Introduction
In 2012, an estimated 338,000 novel cases of renal
cancer were diagnosed worldwide (1).
Clinical stage I disease accounts for ~70% of newly identified
renal tumors, the majority of which are small renal tumors of ≤4 cm
diameter (T1a) (2,3). A 5-year cancer-specific survival rate of
>95% has been demonstrated in clinical T1a disease following the
administration of optimal treatments, including partial nephrectomy
and radiofrequency ablation (4). The
detection rate of renal tumors has increased due to the widespread
use of imaging techniques, such as ultrasonography and computed
tomography (CT). These imaging modalities enable the clinical
diagnosis of almost all tumors pre-operatively. However, the
incidence rate of benign lesions among clinical cases of presumed
T1a renal cell carcinoma (RCC) in surgical specimens ranges from
7.1–19.5% (5–8). It is difficult to distinguish benign
small renal lesions, including oncocytoma, angiomyolipoma (AML) and
compromised cysts, from clinical T1a RCC by routine examinations
prior to surgery (5–8).
CT remains the most widely used and single most
effective modality for the staging of RCC (9). However, there has been a recent trend
toward the performance of detailed pre-operative examinations using
magnetic resonance (MR) imaging for small renal masses. The small
amounts of fat in renal masses may be detected in MR imaging, and
small cystic renal masses may be better categorized in MR imaging
compared with CT (10–13). Combined pre-operative imaging analysis
for small renal masses may lead to a correct pathological
diagnosis. However, there have been few previous studies regarding
the correspondence rate of the diagnosis between clinical
pre-operative imaging and pathological findings.
The present study was performed to determine the
incidence of benign pathological findings for small renal masses
diagnosed as clinical T1a RCC prior to surgery, and to
retrospectively analyze the utility of pre-operative CT and MR
imaging of benign pathological lesions.
Patients and methods
Patients and treatment
Between January 1998 and December 2011, 196 cases of
sporadic renal tumors, with a diameter of ≤4 cm determined on CT
and/or MR imaging for pre-operative evaluation, were surgically
treated as clinical T1a RCC at the Department of Integrative Cancer
Therapy and Urology, Kanazawa University Graduate School of Medical
Science, Kanazawa, Japan. Cases with bilateral and/or multiple
lesions or von Hippel-Lindau disease were excluded from this
study.
Pre-operative evaluation
Pre-operative evaluations, including blood a test,
chest and abdominal CT with dynamic scan, and bone scintigraphy,
were performed. MR imaging was performed in selected patients. CT
and MR imaging findings were reviewed by two radiologists, and
urologists made a diagnosis based on their reports and clinical
findings.
Treatment and post-operative
analysis
All patients underwent radical nephrectomy (RN) or
nephron-sparing surgery (NSS). Pathological stage, Fuhrman nuclear
grade (14) and histological subtype
were assessed according to the Heidelberg classification (15) and the 2002 American Joint Committee on
Cancer version of the tumor-node-metastasis staging system
(16). Pathological diagnosis was
made by various immunohistological procedures, including
cytokeratin AE1/AE3, 7 and CD10 staining, for the selected cases in
which it was difficult to distinguish between RCC and benign
lesions based on pathology.
The patients' medical records were reviewed, and the
rate of correspondence with the pre-operative diagnosis was
calculated. The pre-operative imaging results for benign small
renal lesions were retrospectively examined on pathological
reports.
In our previous study, of the 196 eligible cases in
the present study, the clinical outcomes of 105 cases with cT1aN0M0
renal cell carcinoma were examined, and it was demonstrated that
disease recurrence occurred in 6 patients (5.7%) during a mean
postoperative follow-up period of 41.7 months (17).
In this analysis, the principles of the Helsinki
Declaration were followed, and all patients provided written
informed consent with guarantees of confidentiality. The present
study was approved by the ethics committee of Kanazawa University
(Kanazawa, Japan).
Statistical analysis
Statistical analyses were performed using
commercially available software (GraphPad Prism; GraphPad Software,
Inc., La Jolla, CA, USA). Comparisons between two groups were
performed by Students unpaired t-test and Fisher's exact test. In
all analyses, P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical characteristics of
pre-operatively diagnosed cT1a renal cell carcinoma patients
A pre-operative diagnosis of clinical T1a RCC was
made in 196 cases. Of these 196 tumors, RCC was detected in 183
cases (93.37%) and benign pathological lesions in 13 cases (6.63%)
(Table I). The benign cases were
pathologically diagnosed as AML (n=4; 2.04%), oncocytoma (n=3;
1.53%), renal cysts (n=4; 2.04%), xanthogranulomatous
pyelonephritis (n=1; 0.51%) and leiomyoma (n=1; 0.51%). There were
no significant associations between the age of the patient and the
pathological findings. The benign pathological lesions were found
in 11 female and 2 male patients, and the correlation between
gender and benign pathological findings was statistically
significant (P<0.05). The mean diameter of the benign
pathological lesions was 19.5 mm (range, 12–28 cm), and the
incidences of benign pathological lesions with a diameter of ≤20 mm
and with tumors >20 mm in diameter were 12.7 and 3.2%,
respectively. The tumor diameters of the benign lesions were
significantly smaller than those of RCC. In terms of surgical
procedure, the rate of NSS was significantly higher than RN in the
benign pathological lesions.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variables | RCC | Benign | P-value |
|---|
| Total patients,
n | 183 | 13 |
|
| Mean age, years | 62.8 | 60.7 | 0.5631a |
| Gender, n |
|
|
|
| Male | 129 | 2 | 0.0002b |
|
Female | 54 | 11 |
|
| Diameter of tumor,
mm |
|
|
|
| Mean | 24.7 | 19.5 | 0.0319a |
| ≤20,
n | 62 | 9 | 0.0236b |
| >20,
n | 121 | 4 |
|
| Surgery, n |
|
|
|
| RN | 105 | 2 | 0.0080b |
| NSS | 78 | 11 |
|
Diagnostic accuracy of pre-operative
imaging findings
Table II shows the
accuracy of pre-operative CT and MR imaging findings according to
tumor diameter. All patients underwent CT scans, and 152 (77.55%)
of the total of 196 patients underwent MR imaging prior to surgery.
CT and MR imaging findings were obtained for all 9 of the benign
pathological lesions that were ≤20 mm in diameter; however, MR
imaging was performed in only 1 case with tumors >20 mm in
diameter. The diagnostic accuracies of pre-operative CT and MR
imaging findings of the renal masses ≤20 mm in diameter were
significantly lower than those of masses >20 mm in diameter.
 | Table II.Accuracy of pre-operative CT and MR
imaging findings according to tumor diameter. |
Table II.
Accuracy of pre-operative CT and MR
imaging findings according to tumor diameter.
|
| Tumor diameter,
mm |
|
|---|
|
|
|
|
|---|
| Variables | ≤20 | >20 | P-value |
|---|
| Total patients,
n | 71 | 125 |
|
| Pre-operative CT, n
(%) |
|
|
|
| Images
obtained | 71
(100.0) | 125
(100.0) |
|
|
Accuracy | 62 (87.3) | 121 (96.8) | 0.0236a |
| Pre-operative MR
imaging, n (%) |
|
|
|
| Images
obtained | 59 (83.1) | 93
(74.4) |
|
|
Accuracy | 50 (84.7) | 92
(97.8) | 0.0019a |
Pre-operative imaging findings of
benign renal masses
In the retrospective CT and MR imaging analysis,
none of the 9 benign pathological lesions with a diameter of ≤20 mm
could be distinguished from RCC. A total of 3 cases of AML had no
identifiable macroscopic fat on pre-operative CT and MR imaging.
Another 3 cases of renal cysts classified as category III according
to the Bosniak renal cyst classification system (12,18) showed
enhancement characteristics of septa on contrast-enhanced CT and MR
imaging. In 2 cases of oncocytoma and 1 case of leiomyoma, benign
pathological lesions could not be distinguished from RCC by
retrospective image analysis.
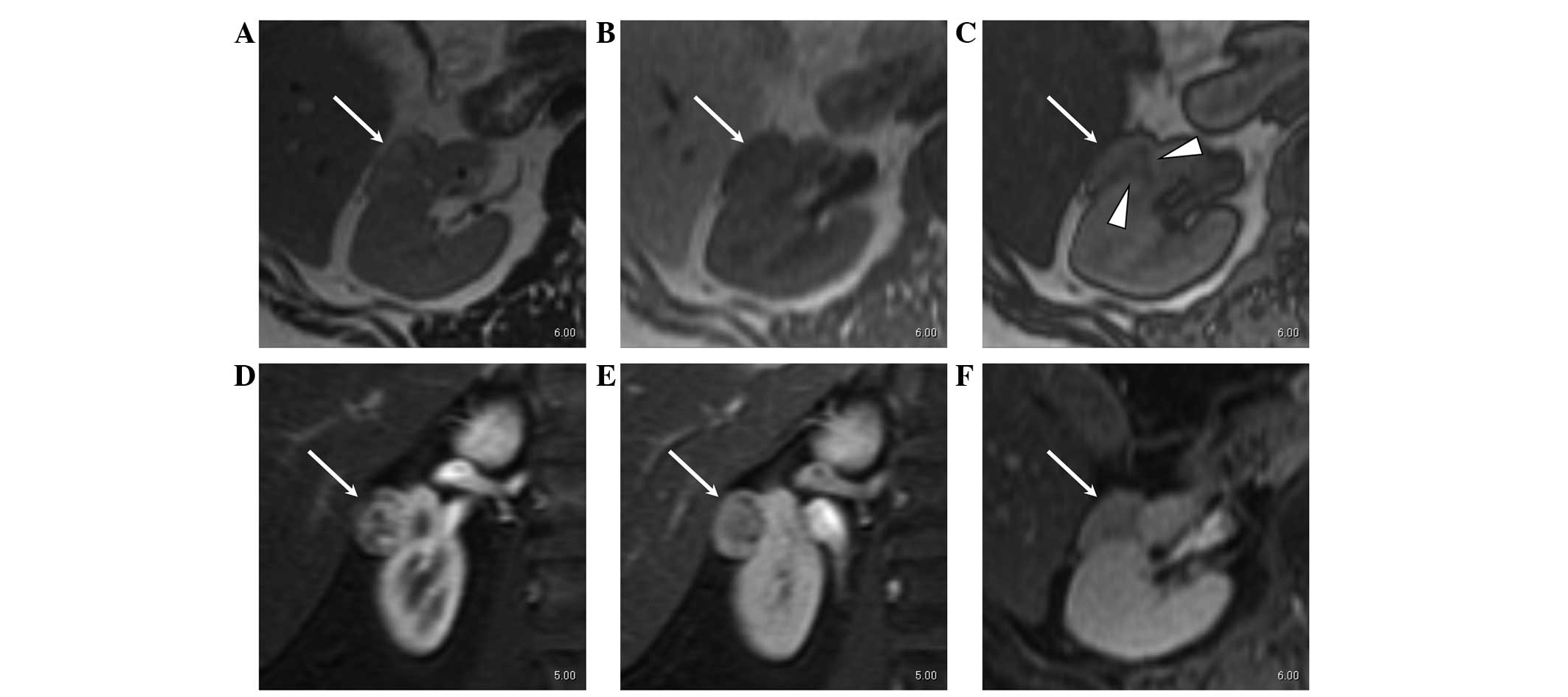
With regard to the 4 benign renal masses with a
diameter of >20 mm, 1 case that underwent pre-operative MR
imaging was AML. Small areas of low attenuation in the renal mass
were found on CT and MR imaging; however, the mass showed marked
contrast enhancement in the arterial phase and washout of contrast
medium in the late phase suggesting RCC (Fig. 1). The other three cases without
pre-operative MR imaging were a hemorrhagic cyst, oncocytoma and
xanthogranulomatous pyelonephritis. Differentiation between these
tumors and RCC based on pre-operative CT findings was impossible
even on retrospective evaluation.
Discussion
The incidence rates of benign pathological lesions
at nephrectomy for presumed small RCC have been reported previously
(5–8,19–23). Various incidence rates were reported,
and differences were indicated based on ethnicity. The reported
incidences of benign pathological lesions with a tumor diameter of
≤40 mm were 17.3–23.4% in studies from Western countries (5,6,19–22) and
7.1–15.0% in studies from Asian countries (7,8,23). Fujii et al suggested that one
possible reason for the lower incidence of benign pathological
findings in Asian than Western countries was the low incidence rate
of oncocytoma in Asia (7), which is
indistinguishable from RCC using the present imaging modalities
(24). The reported incidence rates
of oncocytoma in small renal masses were 5.7–10.7% in Western
countries (5,6,19–22) but 1.3–2.8% in Asian countries
(7,8,23),
consistent with the results of the present study (1.5%). Although
further studies in various countries are required, these
observations suggest that there may be ethnic differences in the
oncocytoma incidence rate.
In the present series, the incidence rate of benign
pathological lesions for presumed clinical T1a RCC was 6.63%, which
was lower than that in previous studies, even considering the low
incidence of oncocytoma. The lower incidence of benign pathological
findings in the present series may have been due to the high rate
(77.6%) of pre-operative MR imaging. In previous studies regarding
the incidence rate of benign lesions in presumed clinical T1a RCC,
the rates of pre-operative MR imaging were low (32%) (7) or not described (5,6). MR
imaging has advantages with regard to detecting small amounts of
fat in small renal masses (10–13), which
may aid in distinguishing AMLs from RCCs.
With regard to the correlation between the clinical
characteristics of the patients and pathological features in
surgically resected specimens from cases with small renal masses,
tumor size appears to be the strongest predictor of malignant
pathological features. Previous studies indicated that 12.4–30.0%
of tumors smaller than 2 cm were benign compared with 4.8–20.9% of
those larger than 2 cm in cases with small renal masses presumed to
be T1a RCC (5,6,19–23). Furthermore, the incidence of benign
pathological lesions in the renal tumors of ≤20 mm in diameter was
significantly higher (12.7%) than in tumors >20 mm (3.9%) in the
present series.
The incidence of small benign pathological lesions
may depend on the accuracy of pre-operative screening by the
radiological approach. A recent study suggested that the accuracy
of radiological examination for nodules <2 cm in diameter must
be improved due to the low incidence of benign lesions in resected
suspicious renal masses with diameters >2 cm (8). Although there have been no studies
regarding the accuracy of pre-operative radiological screening for
small renal masses, in the present series, the diagnostic accuracy
of pre-operative CT and MR imaging of the renal masses ≤20 mm in
diameter were significantly lower than those of the masses >20
mm in diameter. Moreover, benign pathological lesions of ≤20 mm in
diameter could not be distinguished from RCC in retrospective CT
and MR imaging analysis. There may be a limitation of imaging
modalities for the pre-operative diagnosis of several small renal
masses with diameters of ≤20 mm, such as AML without identifiable
macroscopic fat on pre-operative imaging (25) and category III renal cysts with the
enhancement characteristics of septa on contrast-enhanced CT and MR
imaging (12,18).
Analysis of false-negative cases, i.e., pathological
T1a RCC clinically diagnosed as benign lesions by pre-operative CT
and MR imaging, could not be performed in the present study. This
was one of the limitations of this study, and there may have been
cases of small RCC missed or followed up as benign disease without
pathological diagnosis. There have been few reports regarding such
cases, and further challenging issues must be resolved by the
development of improved methodologies for percutaneous renal biopsy
and molecular pathological analyses (26–28).
In conclusion, in the present study pre-operative CT
and MR findings demonstrated high diagnostic accuracy for renal
masses >20 mm in diameter, however, pre-operative imaging for
small renal masses, particularly those of ≤20 mm in diameter, was
limited. Therefore, these findings may be useful for the
preoperative diagnosis of RCC in clinical practice.
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0. Cancer Incidence and Mortality
Worldwide: IARC Cancer Base No. 11. http://globocan.iarc.frAccessed. November
25–2015
|
|
2
|
Laguna MP, Algaba F, Cadeddu J, Clayman R,
Gill I, Gueglio G, Hohenfellner M, Joyce A, Landman J, Lee B and
van Poppel H: Clinical Research Office of the Endourological
Society Renal Mass Study: Current patterns of presentation and
treatment of renal masses: A clinical research office of the
endourological society prospective study. J Endourol. 28:861–870.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
King SC, Pollack LA, Li J, King JB and
Master VA: Continued increase in incidence of renal cell carcinoma,
especially in young patients and high grade disease: United States
2001 to 2010. J Urol. 191:1665–1670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang X, Liu T, Zhang F, Ji C, Zhao X,
Wang W and Guo H: Radiofrequency ablation versus partial
nephrectomy for clinical T1a renal-cell carcinoma: Long-term
clinical and oncologic outcomes based on a propensity score
analysis. J Endourol. 29:518–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Remzi M, Özsoy M, Klingler HC, Susani M,
Waldert M, Seitz C, Schmidbauer J and Marberger M: Are small renal
tumors harmless? Analysis of histopathological features according
to tumors 4 cm or less in diameter. J Urol. 176:896–899. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pahernik S, Ziegler S, Roos F, Melchior SW
and Thüroff JW: Small renal tumors: Correlation of clinical and
pathological features with tumor size. J Urol. 178:414–417;
discussion 416–417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujii Y, Komai Y, Saito K, Iimura Y,
Yonese J, Kawakami S, Ishikawa Y, Kumagai J, Kihara K and Fukui I:
Incidence of benign pathologic lesions at partial nephrectomy for
presumed RCC renal masses: Japanese dual-center experience with 176
consecutive patients. Urology. 72:598–602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soga N, Nishikawa K, Takaki H, Yamada Y,
Arima K, Hayashi N and Sugimura Y: Low incidence of benign lesions
in resected suspicious renal masses greater than 2 cm:
Single-center experience from Japan. Int J Urol. 19:729–734. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sheth S, Scatarige JC, Horton KM, Corl FM
and Fishman EK: Current concepts in the diagnosis and management of
renal cell carcinoma: Role of multidetector ct and
three-dimensional CT. Radiographics. 21(suppl_1): S237–S254. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hecht EM, Israel GM, Krinsky GA, Hahn WY,
Kim DC, Belitskaya-Levy I and Lee VS: Renal masses: Quantitative
analysis of enhancement with signal intensity measurements versus
qualitative analysis of enhancement with image subtraction for
diagnosing malignancy at MR imaging. Radiology. 232:373–378. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ho VB, Allen SF, Hood MN and Choyke PL:
Renal masses: Quantitative assessment of enhancement with dynamic
MR imaging. Radiology. 224:695–700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Israel GM, Hindman N and Bosniak MA:
Evaluation of cystic renal masses: Comparison of CT and MR imaging
by using the Bosniak classification system. Radiology. 231:365–371.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Silverman SG, Mortele KJ, Tuncali K,
Jinzaki M and Cibas ES: Hyperattenuating renal masses: Etiologies,
pathogenesis, and imaging evaluation. Radiographics. 27:1131–1143.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Furman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. AM J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kovacs G, Akhtar M, Beckwith BJ, Bugert P,
Cooper CS, Delahunt B, Eble JN, Fleming S, Ljungberg B, Medeiros
LJ, et al: The Heidelberg classification of renal cell tumours. J
Pathol. 183:131–133. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: AJCC Cancer Staging Manual (6th).
Springer Science and Business Media. New York, NY: 2002. View Article : Google Scholar
|
|
17
|
Kitagawa Y, Nakashima K, Shima T, Izumi K,
Narimoto K, Miwa S, Miyagi T, Maeda Y, Kadono Y, Konaka H, et al:
Clinicopathological outcomes of clinical T1a renal cell carcinoma
by tumor size. Jpn J Clin Oncol. 41:637–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Israel GM and Bosniak MA: An update of the
Bosniak renal cyst classification system. Urology. 66:484–488.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Snyder ME, Bach A, Kattan MW, Raj GV,
Reuter VE and Russo P: Incidence of benign lesions for clinically
localized renal masses smaller than 7 cm in radiological diameter:
Influence of sex. J Urol. 176:2391–2395; discussion 2395–2396.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duchene DA, Lotan Y, Cadeddu JA,
Sagalowsky AI and Koeneman KS: Histopathology of surgically managed
renal tumors: Analysis of a contemporary series. Urology.
62:827–830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schlomer B, Figenshau RS, Yan Y, Venkatesh
R and Bhayani SB: Pathological features of renal neoplasms
classified by size and symptomatology. J Urol. 176:1317–1320;
discussion 1320. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Frank I, Blute ML, Cheville JC, Lohse CM,
Weaver AL and Zincke H: Solid renal tumors: An analysis of
pathological features related to tumor size. J Urol. 170:2217–2220.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong YH, Zhang ZL, Li YH, Liu ZW, Hou GL,
Liu Q, Yun JP, Zhang XQ and Zhou FJ: Benign pathological findings
in 303 Chinese patients undergoing surgery for presumed localized
renal cell carcinoma. Int J Urol. 17:517–521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marhuenda A, Martín MI, Deltoro C, Santos
J and Briones Rubio J: Radiologic evaluation of small renal masses
(I): Pretreatment management. Adv Urol. 415848:4158482008.
|
|
25
|
Prasad SR, Surabhi VR, Menias CO, Raut AA
and Chintapalli KN: Benign renal neoplasms in adults:
Cross-sectional imaging findings. AJR Am J Roentgenol. 190:158–164.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lane BR, Samplaski MK, Herts BR, Zhou M,
Novick AC and Campbell SC: Renal mass biopsy - a renaissance? J
Urol. 179:20–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Volpe A, Kachura JR, Geddie WR, Evans AJ,
Gharajeh A, Saravanan A and Jewett MA: Techniques, safety and
accuracy of sampling of renal tumors by fine needle aspiration and
core biopsy. J Urol. 178:379–386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Izumi K, Narimoto K, Sugimoto K, Kobori Y,
Maeda Y, Mizokami A, Koh E, Yamada T, Yano S and Namiki M: The role
of percutaneous needle biopsy in differentiation of renal tumors.
Jpn J Clin Oncol. 40:1081–1086. 2010. View Article : Google Scholar
|