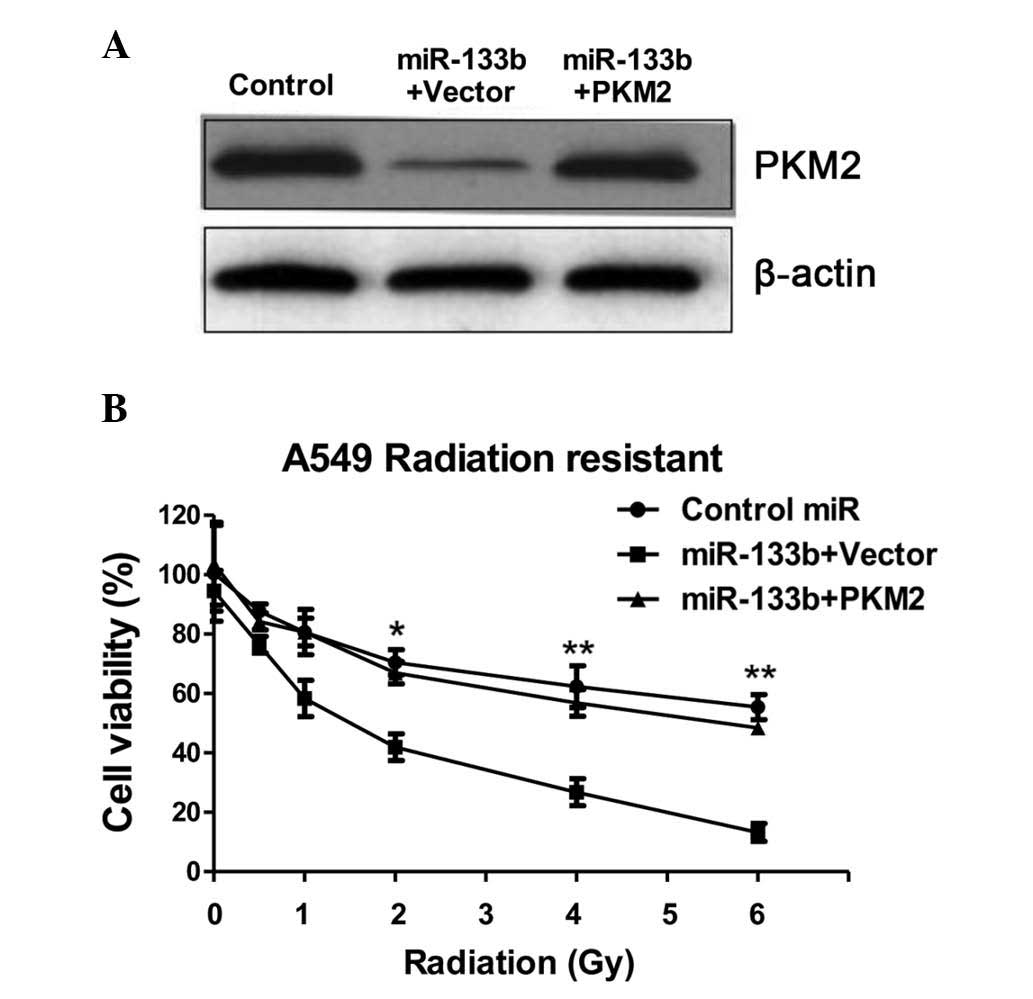
restored the PKM2 to the initial level (Fig. 5A), indicating that the activities of
glycolysis may be recovered by the overexpression of PKM2 in
miR-133b-overexpressing cells. Overexpression of miR-133b
significantly sensitized the radioresistant cells to radiation
(Fig. 5B), indicating that miR-133b
may serve as a therapeutic target aiding the the development of
drugs. Additionally, restoration of the activity of glycolysis by
the overexpression of PKM2 in the miR-133b overexpressing cells led
to a significant resistance to irradiation when compared with the
transfection of the control vector in radioresistant cells
(Fig. 5B), suggesting that the
overexpression of miR-133b sensitized lung cancer radioresistant
cells to irradiation by the inhibition of PKM2-mediated
glycolysis.
Discussion
miR-133b has been reported as a tumor suppressor
functioning in numerous types of cancer that demonstrate low
miR-133b expression, as noted in colorectal (13), gastric (14), bladder (15), lung (16), esophageal (17), ovarian (18) and breast (19) cancer. However, the role of miR-133b
within radiosensitivity remains under investigation. During the
present study, a significant downregulation of miR-133b in
radioresistant NSCLC cells was observed. Additionally, it was
demonstrated that miR-133b suppresses glycolysis within lung cancer
cells by targeting PKM2, which is an essential enzyme involved in
glycolysis. As aforementioned, cancer cells exhibit an upregulated
rate of glycolysis when compared with normal cells. Together, the
results of the present study are consistent with those of previous
studies reporting that miR-133b functions as a tumor
suppressor.
Pyruvate kinase catalyzes the production of pyruvate
from phosphoenol pyruvate through a glycolytic cascade (20). Pyruvate kinase has 4 different
isoforms (L, R, M1 and M2), with each isoform expressed in a
tissue-specific manner (21).
Additionally, PKM2 upregulation has been observed in numerous types
of cancer (21). The current study
reported that PKM2 is a target of miR-133b. Notably, the expression
of PKM2 was correlated with radioresistance in the assessed lung
cancer cells. Radioresistant lung cancer cells demonstrated
upregulated PKM2 expression, indicating that PKM2 may serve as a
target to overcome radiation resistance.
The altered energy metabolism of cancer cells has
recently been studied and recognized as a novel hallmark of cancer
(7). Glucose metabolism in cancer
cells is primarily characterized by two major biochemical events:
i) The increased uptake of glucose and ii) the increased production
of lactate. To the best of our knowledge, the present study is the
first to demonstrate that miR-133b significantly suppresses glucose
metabolism within lung cancer cells, identifying a novel mechanism
of miR-133b as a tumor suppressor. It has been reported that the
increased accumulation of lactate may act as an anti-oxidant in
cancer cells and contribute to radioresistance (22). The results of the current study
demonstrated that radioresistant lung cancer cells have increased
levels of lactate product, which may be the mechanism underlying
radioresistance. However, further understanding is required with
regard to the signaling pathway and molecular mechanisms. In
summary, a novel function of miR-133b associated with the
radiosensitivity of lung cancer cells was identified. Furthermore,
it was observed that PKM2 serves as a target of miR-133b, and the
glucose metabolism of lung cancer cells was positively correlated
with radioresistance. Overexpression of miR-133b resensitized
radioresistant lung cancer cells through the inhibition of
PKM2-mediated glycolysis. The present study provides further
insight into the cellular and molecular mechanisms that may be
involved in the exhibition of radiation resistance in NSCLC.
Acknowledgements
The authors would like to thank Dr Yi Li for
providing editorial assistance (Department of Internal Medicine,
Tianjin Huanhu Hospital, Tianjin, China).
References
|
1
|
Sechler M, Cizmic AD, Avasarala S, Van
Scoyk M, Brzezinski C, Kelley N, Bikkavilli RK and Winn RA:
Non-small-cell lung cancer: Molecular targeted therapy and
personalized medicine - drug resistance, mechanisms, and
strategies. Pharmgenomics Pers Med. 6:25–36. 2013.PubMed/NCBI
|
|
2
|
Doebele RC, Pilling AB, Aisner DL,
Kutateladze TG, Le AT, Weickhardt AJ, Kondo KL, Linderman DJ,
Heasley LE, Franklin WA, et al: Mechanisms of resistance to
crizotinib in patients with ALK gene rearranged non-small cell lung
cancer. Clin Cancer Res. 18:1472–1482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang A: Chemotherapy, chemoresistance and
the changing treatment landscape for NSCLC. Lung Cancer. 71:3–10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Willers H, Azzoli CG, Santivasi WL and Xia
F: Basic mechanisms of therapeutic resistance to radiation and
chemotherapy in lung cancer. Cancer J. 19:200–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimura T, Noma N, Sano Y, Ochiai Y,
Oikawa T, Fukumoto M and Kunugita N: AKT-mediated enhanced aerobic
glycolysis causes acquired radioresistance by human tumor cells.
Radiother Oncol. 112:302–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Y, Butler EB and Tan M: Targeting
cellular metabolism to improve cancer therapeutics. Cell Death Dis.
4:e5322013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng T, Wang J, Chen X and Liu L: Role of
microRNA in anticancer drug resistance. Int J Cancer. 126:2–10.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma J, Dong C and Ji C: MicroRNA and drug
resistance. Cancer Gene Ther. 17:523–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gwak HS, Kim TH, Jo GH, Kim YJ, Kwak HJ,
Kim JH, Yin J, Yoo H, Lee SH and Park JB: Silencing of microRNA-21
confers radio-sensitivity through inhibition of the PI3K/AKT
pathway and enhancing autophagy in malignant glioma cell lines.
PLoS One. 7:e474492012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin CW, Li XR, Zhang Y, Hu G, Guo YH, Zhou
JY, Du J, Lv L, Gao K, Zhang Y and Deng H: TAp63 suppress
metastasis via miR-133b in colon cancer cells. Br J Cancer.
110:2310–2320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Huang J, Zhang L, Qu Y, Li J, Yu
B, Yan M, Yu Y, Liu B and Zhu Z: MiR-133b is frequently decreased
in gastric cancer and its overexpression reduces the metastatic
potential of gastric cancer cells. BMC Cancer. 14:342014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen XN, Wang KF, Xu ZQ, Li SJ, Liu Q, Fu
DH, Wang X and Wu B: MiR-133b regulates bladder cancer cell
proliferation and apoptosis by targeting Bcl-w and Akt1. Cancer
Cell Int. 14:702014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crawford M, Batte K, Yu L, Wu X, Nuovo GJ,
Marsh CB, Otterson GA and Nana-Sinkam SP: MicroRNA 133B targets
pro-survival molecules MCL-1 and BCL2L2 in lung cancer. Biochem
Biophys Res Commun. 388:483–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kano M, Seki N, Kikkawa N, Fujimura L,
Hoshino I, Akutsu Y, Chiyomaru T, Enokida H, Nakagawa M and
Matsubara H: miR-145, miR-133a and miR-133b: Tumor-suppressive
miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J
Cancer. 127:2804–2814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai A, Sun H, Fang T, Zhang Q, Wu S, Jiang
Y, Ding L, Yan G and Hu Y: MicroRNA-133b stimulates ovarian
estradiol synthesis by targeting Foxl2. FEBS Lett. 587:2474–2482.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen L, Li J, Xu L, Ma J, Li H, Xiao X,
Zhao J and Fang L: miR-497 induces apoptosis of breast cancer cells
by targeting Bcl-w. Exp Ther Med. 3:475–480. 2012.PubMed/NCBI
|
|
20
|
Wong N, Ojo D, Yan J and Tang D: PKM2
contributes to cancer metabolism. Cancer Lett. 356:184–191. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Israelsen WJ, Dayton TL, Davidson SM,
Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW,
et al: PKM2 isoform-specific deletion reveals a differential
requirement for pyruvate kinase in tumor cells. Cell. 155:397–409.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koukourakis MI, Giatromanolaki A,
Panteliadou M, Pouliliou SE, Chondrou PS, Mavropoulou S and
Sivridis E: Lactate dehydrogenase 5 isoenzyme overexpression
defines resistance of prostate cancer to radiotherapy. Br J Cancer.
110:2217–2223. 2014. View Article : Google Scholar : PubMed/NCBI
|