Introduction
Breast cancer is the most frequently diagnosed
cancer and the leading cause of cancer-associated mortality among
females (1,2), accounting for 23% of the total cancer
cases and 14% of cancer mortalities (3). Chemotherapy serves an important role in
the treatment of breast cancer. However, resistance to
chemotherapeutic agents, in particular, multi-drug resistance
(MDR), is an major cause of treatment failure in cancer. The MDR
mechanisms are complicated, involving increased drug efflux,
reduced drug uptake, altered metabolism of drugs, altered
expression of drug targets, reduced affinity of drug targets,
activation of the detoxification system, enhanced repair of
drug-induced defects and blocked apoptosis (4). Hence, identification of novel and
effective agents that reverse drug resistance in breast cancer is
urgently needed.
Pristimerin is a quinonemethide triterpenoid
compound isolated from Celastraceae and Hippocrateaceae and has
long been used as anti-inflammatory, antioxidant, antimalarial and
insecticidal agents (5). In addition,
it has been reported that pristimerin has promising clinical
potential as both a therapeutic and chemopreventive agent for
various types of cancer such as pancreatic cancer, glioma,
leukemia, cervical cancer, prostate cancer and breast cancer
(6–11). Pristimerin has been shown to induce
cell death via a number of several mechanisms, including proteosome
inhibition, caspase activation, inhibition of cell cycle
progression, and suppression of antiapoptotic nuclear factor kappa
B (NF-κB) and Akt signaling pathways (6,9). In breast
cancer, it has been reported that pristimeirin induces apoptotic
cell death in MDA-MB-231 breast cancer cells in a caspase-dependent
manner (11). In addition,
prestimerin has been demonstrated to inhibit the migration and
invasion of breast cancer cells (12). However, to the best of our knowledge,
the function of pristimerin on chemoresistance in human breast
cancer has not yet been investigated.
The present study aims to address whether
pristimerin can reverse chemoresistance in human breast cancer
cells. The results demonstrated that pristimerin indeed suppressed
the proliferation of ADR-resistant MCF-7/ADR breast cancer cells,
and that these effects occurred through the suppression of Akt
signaling, which in turn led to the downregulation of antiapoptotic
effectors and increased apoptosis. These findings indicate that
pristimerin may have potential in the treatment of ADR-resistant
breast cancer.
Materials and methods
Chemicals
Pristimerin and ADR were obtained from Sigma-Aldrich
(St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO) to
give a stock solution of 1.0 mM and stored at −20°C in small
aliquots.
Cell culture
MCF-7 and MCF-7/ADR human breast cancer cells were
purchased from Nanjing KeyGen Biotech. Co. Ltd. (Nanjing, China).
The cells were grown in Dulbecco's modified Eagle medium
(Invitrogen™; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (HyClone; GE Healthcare
Life Sciences, Logan, UT, USA). MCF-7/ADR cells were cultured in
the above medium containing 1.0 µM ADR in order to maintain the
ADR-resistant phenotype, upon treatment with or without
pristimerin.
Cell viability analysis
Cells were seeded in 96-well flat-bottom plates at a
density of 1×104 cells per well and cultured in a
humidified incubator for 24 h in the absence or presence of the
indicated concentrations of pristimerin and 1.0 µM ADR for
additional 48 h. Cell viability was measured by using the MTS assay
as described previously (13). The
concentration of drug that inhibited cell survival by 50% (IC50)
was calculated by Bliss's software (14). The resistance factor (RF) was
determined from the ratio of the IC50 for MCF-7/ADR to the IC50 for
the sensitive cell line MCF-7. The data are presented as mean ±
standard deviation from 3 independent experiments.
Annexin V-FITC (fluorescein
isothiocyanate)/propidium iodide (PI) staining assay
MCF-7/ADR cells (2×105 cells/well) were
seeded in 60 mm plates and cultured in a humidified incubator for
24 h before treatment with the indicated concentrations (0, 0.5 or
1.0 µM) of pristimerin and 1.0 µM ADR for an additional 8 h.
Apoptosis was determined by flow cytometry using Annexin V-FITC
Apoptosis Detection Kit II (BD Pharmingen, San Diego, CA, USA), as
described previously (13), and a BD
FACSCanto™ II (BD Biosciences, San Jose, CA, USA).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
MCF-7/ADR cells were plated at a density of
1×105 cells per well in 24-well flat-bottom plates,
treated with 1.0 µM pristimerin and 1.0 µM ADR for 24 h. The TUNEL
assay for in situ detection of apoptosis was performed by
using the DeadEnd™ Fluorometric TUNEL System assay kit (Promega
Corporation, Madison, WI, USA) as previously reported (13).
Caspase activity assay
After treatment of pristimerin with the indicated
concentrations (0, 0.5 or 1.0 µM) and 1.0 µM ADR for 24 h, activity
of caspase-8 and-9 was measured using a Caspase Colorimetric Assay
kit (Nanjing KeyGen Biotech. Co. Ltd.) according to the
manufacturer's protocol as described previously (13).
Western blotting analysis
Following treatment with pristimerin and/or 1.0 µM
ADR at various concentrations (0, 0.5 and 1.0 µM) for 48 h, cells
in each dish, including dead cells floating in medium, were
harvested and lysed in 1X sampling buffer (Nanjing KeyGen Biotech.
Co. Ltd.). Protein concentrations of the lysates were determined
using the Pierce Bicinchoninic Acid Protein Assay kit (Thermo
Fisher Scientific, Inc.). An aliquot of the denatured supernatant
containing 30 µg of protein was subjected to 12% sodium dodecyl
sulphate polyacrylamide gel electrophoresis, and subsequently
transferred to polyvinylidene fluoride membranes (Millipore,
Billerica, MA, USA). After blocking with blocking buffer
(Tris-buffered saline containing 5% non-fat milk) for 1 h at room
temperature, the membranes were incubated overnight at 4°C with the
following specific primary antibodies: Monoclonal mouse anti-human
caspase-8 (catalog no. 9746), polyclonal rabbit anti-human
caspase-9 (catalog no. 9502), polyclonal rabbit anti-human PARP
(catalog no. 9542), polyclonal rabbit anti-human p-Akt (Ser473;
catalog no. 9271), polyclonal rabbit anti-human Akt (catalog no.
9272), monoclonal rabbit anti-human p-Bad (Ser136; catalog no.
4366), polyclonal rabbit anti-human Bad (catalog no. 9292),
polyclonal rabbit anti-human Bcl-xL (catalog no. 2762) and
monoclonal mouse anti-human GAPDH (catalog no. 60004-1; dilution,
1:10,000; ProteinTech Group, Inc., Chicago, IL, USA). All the
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA) and were diluted at 1:1,000, unless otherwise
specified. Further incubation with appropriate horseradish
peroxidase-conjugated goat anti-rabbit (catalog. no. SA00001-2;
1:4,000) or goat anti-mouse (catalog no. SA00001-1: 1:4,000) IgG
secondary antibodies (ProteinTech Group, Inc.), depending on the
primary antibody used, was performed for 1 h at room temperature.
Detection of staining signals was performed using the Pierce
Enhanced Chemiluminescence Western Blotting Substrate (Thermo
Fisher Scientific, Inc.).
Statistical analysis
The data given in the text are expressed as the mean
± standard deviation (SD). Comparisons between groups for
statistical significance were carried out with a two-tailed
Student's t-test using SPSS version 17.0 software (SPSS, Inc.,
Chicago, IL, USA). In all cases, P<0.05 was considered to
indicate a statistically significant difference.
Results
Growth inhibition of ADR-resistant
MCF-7 cell by pristimerin
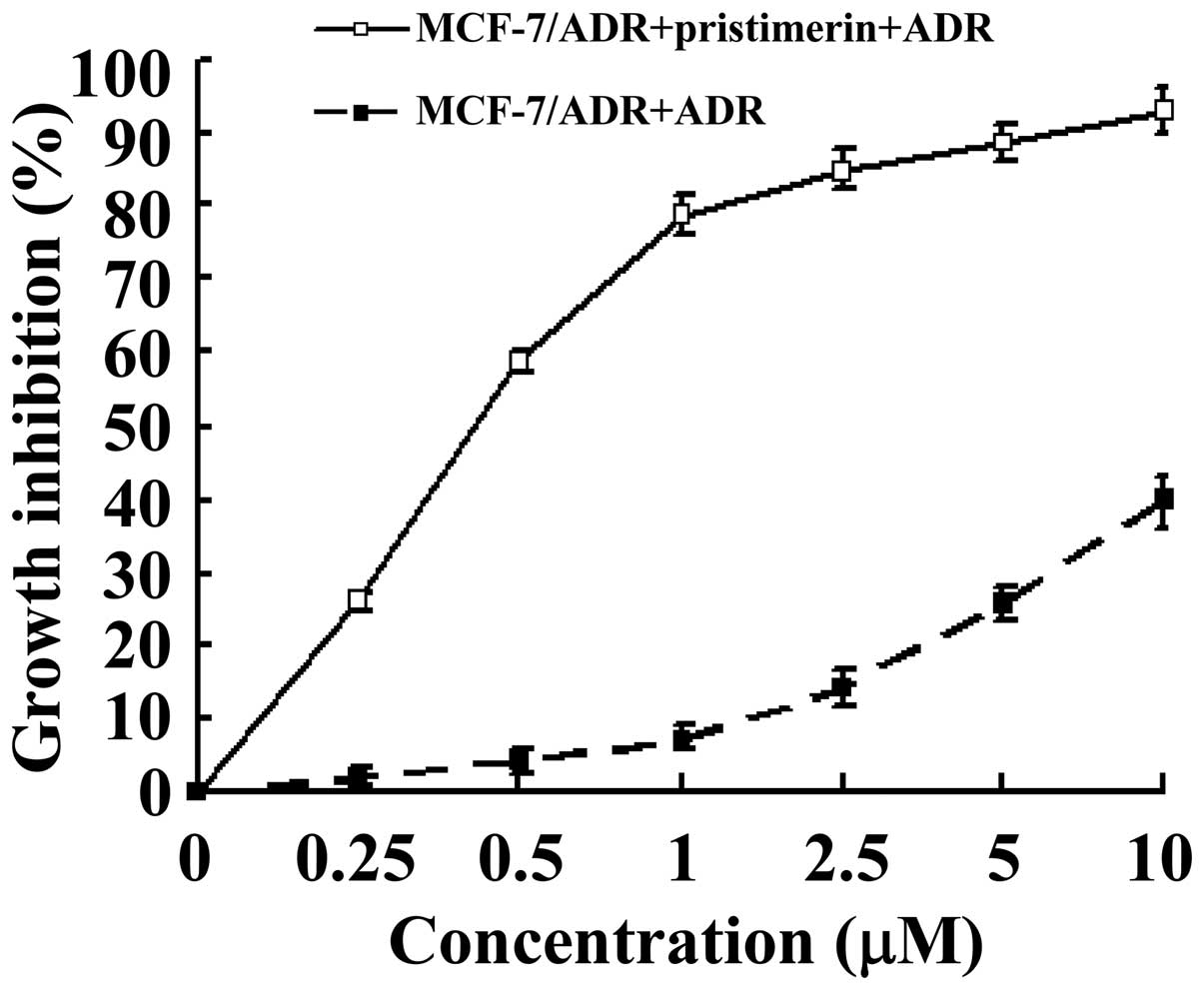
The effect of pristimerin on the growth of the
ADR-resistant human breast cancer cell line MCF-7/ADR and the
parental sensitive MCF-7 cells was investigated using the MTS
assay. After treatment with pristimerin for 48 h, MCF-7/ADR cells
displayed markedly inhibited growth, compared with control cells
treated with vehicle, in a dose-dependent manner (Fig. 1). The calculated IC50 values, i.e. the
concentrations of pristimerin that inhibited cell survival by 50%,
were 0.43 µM for MCF-7/ADR and 0.59 µM for MCF-7, respectively.
Pristimerin reduced the RF from 14.54 to 0.73 (Table I), which revealed that pristimerin
reversed the ADR resistance of MCF-7/ADR cells.
 | Table I.IC50 values of pristimerin
on MCF-7/ADR cells. |
Table I.
IC50 values of pristimerin
on MCF-7/ADR cells.
|
| ADR | Pristimerin |
|---|
|
|
|
|
|---|
| Cell lines | IC50 | RF | IC50 | RF |
|---|
| MCF-7 | 1.12 | – | 0.59 | – |
| MCF-7/ADR | 16.29 | 14.54 | 0.43 | 0.73 |
Pristimerin induced apoptosis in
MCF-7/ADR cells
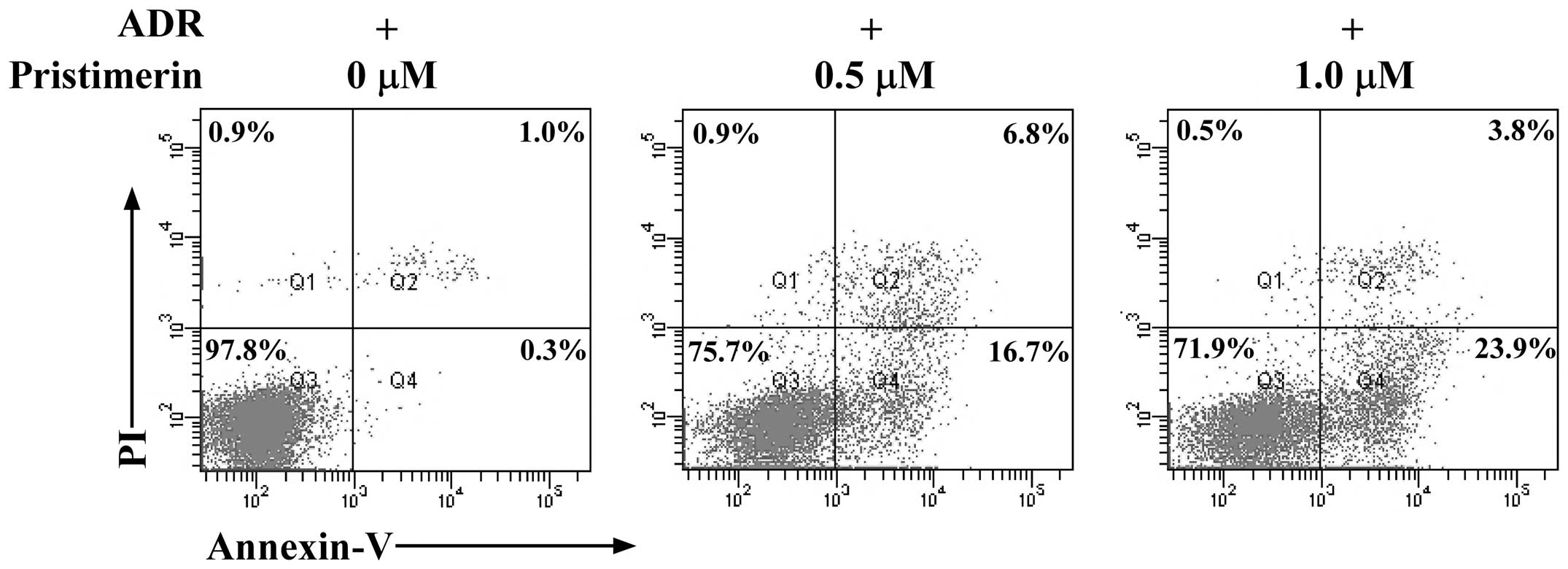
To investigate whether pristimerin was able to
induce apoptosis in MCF-7/ADR cells, an Annexin V-FITC/PI binding
assay was performed. As shown in Fig.
2, after 8 h treatment with pristimerin at 0.5, 1.0 µM, the
percentage of apoptotic MCF-7/ADR cells (Annexin
V+/PI−), markedly increased in a
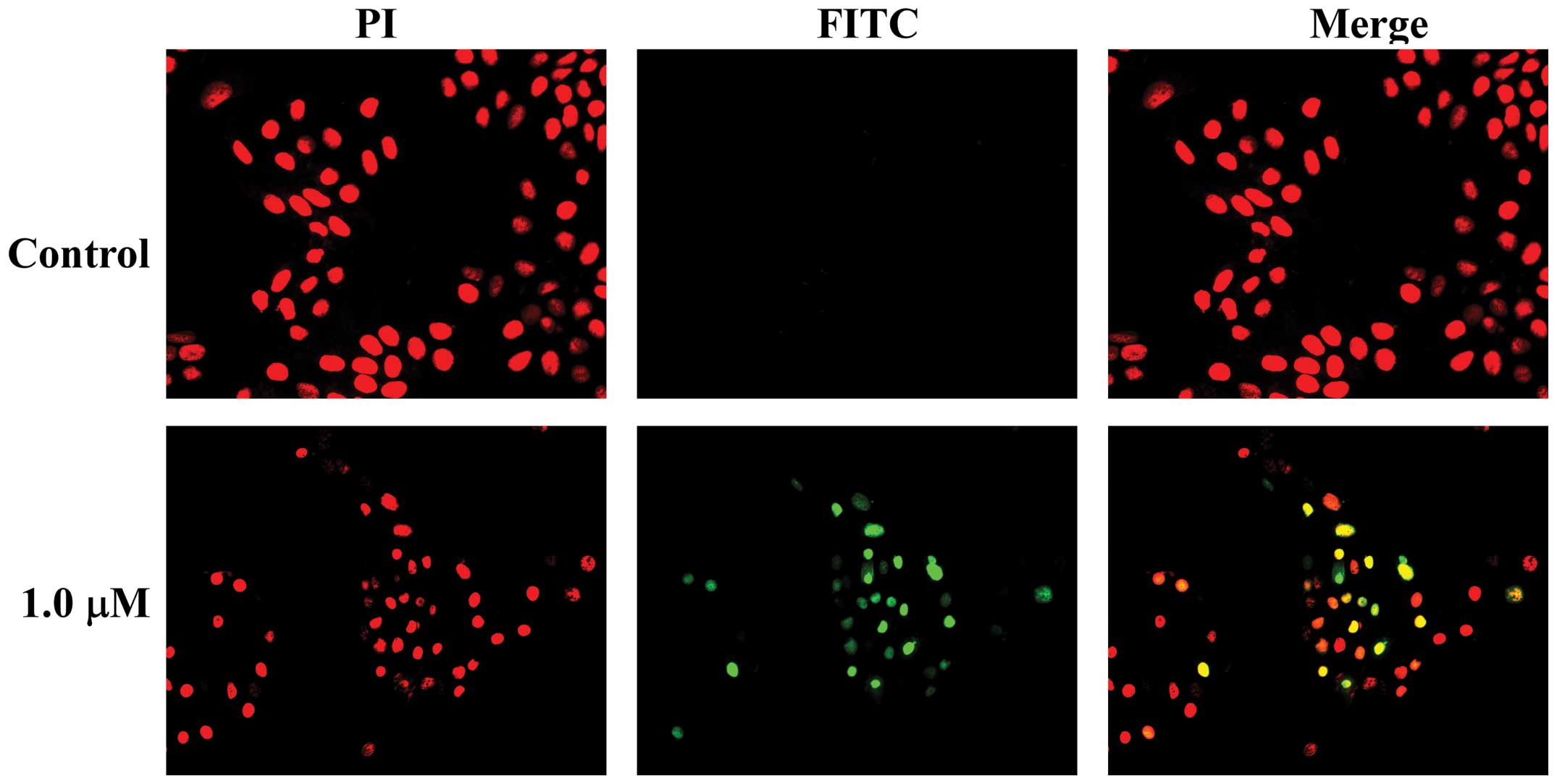
dose-dependent manner. In addition, when the TUNEL assay was used
to evaluate apoptosis-induced nuclear DNA fragmentation as a late
event during the apoptotic process, a higher amount of
TUNEL-positive cells was demonstrated in MCF-7/ADR cells treated
for 24 h with pristimerin at 1.0 µM compared to the control
(Fig. 3). These results strongly
indicated a proapoptotic effect of pristimerin on MCF-7/ADR
cells.
Activation of both the intrinsic and
extrinsic apoptotic pathways by pristimerin in MCF-7/ADR cells
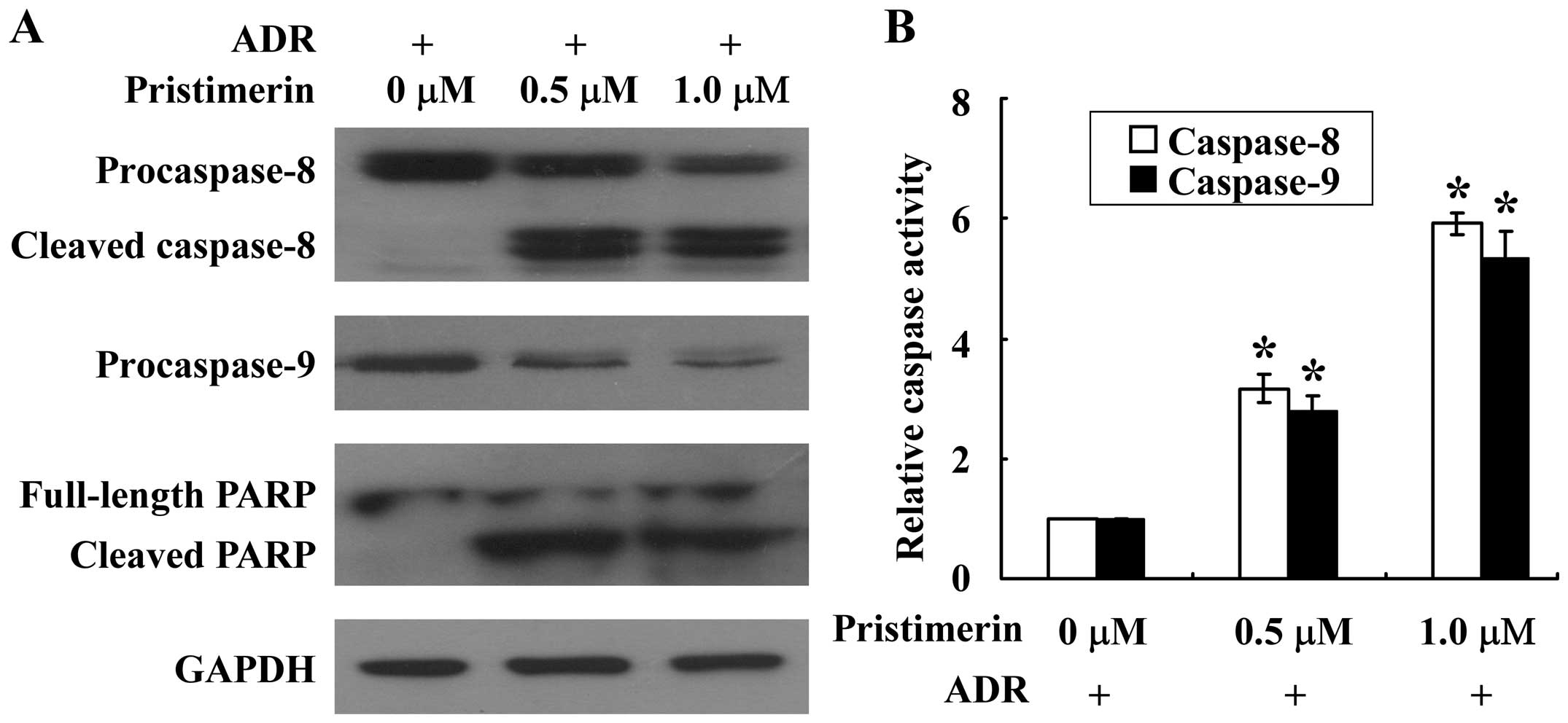
Caspase activation serves a central role in the
execution of apoptosis (15). To
further understand the apoptotic process in MCF-7/ADR cells,
caspase-9 and −8, the proapoptotic caspases in intrinsic and
extrinsic apoptotic pathways, respectively, were examined using
western blotting and enzymatic activity assays. As shown in
Fig. 4A, pristimerin treatment
dose-dependently activated the cleavage of inactive procaspase-9
and −8 in MCF-7/ADR cells. Consistent with the results of western
blotting analysis, the enzymatic activity of caspase-9 and −8
markedly increased in a dose-dependent manner after treatment with
pristimerin at 0.5, 1.0 µM (Fig. 4B).
The effector molecule PARP was cleaved into the 85 kDa fragment
after pristimerin treatment in MCF-7/ADR cells (Fig. 4A). These results indicated that
apoptosis induced by pristimerin in MCF-7/ADR cells may involve
both intrinsic and extrinsic pathways.
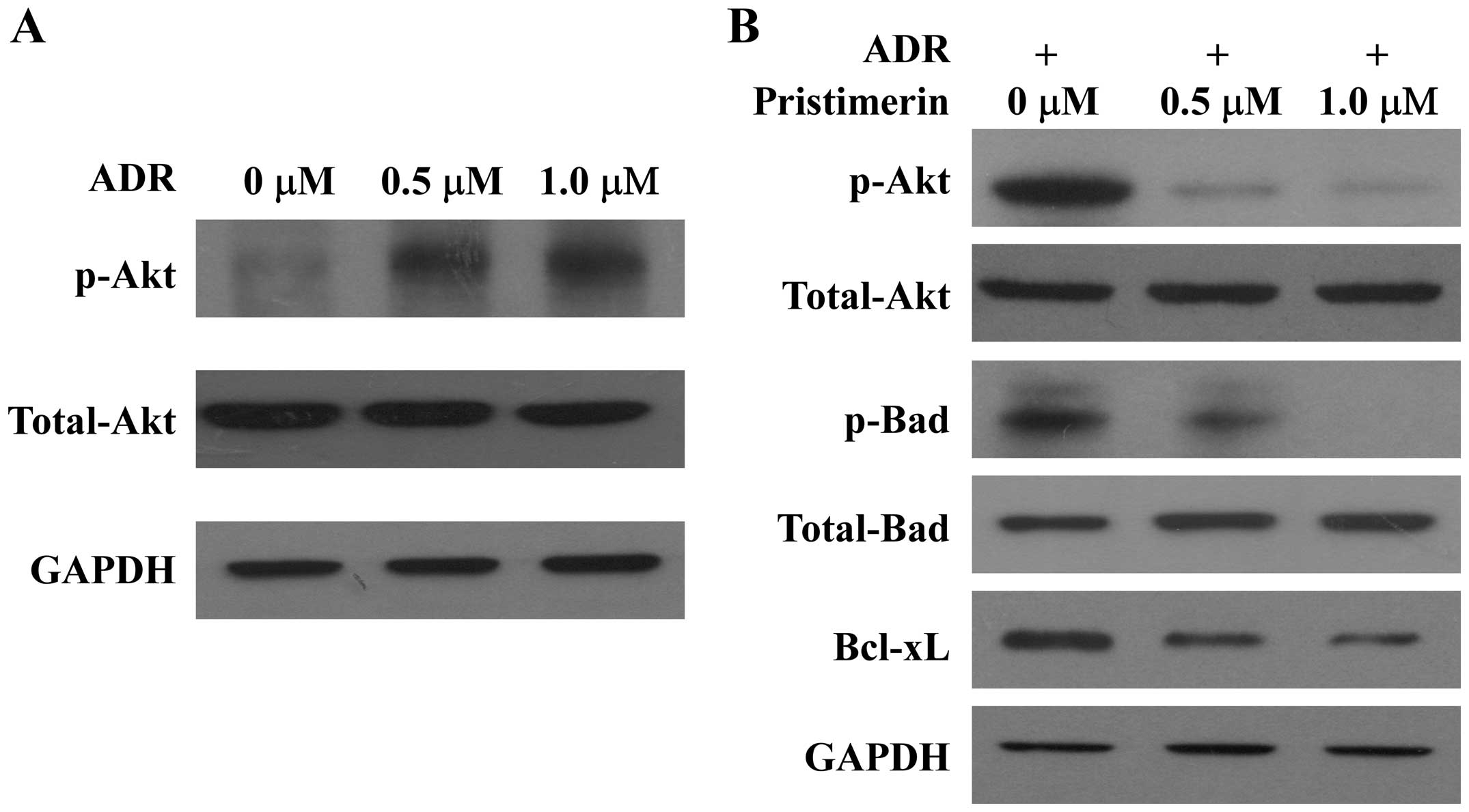
Suppression of Akt activation and its
downstream substrates by pristimerin
In an effort to further understand the signaling
cascade that mediates the proapoptotic effect of pristimerin on
MCF-7/ADR cells, whether pristimerin modulates the activation of
Akt was next investigated. As shown in Fig. 5, Akt was found to be constitutively
activated upon treatment with ADR in MCF-7/ADR cells (Fig. 5A), and pristimerin treatment
suppressed constitutively phosphorylated Akt levels in a
dose-dependent manner without an effect on total Akt expression
(Fig. 5B). Bad is a proapoptotic
member of the Bcl-2 family. Dephosphorylated Bad forms a
heterodimer with Bcl-2 and Bcl-xL. When Bad is phosphorylated by
Akt, it promotes binding of Bad to 14-3-3 proteins (16). This leaves Bcl-2 free to inhibit
apoptosis and then promote cell survival. Consistent with the
suppression of Akt activation, pristimerin treatment reduced
phosphorylation of Bad (Ser136) dose-dependently without an effect
on total Bad expression (Fig. 5B). In
addition, Bcl-xL, another antiapoptotic effector downstream of Akt
signaling, was found to be downregulated upon treatment with
pristimerin in MCF-7/ADR cells (Fig.
5B).
Discussion
Chemoresistance remains a major therapeutic
challenge in breast cancer. In recent years, increasing effort has
been focused on the identification of novel anticancer agents.
Triterpenoids, possessing a wide range of unique biological
activities, have received the most attention in this area (17). Triterpenoids such as 2-cyano-3,
12-dioxooleana-1, 9-dien-28-oic acid (CDDO), its methyl ester
(CDDO-Me), betulinic acid and the ginsenosides, have shown
promising anticancer activities and are presently being evaluated
in clinical trials for the treatment of leukemia, lymphoma, and
solid tumors (18). Pristimerin is a
triterpenoid isolated from Celastraceae and Hippocrateaceae and has
been previously demonstrated to be a promising therapeutic agent
against several types of cancer. Previous studies have shown that
pristimerin is able to inhibit breast cancer cell proliferation and
migration (11,12). However, the function of pristimerin on
MDR in human breast cancer has yet to be elucidated. Therefore, in
the present study, the effect of pristimerin on MCF-7/ADR human
breast cancer MDR cells was investigated.
Consistent with previous reports, in the present
study, pristimerin displayed substantial cytotoxic activity on
MCF-7 breast cancer cells, with greater potency than that of other
triterpenoids for solid organ malignancies such as CDDO or
betulinic acid (8,19), which are currently in clinical trials.
For MDR MCF-7/ADR cells (IC50, 0.43 µM), pristimerin
displayed equally, if not more, effective cytocidal effects as
compared with that against sensitive MCF-7 cells (IC50,
0.59 µM), which indicates that pristimerin is able to reverse the
ADR resistance of MCF-7/ADR cells.
The precise molecular mechanism underlying
chemoresistance in breast cancer is not clear, but the activation
of the phosphoinositide-3-kinases (PI3K)/Akt signaling and
dysregulation of apoptosis have been implicated as the major
players involved in chemoresistance (19–22).
Apoptosis is a key mechanism by which anticancer drugs kill tumor
cells, which is a cellular suicide program through the
mitochondrial (intrinsic) or death receptor pathways (extrinsic).
In the extrinsic pathway, binding of death ligands (e.g. TNF-α,
FasL, TRAIL) with their cognate receptors activates initiator
caspase-8 which then cleaves and activates effector caspases-3, −6,
and −7 leading to apoptosis. In mitochondrial or intrinsic pathway,
undefined signals induce release of cytochrome c from mitochondria,
which in conjunction with Apaf-1 causes activation of initiator
caspase-9. Activated caspase-9, in turn, activates effector
caspases-3, −6, and −7. In the present study, it was confirmed that
pristimerin induced significant apoptosis in MCF-7/ADR cells
through both the extrinsic and intrinsic apoptotic pathways as
evidenced by activation of caspases, PARP cleavage, Annexin
V-positive binding and TUNEL-positive staining.
Chemoresistance in several types of cancer has been
linked to activation of the PI3K/Akt signaling pathway. The
PI3K/Akt pathway is involved in the regulation of a number of
cellular processes including cell death and survival, cell
proliferation, protein synthesis and cell metabolism (23,24).
Accumulating evidence has indicated that activation of the PI3K/Akt
pathway may confer acquired resistance to chemotherapeutic drugs
with different mechanisms of actions: ADR, mitoxantrone,
5-fluorourocil, etoposide, camptothecin, etc (20,21). In
breast cancer cells, activation of Akt by HER2/PI3K is involved in
conferring broad-spectrum chemoresistance. Therefore, Akt is
considered to be a novel target to develop therapeutic strategy to
improve the outcome of breast cancer chemotherapy (25). In agreement with previous reports, the
present study revealed that Akt was indeed constitutively activated
upon ADR treatment in MCF-7/ADR cells. The ability of pristimerin
to suppress the Akt signaling pathways has been previously reported
in a number of sensitive cancer cell lines (6,26). For
ADR-resistant MCF-7/ADR cells, it was demonstrated that pristimerin
treatment also resulted in a dose-dependent reduction in
phospho-Ser473-Akt without an effect on total Akt expression. Bad
and Bcl-xL are two crucial mediators downstream of Akt signaling,
and dysregulation of Bad and Bcl-xL has been linked to
chemoresistance (27–30). In the present study, in parallel with
the observed reduction of Akt phosphorylation, phosphorylation of
Bad was inhibited and Bcl-xL was downregulated in response to
pristimerin treatment in MCF-7/ADR cells.
In conclusion, the results of the present study
indicate that pristimerin is a potent apoptosis inducer in breast
cancer cells resistant to ADR treatments, and its mechanism is
relevant with the suppression of Akt signaling, which indicates the
therapeutic value of pristimerin as a potential MDR reversing agent
for breast cancer chemotherapy.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81101682).
References
|
1
|
Friedenreich CM: Physical activity and
breast cancer: Review of the epidemiologic evidence and biologic
mechanisms. Recent Results Cancer Res. 188:125–139. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng L, Zhou B, Meng X, Zhu W, Zuo A,
Wang X, Jiang R and Yu S: A model of spontaneous mouse mammary
tumor for human estrogen receptor- and progesterone
receptor-negative breast cancer. Int J Oncol. 45:2241–2249.
2014.PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gottesman MM: Mechanisms of cancer drug
resistance. Annu Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dirsch VM, Kiemer AK, Wagner H and Vollmar
AM: The triterpenoid quinonemethide pristimerin inhibits induction
of inducible nitric oxide synthase in murine macrophages. Eur J
Pharmacol. 336:211–217. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deeb D, Gao X, Liu YB, Pindolia K and
Gautam SC: Pristimerin, a quinonemethide triterpenoid, induces
apoptosis in pancreatic cancer cells through the inhibition of
pro-survival Akt/NF-κB/mTOR signaling proteins and anti-apoptotic
Bcl-2. Int J Oncol. 44:1707–1715. 2014.PubMed/NCBI
|
|
7
|
Yan YY, Bai JP, Xie Y, Yu JZ and Ma CG:
The triterpenoid pristimerin induces U87 glioma cell apoptosis
through reactive oxygen species-mediated mitochondrial dysfunction.
Oncol Lett. 5:242–248. 2013.PubMed/NCBI
|
|
8
|
Tiedemann RE, Schmidt J, Keats JJ, Shi CX,
Zhu YX, Palmer SE, Mao X, Schimmer AD and Stewart AK:
Identification of a potent natural triterpenoid inhibitor of
proteosome chymotrypsin-like activity and NF-kappaB with
antimyeloma activity in vitro and in vivo. Blood. 113:4027–4037.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Byun JY, Kim MJ, Eum DY, Yoon CH, Seo WD,
Park KH, Hyun JW, Lee YS, Lee JS, Yoon MY and Lee SJ: Reactive
oxygen species-dependent activation of Bax and poly(ADP-ribose)
polymerase-1 is required for mitochondrial cell death induced by
triterpenoid pristimerin in human cervical cancer cells. Mol
Pharmacol. 76:734–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu YB, Gao X, Deeb D, Brigolin C, Zhang
Y, Shaw J, Pindolia K and Gautam SC: Ubiquitin-proteasomal
degradation of antiapoptotic survivin facilitates induction of
apoptosis in prostate cancer cells by pristimerin. Int J Oncol.
45:1735–1741. 2014.PubMed/NCBI
|
|
11
|
Wu CC, Chan ML, Chen WY, Tsai CY, Chang FR
and Wu YC: Pristimerin induces caspase-dependent apoptosis in
MDA-MB-231 cells via direct effects on mitochondria. Mol Cancer
Ther. 4:1277–1285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mu XM, Shi W, Sun LX, Li H, Wang YR, Jiang
ZZ and Zhang LY: Pristimerin inhibits breast cancer cell migration
by up- regulating regulator of G protein signaling 4 expression.
Asian Pac J Cancer Prev. 13:1097–1104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie G, Zhu X, Li Q, Gu M, He Z, Wu J, Li
J, Lin Y, Li M, She Z and Yuan J: SZ-685C, a marine anthraquinone,
is a potent inducer of apoptosis with anticancer activity by
suppression of the Akt/FOXO pathway. Br J Pharmacol. 159:689–697.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bliss CI: The calculation of the
dose-mortality curve. Ann Appl Biol. 22:134–167. 1935. View Article : Google Scholar
|
|
15
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Setzer WN and Setzer MC: Plant-derived
triterpenoids as potential antineoplastic agents. Mini Rev Med
Chem. 3:540–556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Petronelli A, Pannitteri G and Testa U:
Triterpenoids as new promising anticancer drugs. Anticancer Drugs.
20:880–892. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clark AS, West K, Streicher S and Dennis
PA: Constitutive and inducible Akt activity promotes resistance to
chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol
Cancer Ther. 1:707–717. 2002.PubMed/NCBI
|
|
20
|
Knuefermann C, Lu Y, Liu B, Jin W, Liang
K, Wu L, Schmidt M, Mills GB, Mendelsohn J and Fan Z:
HER2/PI-3K/Akt activation leads to a multidrug resistance in human
breast adenocarcinoma cells. Oncogene. 22:3205–3212. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raguz S and Yagüe E: Resistance to
chemotherapy: New treatments and novel insights into an old
problem. Br J Cancer. 99:387–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kashkar H: X-linked inhibitor of
apoptosis: A chemoresistance factor or a hollow promise. Clin
Cancer Res. 16:4496–4502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katso R, Okkenhaug K, Ahmadi K, White S,
Timms J and Waterfield MD: Cellular function of phosphoinositide
3-kinases: Implications for development, homeostasis and cancer.
Annu Rev Cell Dev Biol. 17:615–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sui T, Ma L, Bai X, Li Q and Xu X:
Resveratrol inhibits the phosphatidylinositide 3-kinase/protein
kinase B/mammalian target of rapamycin signaling pathway in the
human chronic myeloid leukemia K562 cell line. Oncol Lett.
7:2093–2098. 2014.PubMed/NCBI
|
|
25
|
Knight ZA and Shokat KM: Chemically
targeting the PI3K family. Biochem Soc Trans. 35:245–249. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao X, Liu Y, Deeb D, Arbab AS and Gautam
SC: Anticancer activity of pristimerin in ovarian carcinoma cells
is mediated through the inhibition of prosurvival Akt/NF-κB/mTOR
signaling. J Exp Ther Oncol. 10:275–283. 2014.PubMed/NCBI
|
|
27
|
Marchion DC, Cottrill HM, Xiong Y, Chen N,
Bicaku E, Fulp WJ, Bansal N, Chon HS, Stickles XB, Kamath SG, et
al: BAD phosphorylation determines ovarian cancer chemosensitivity
and patient survival. Clin Cancer Res. 17:6356–6366. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Q, Yogosawa S, Iizumi Y, Sakai T and
Sowa Y: The alkaloid emetine sensitizes ovarian carcinoma cells to
cisplatin through downregulation of bcl-xL. Int J Oncol.
46:389–394. 2015.PubMed/NCBI
|
|
29
|
Villedieu M, Louis MH, Dutoit S, Brotin E,
Lincet H, Duigou F, Staedel C, Gauduchon P and Poulain L: Absence
of Bcl-xL down-regulation in response to cisplatin is associated
with chemoresistance in ovarian carcinoma cells. Gynecol Oncol.
105:31–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Williams J, Lucas PC, Griffith KA, Choi M,
Fogoros S, Hu YY and Liu JR: Expression of Bcl-xL in ovarian
carcinoma is associated with chemoresistance and recurrent disease.
Gynecol Oncol. 96:287–295. 2005. View Article : Google Scholar : PubMed/NCBI
|