Introduction
Ovarian carcinoma is a lethal gynecological
malignancy with a 5-year survival rate of 25–30% (1). At present, 60% of advanced-stage
patients will experience disease recurrence (2), whilst the average life expectancy is 12
to 18 months (3). The primary
treatment for ovarian cancer is cytoreductive surgery and adjuvant
platinum-based chemotherapy (3). The
low ovarian carcinoma survival rates are known to be largely
impacted by the development of chemotherapy resistance (4), with improved treatments now required for
patients.
Human epidermal growth factor receptor 2 (HER2), a
member of the epidermal growth factor receptor (EGFR) family, is
located on the chromosome 17 q21 band. As a key gene involved in
cell survival, differentiation, proliferation, apoptosis and
migration, HER2 gene amplification and protein overexpression may
result in malignant transformation (5–8). The
present study demonstrates that the HER2 gene is highly expressed
in ovarian carcinoma and is closely associated with cancer cell
growth, as well as differentiation, chemical resistance, malignant
transformation and patient prognosis (9–12). RNA
interference (RNAi) technology is now recognized as a common
research tool for the silencing of target genes (13), and as a revolutionary class of
therapeutics for the treatment of cancer (14). RNAi may be activated by
double-stranded RNAs, which are processed into small fragments
(21–23 base pairs) by the Dicer enzyme (15);these fragments are then loaded into the
RNA-induced silencing complex (RISC). Following this, the activated
RISC-guide strand complex is directed to the target mRNA
complementary region, which is then degraded and therefore
inhibited from being translated (16). A previous study demonstrated that the
transfection of HER2 small interfering RNA (siRNA) into SKOV3 cells
induced apoptosis, and inhibited proliferation and the invasive and
migratory phenotypes of the SKOV3 cells (4).
In the present study, RNAi technology was utilized
to design siRNAs that targeted different regions within the open
reading frame of HER2 mRNA, in order to examine the
chemosensitivity of ovarian carcinoma.
Materials and methods
Cell lines and culture
The ovarian cancer SKOV3 cell line was obtained from
the Jiangsu Institute of Hematology (Suzhou, China). Cell culture
was performed according to the manufacturer's protocols. The cells
were routinely maintained in RPMI 1640 Medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA), supplemented with 10%
fetal bovine serum (HyClone; GE Healthcare Life Sciences), 1%
penicillin and 1% streptomycin in a well-humidified incubator with
5% CO2 at 37°C. SKOV3 cells were transfected with
lentiviral-mediated HER2-small hairpin RNA (shRNA) molecules to
establish the stable expression of HER2-shRNA in the SKOV3 cell
line (knockdown cells; KD) and negative control cell line (NC). The
untransfected SKOV3 cell line served as the blank control (CON)
group.
siRNA preparation and infection
HER2 cDNA sequences (NM_004448) were selected based
on HER2 target sites (obtained from GenBank; www.ncbi.nlm.nih.gov/genbank) using the BLOCK-iT™ RNAi
Designer (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). siRNA design principles described by Tuschl (17) were used for the design of the three
target sequences of RNAi and to aid the selection of a negative
control (NC) sequence. In previous experiments, the present study
designed three target sequences of RNAi and an NC sequence:
HER2-RNAi-1, sense TCTGCGGTGGTTGGCATTC (2204–2222; 57.89% GC);
HER-2-RNAi-1, sense ATATGTGAACCAGCCAGAT (3652–3670; 42.11% GC);
HER2-RNAi-3, sense GTGCCAATATCCAGGAGTT (1311–1329; 47.37% GC); and
HER2-RNAi-NC, sense TTCTCCGAACGTGTCACGT. Following screening the
most effective interference sequence was HER2-RNAi-1 sense. Using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.),
the siRNAs were transfected into 293T cells, and viral supernatants
were harvested and infected into the human ovarian cancer SKOV3
cell line. Following a total of 12 h post-infection, the medium was
replaced with medium containing puromycin (1.5 µg/ml), and 2 weeks
later the medium was replaced by normal medium and cells were
cultured to expand.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols, and was stored at −80°C. The RNA
concentration and purity was measured using a spectrophotometer
(BioPhotometer Plus; Eppendorf, Hamburg, Germany). According to the
Transcriptor One-Step RT-PCR kit (Roche Diagnostics, Basel,
Switzerland) protocols, 1 µg total RNA (Promega Corporation,
Madison, WI, USA) was reverse-transcribed. Reverse transcriptase
was purchased from Roche Diagnostics, (Basel, Switzerland). β-actin
was used as an internal reference. DNA polymerase/DNase was
purchased from Promega Corp. (Madison, WI, USA). The primer
sequences (Shanghai Sangon Biological Engineering Technology
Services Ltd., Shanghai, China) used were as follows: Forward,
5′-CTGAACAATACCACCCCTGTC-3′; and reverse,
5′-AGATGTCCTTCCACAAAATCGT-3′. The reaction conditions were a
two-step procedure hot-start activation at 95°C for 2 min and 40
cycles, with denaturation at 95°C for 15 sec, followed by
annealing/elongation at 60°C for 60 sec and finally dissociation at
60–95°C. Abosorbance values were read and the 2−ΔΔCq was
used to calculate target gene expression. PCR was performed on the
ABI 7500 PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Furthermore, each sample was subjected to RT-qPCR testing
using the same conditions in triplicate, and the average values
were used for data analysis.
Western blot analysis
Extraction of total protein from the cells was
performed using radioimmunoprecipitation assay buffer and protein
quantification was conducted using Coomassie brilliant blue stain.
A total of 50 µg protein was loaded onto sodium dodecyl sulfate
polyacrylamide gel electrophoresis gels (8%), electrophoresed at
200 V for 2 h and subsequently transferred onto
Immobilon®-P polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were then incubated
with the primary antibodies against HER2 (rabbit polyclonal;
catalog no., ab58616; dilution, 1:150; Abcam, Cambridge, MA, USA)
and β-actin (mouse monoclonal; catalog no., AA128; dilution,
1:1,000; Beyotime Institute of Biotechnology, Shanghai, China),
followed by incubation with the appropriate goat monoclonal
anti-rabbit and rabbit polyclonal anti-mouse secondary antibodies
conjugated with alkaline phosphatase (catalog nos., A0208 and
A0216, respectively; dilution, 1:1,000; Beyotime Institute of
Biotechnology). Enhanced chemiluminescence chromogenic exposure and
X-ray exposure were used for visualization.
Cell inhibition assay
Cell inhibition was analyzed using Cell Counting
Assay kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Gaithersburg, MD, USA) according to the manufacturer's protocols.
Briefly, the cell suspension was seeded in 96-well plates at a
density of 5×103 cells, with varying concentrations of
cisplatin (DDP) added (0.312, 0.625, 1.25, 2.5, 5, 20, 40 and 20
µg/ml), and cultured for 24 h. A total of 10 µl WST-8 was added to
each well. The cells were incubated at 37°C for 1 h with 5%
CO2. An automatic microplate reader (Multiskan™ MK3
Microplate Photometer; Thermo Fisher Scientific, Inc.) measured the
absorbance (A) of each well at 450 nm. The inhibition rate was
calculated using the following equation: Inhibition rate = (1 -
Adosing group / Acell control group) ×
100.
Tumorigenicity assay in nude mice
Thymus-null BALB/c nude mice (female, 3–4 weeks old)
were purchased from the Experimental Animal Center of Lake Hayes
(Shanghai, China). All animal procedures were performed according
to approved protocols and were in accordance with recommendations
for the proper use and care of laboratory animals at the
Experimental Animal Center of Soochow University (Suzhou, China). A
total of 100 µl of CON group and KD group cell solutions
(~4×106 cells) were seeded in 25 nude mice
subcutaneously, near the right armpit, to form three groups: The
CON, CON + DDP and KD + DDP groups. The transplanted tumor
diameters of the five nude mice were ~5 mm. The CON + DDP and KD +
DDP mice groups were intravenously injected with DDP at a dose of 5
mg/kg, once per week, over a period of 4 weeks. Tumor diameter was
measured using Vernier calipers, and was used to calculate the
tumor volume as 0.5ab2 (with a and b as the long and
short diameters, respectively). At the end of the experiment, the
mice were sacrificed by cervical dislocation. The tumors were
excised, and immunohistochemical detection of HER2 protein
expression and hematoxylin and eosin staining of tumor tissue was
performed.
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical significance was determined by conducting
analysis of variance or χ2 tests, using SPSS software,
version 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Stable expression of HER2-shRNA in the
SKOV3 and NC cells
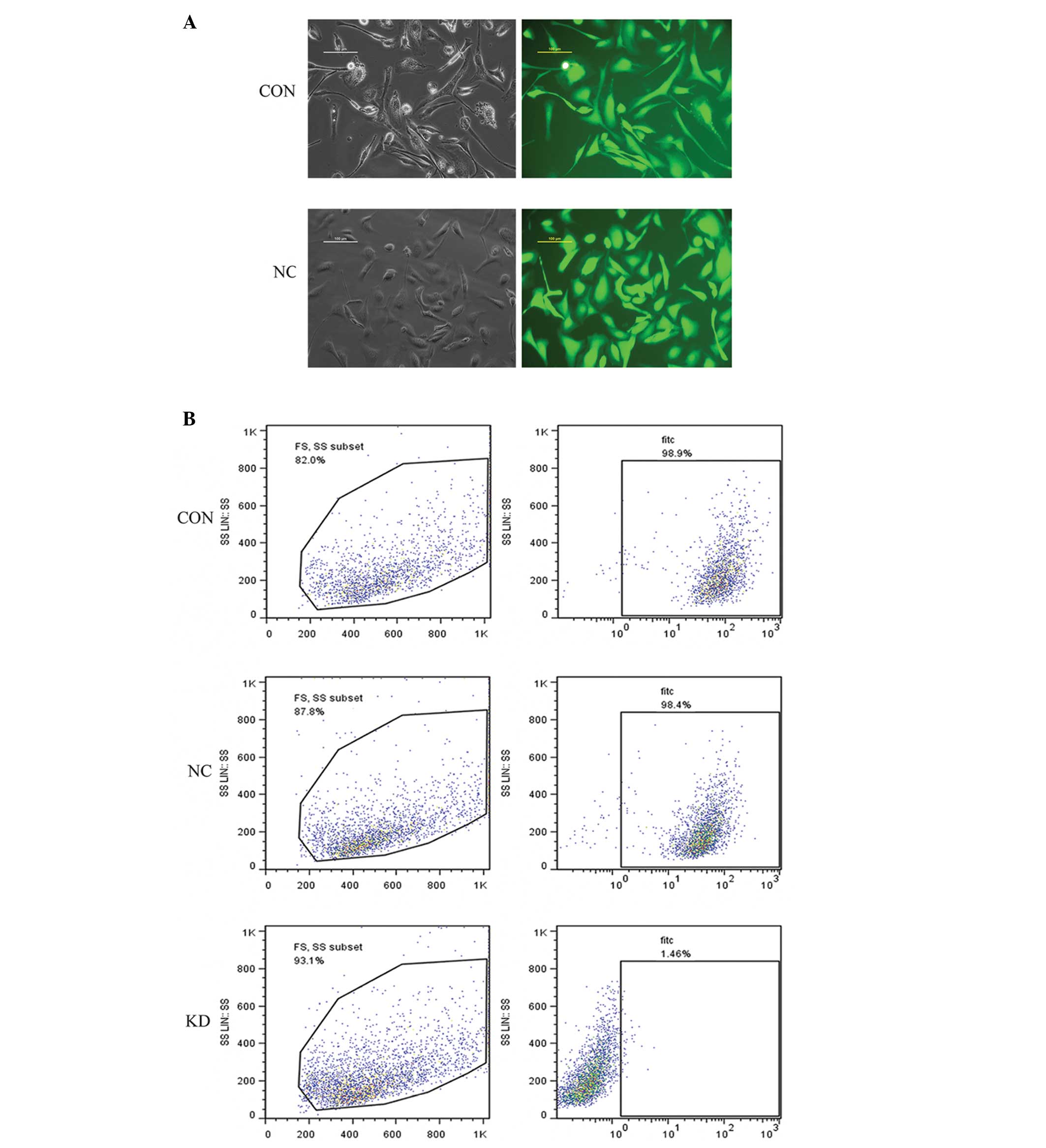
Lentiviral vector-infected SKOV3 cells were selected
with puromycin for 2 weeks. Fluorescence microscopy (Fig. 1A) and flow cytometry (Fig. 1B) determined that the infection
efficiency of the NC and KD groups was >98%. The results
demonstrate the successful establishment of stable HER2-shRNA and
NC expression in the SKOV3 cell lines.
Stable expression of HER2-shRNA in the
SKOV3 cells downregulates the HER2 gene
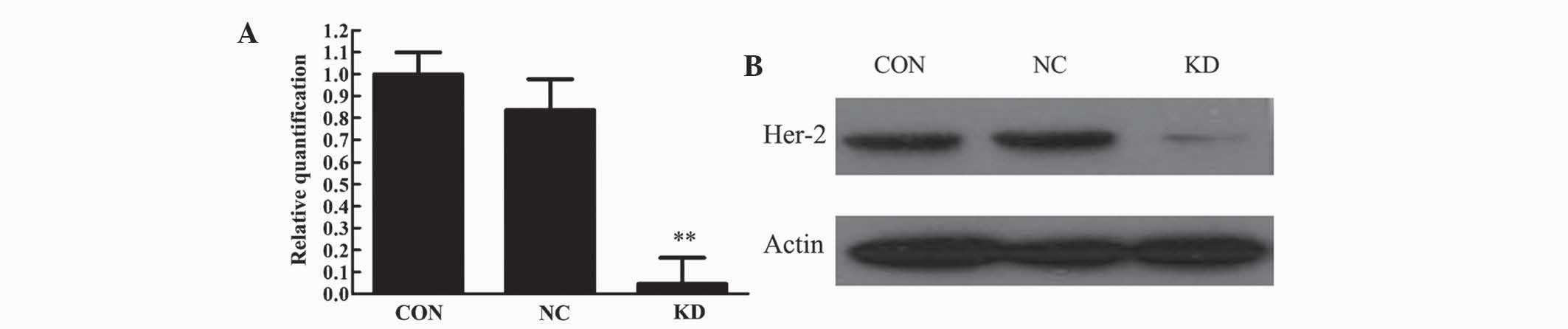
The relative mRNA content (HER2/β-actin) in the CON,
NC and KD cells was 1.000, 0.837 and 0.045, respectively. HER2 mRNA
levels in the KD group compared with the CON and NC group were
lower, and the difference was statistically significant (P<0.01)
(Fig. 2A). Western blot analysis of
HER2 protein expression following transfection of the three cell
groups delivered results consistent with the mRNA levels (Fig. 2B).
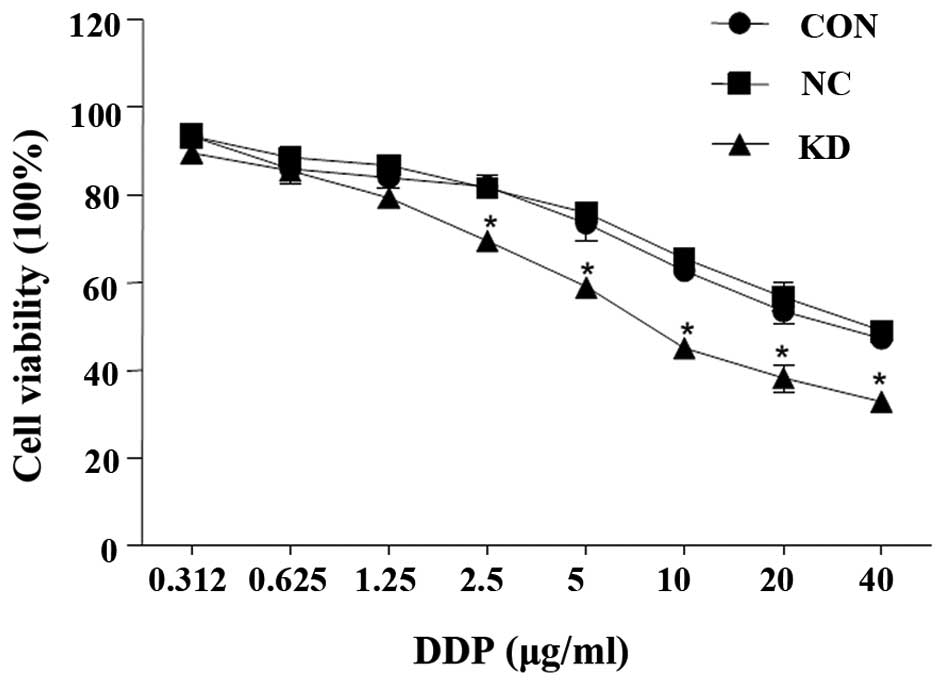
Chemosensitivity to DDP in the SKOV3
cells
Following RNAi, chemosensitivity to DDP in the SKOV3
cells increased. With increasing concentrations of DDP
administered, the proliferation rate of all three cell groups
decreased; however, the decrease in the KD cell proliferation curve
was more significant compared with the NC and CON groups
(P<0.01; Fig. 3).
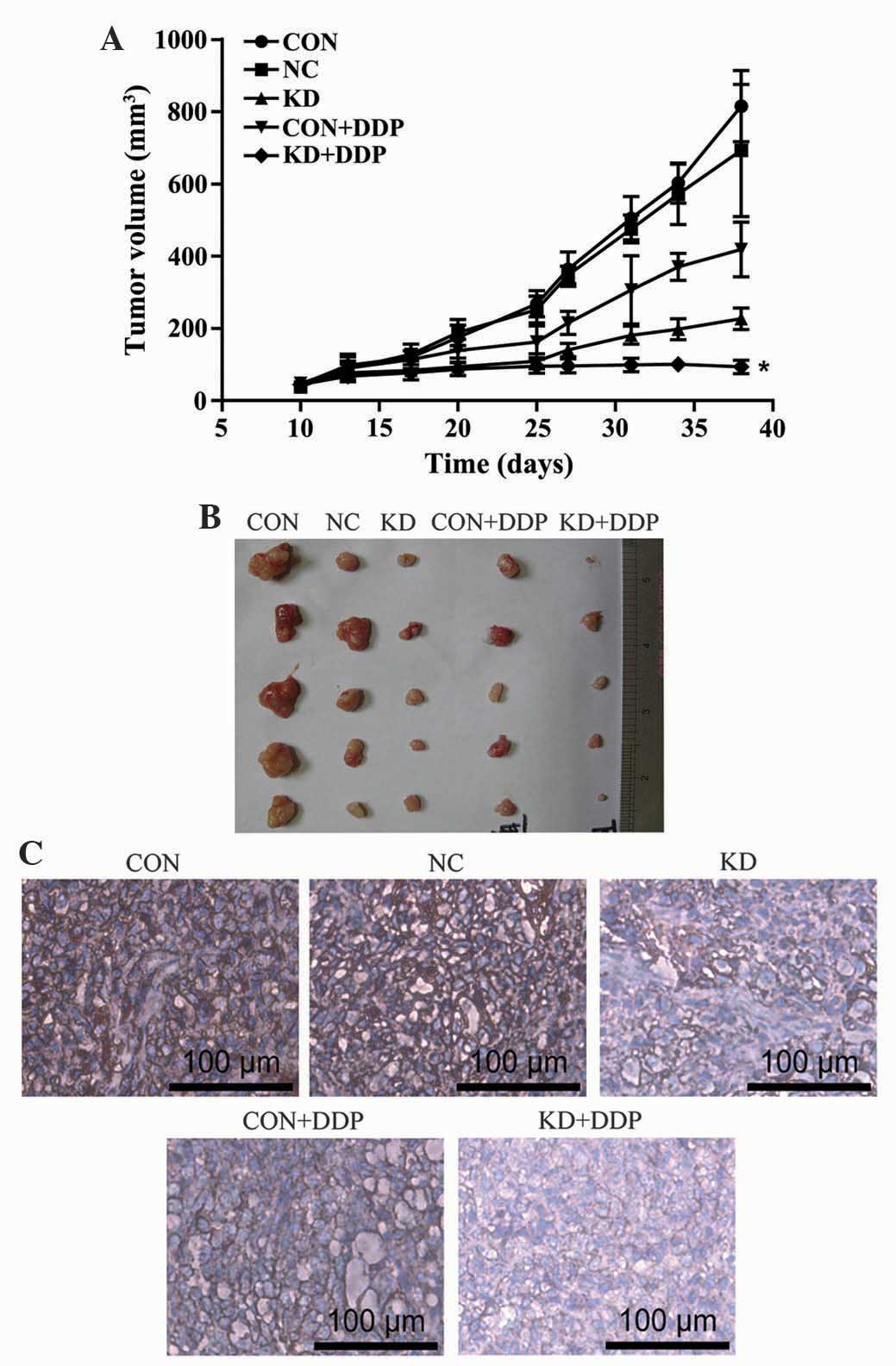
HER2 silencing inhibits ovarian cancer
growth in vivo
The results demonstrated that the tumor volume in
the KD + DDP group was significantly less than that of the CON and
CON + DDP groups (P<0.05), therefore indicating that DDP
significantly inhibited the tumor growth rate. Lentivirus-mediated
HER2-shRNA increased the sensitivity of the ovarian cancer
xenografts to DDP (Fig. 4A and
B).
Immunohistochemical staining in the
SKOV3 cells
Immunohistochemical staining of the CON group
revealed that the area surrounding the nucleus was a deep brown
color, showing positive staining for HER2. In the CON + DDP group,
there were fewer of the brown particles compared with the CON
group, showing weakly positive staining (P<0.05). The KD + DDP
group had a significantly decreased number of brown particles,
indicating that HER2 protein expression was significantly reduced
(Fig. 4C).
Discussion
The treatment of ovarian cancer remains a challenge
for clinicians (18). Despite the
introduction of platinum-based chemotherapy and its improvement of
the ovarian cancer patient survival rates, the long-term survival
of patients remains low due to tumor recurrence and chemoresistance
(19). Resistance to
chemotherapeutics has been studied thoroughly and a number of
mechanisms as to how this may occur have been proposed. A potential
molecular marker that may improve the prediction of the patient
response to a given treatment is HER2 overexpression, which affects
the growth, proliferation, invasion and metastasis of cells
(20). HER2 is the predominant cause
of gynecological cancer mortality, and it has been demonstrated
in vitro that the reduction of HER2 expression, by antisense
or siRNA, resulted in the inhibition of growth and the initiation
of apoptosis in HER2+ breast and ovarian cancer cells
(4,21,22).
Despite chemotherapeutic agents such as trastuzumab benefiting a
large number of HER+ patients, the development of drug
resistance and toxic side effects may compromise the therapeutic
effect (9).
In the present study, ovarian carcinoma SKOV3 cells
were utilized as a model to analyze the effect of HER2 expression
and signaling levels on DDP sensitivity. RNAi was used to produce
stable cell lines and the inhibition of HER2 gene expression was
detected following the inhibition of the HER2 gene; furthermore,
SKOV3 cell chemosensitivity to DDP was significantly enhanced.
In vivo experiments demonstrated that the tumor volume in
the KD + DDP group was significantly smaller than that of the other
four groups. Tumor tissue immunohistochemistry indicated that the
HER2 protein expression in the KD + DDP group was significantly
lower than that in the other two groups, suggesting that lentiviral
vector-mediated HER2-shRNA increases cell sensitivity to DDP in
ovarian cancer. Such results provide a theoretical basis for novel
therapies for chemotherapy-resistant ovarian cancers.
In the current study, lentiviral-mediated shRNA
expression vectors, as compared with plasmid-mediated siRNA, were
stably expressed for a long period of time, and the preparation of
cell lines stably expressing shRNA was the most effective means for
the in vivo experiments. The use of lentivirus in an
organism may induce gene mutations and function as a potential
biological hazard; therefore, it is important to demonstrate that
they can be safely applied to the human body (23). As technology continues to advance, the
use of RNAi may become an important means for future cancer gene
therapy.
In conclusion, the present study demonstrated that
HER2 serves an important role in the chemoresistance of ovarian
cancer. However, further clarification of its functional
characterization is required. The results of the present study
provide support for the possible development of a novel gene
therapy targeting HER2, ultimately aiming to prevent
chemoresistance in human ovarian cancer.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Salani R, Santillan A, Zahurak ML, et al:
Secondary cytoreductive surgery for localized, recurrent epithelial
ovarian cancer: Analysis of prognostic factors and survival
outcome. Cancer. 109:685–691. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Helm CW: Current status and future
directions of cytoreductive surgery and hyperthermic
intraperitoneal chemotherapy in the treatment of ovarian cancer.
Surg Oncol Clin N Am. 21:645–663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu YM, Rong ML, Shang C, et al:
Suppression of HER-2 via siRNA promotes apoptosis and decreases
metastatic potential of SKOV3 human ovarian carcinoma cells. Oncol
Rep. 29:1133–1139. 2013.PubMed/NCBI
|
|
5
|
Baselga J and Arteaga CL: Critical update
and emerging trends in epidermal growth factor receptor targeting
in cancer. J Clin Oncol. 23:2445–2459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Camilleri-Broët S, Hardy-Bessard AC, Le
Tourneau A, Paraiso D, Levrel O, Leduc B, Bain S, Orfeuvre H,
Audouin J and Pujade-Lauraine E: GINECO group: HER-2 overexpression
is an independent marker of poor prognosis of advanced primary
ovarian carcinoma: A multicenter study of the GINECO group. Ann
Oncol. 15:104–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lanitis E, Dangaj D, Hagemann IS, et al:
Primary human ovarian epithelial cancer cells broadly express HER2
at immunologically-detectable levels. PLoS One. 7:e498292012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neve RM, Lane HA and Hynes NE: The role of
overexpressed HER2 in transformation. Ann Oncol. 12(Suppl 1):
S9–S13. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tai W, Mahato R and Cheng K: The role of
HER2 in cancer therapy and targeted drug delivery. J Control
Release. 146:264–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu D and Hung MC: Role of erbB2 in breast
cancer chemosensitivity. BioEssays. 22:673–680. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berchuck A, Kamel A, Whitaker R, et al:
Overexpression of HER-2/neu is associated with poor survival in
advanced epithelial ovarian cancer. Cancer Res. 50:4087–4091.
1990.PubMed/NCBI
|
|
12
|
Tan DS, Ang JE and Kaye SB: Ovarian
cancer: Can we reverse drug resistance? Adv Exp Med Biol.
622:153–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fire A, Xu S, Montgomery MK, et al: Potent
and specific genetic interference by double-stranded RNA in
Caenorhabditis elegans. Nature. 391:806–811. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Draz MS, Fang BA, Zhang P, et al:
Nanoparticle-mediated systemic delivery of siRNA for treatment of
cancers and viral infections. Theranostics. 4:872–892. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meister G and Tuschl T: Mechanisms of gene
silencing by double-stranded RNA. Nature. 431:343–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brodersen P, Sakvarelidze-Achard L,
Bruun-Rasmussen M, et al: Widespread translational inhibition by
plant miRNAs and siRNAs. Science. 320:1185–1190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tuschl T: Expanding small RNA
interference. Nat Biotechnol. 20:446–448. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bristow RE, Tomacruz RS, Armstrong DK,
Trimble EL and Montz FJ: Survival effect of maximal cytoreductive
surgery for advanced ovarian carcinoma during the platinum era: A
meta-analysis. J Clin Oncol. 20:1248–1259. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ali AY, Farrand L, Kim JY, et al:
Molecular determinants of ovarian cancer chemoresistance: New
insights into an old conundrum. Ann N Y Acad Sci. 1271:58–67. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takehana T, Kunitomo K, Suzuki S, et al:
Expression of epidermal growth factor receptor in gastric
carcinomas. Clin Gastroenterol Hepatol. 1:438–445. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang G, Cai KQ, Thompson-Lanza JA, Bast RC
Jr and Liu J: Inhibition of breast and ovarian tumor growth through
multiple signaling pathways by using retrovirus-mediated small
interfering RNA against Her-2/neu gene expression. J Biol Chem.
279:4339–4345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roh H, Pippin J and Drebin JA:
Down-regulation of HER2/neu expression induces apoptosis in human
cancer cells that overexpress HER2/neu. Cancer Res. 60:560–565.
2000.PubMed/NCBI
|
|
23
|
Dullaers M, Van Meirvenne S, Heirman C, et
al: Induction of effective antitumor immunity by direct in
vivo administration of lentiviral vectors. Gene Ther.
13:630–640. 2005. View Article : Google Scholar
|