Introduction
In industrialized countries, including the USA,
Japan and UK, ovarian carcinoma is one of the most common
gynecological malignancies diseases and the leading cause of
gynecological cancer mortality (1,2). There are
multiple reasons for this, one cause may be that ovarian carcinoma
is generally detected late, almost 70% of all patients present with
advanced stage III and IV cancer, and a number of patients are
misdiagnosed (3–5). The majority of patients suffer from
abdominal, gastrointestinal, urinary, or pelvic pain, which rarely
lead to timely definitive diagnosis, leading to the generally late
detection of ovarian carcinomas (6,7).
Ovarian carcinoma is relatively asymptomatic at its
early stages, and this results in a low chance of early detection
(8,9).
The majority of ovarian carcinoma patients already have tumor cells
throughout the abdomen [International Federation of Gynaecologists
and Obstetricians (FIGO) stages III–IV] and there is a low 5-year
overall survival (OS) rate (10).
Multiple genetic changes are involved in ovarian carcinoma
development, which are not well characterized. To understand the
pathogenesis of ovarian carcinoma is an important challenge,
involving the identification of novel oncogenes and tumor
suppressor genes (11,12).
In 2003, Zeller et al (13) discovered SAM and SH3- domain
containing 1 (SASH1), a potential target gene on chromosome
6q24.3, through systematic comparison of candidate expressed
sequence tags with genomic sequences from the genomic interval
6q23-25 (13). It has been
demonstrated that SASH1 is down-regulated in the majority (74%) of
breast tumors in comparison with the corresponding normal breast
epithelial tissues. The SASH1 gene encodes one signal
adapter protein consisting of several protein-protein interaction
domains (14). The SAM domain can
exhibit more complex functions in these protein-protein interaction
domains (15). The SAM domain
mediates protein-protein interactions through homologous and
heterologous oligomerization with the SAM domains of other
proteins, and it can mediate Smaug protein and mRNA binding to
facilitate transcriptional regulation (16,17). SASH1
is a member of a recently described family of SH3/SAM adapter
molecules according to its domain structure, thus suggesting a role
in signaling pathways (18).
The carcinogenesis and tumor progression of ovarian
carcinoma is a complex process with multiple factors and stages
(19). The activation of oncogenes
and mutation or deletion of tumor suppressor genes are the leading
causes of ovarian carcinoma (7,20,21). Therefore, the study of suppressor
genes and apoptosis-related genes in carcinogenesis and tumor
progression in ovarian carcinoma has drawn increasing attention. A
previous study indicated that SASH1 is a tumor suppressor
gene, located on chromosome 6q24.3 (22). Zeller et al (13) demonstrated reduced or absent
SASH1 expression in 6 breast cancer cell lines, which
exhibit a chromosome 6q24.3 deletion. These results indicated that
the down-regulation of SASH1 is at least in part due to gene
deletion (13). Down-regulation of
SASH1 expression was closely correlated with tumor invasion,
metastasis, and poor prognosis (22–24).
However, the specific role of SASH1 in ovarian carcinoma has not
yet been reported in the literature. In the present study, the
expression of SASH1 in ovarian carcinoma tissues was determined and
its correlation to the clinical pathology of ovarian carcinoma by
using reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) or western blot analysis. In addition, SKOV3 ovarian
carcinoma cells were transfected with a eukaryotic expression
vector expressing the full-length SASH1 cDNA, and the
changes in SKOV3 cell viability, proliferation, apoptosis and
migration were assessed. These data might provide information for
the prediction of ovarian carcinoma prognosis and the establishment
of targeted therapies.
Materials and methods
Specimens
Fresh resection tissue specimens were collected from
79 patients with ovarian carcinoma at the Maternal and Child Health
Care Hospital of Nantong (Nantong, China) from June 2004 to
December 2013. All patients agreed to the procedure and signed
informed consent forms. The samples were preserved in liquid
nitrogen immediately, stored for analysis, and made anonymous
according to the ethical and legal standard. No patients had
received prior chemotherapy, radiotherapy or other preoperative
treatments, and none had any other associated inflammatory disease.
All tumor tissue and the adjacent normal ovarian tissue from 79
ovarian carcinoma cases were pathologically verified by the
Department of Pathology of the Maternal and Child Health Care
Hospital of Nantong. The clinicopathological characteristics of 79
ovarian patients were collected, including age at diagnosis, FIGO
stage (25), histological type, and
lymph node status. Histological type was classified according to
the World Health Organization (WHO) criteria (26). The patient characteristics are
summarized in Table I.
 | Table I.Association between
clinicopathological character and SASH1 expression in
patients with ovarian cancer. |
Table I.
Association between
clinicopathological character and SASH1 expression in
patients with ovarian cancer.
| Clinical
feature | Cases | SASH1 mRNA
positive rate (%) | P |
|---|
| Normal tissue | 79 | 79 (100.0) |
0.000a |
| Carcinoma
tissue | 79 | 48 (60.8) |
|
| Age (year) |
|
|
|
|
<50 | 36 | 22 (61.1) | 0.953 |
|
≥50 | 43 | 26 (60.5) |
|
| Histological
type |
|
|
|
|
Serous | 63 | 36 (57.1) | 0.344 |
|
Non-serous | 26 | 12 (46.2) |
|
| Residual tumor |
|
|
|
| <1
cm | 41 | 21 (51.2) | 0.127 |
| ≥1
cm | 38 | 13 (34.2) |
|
| FIGO stage |
|
|
|
|
I–II | 46 | 32 (69.6) |
0.016a |
|
III–IV | 33 | 14 (42.4) |
|
| Lymph nodes
metastasis |
|
|
|
|
Negative | 42 | 30 (71.4) |
0.021a |
|
Positive | 37 | 17 (46.0) |
|
Reagents
Ovarian carcinoma SKOV3 cells were obtained from the
American Type Culture Collection (Rockville, MD, USA). Fetal bovine
serum, RPMI 1640 and cell culture plates and cell culture dishes
were purchased obtained from Corning Incorporated (New York, NY,
USA).
TRIzol reagent was obtained from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). FastStart Universal SYBR Green
Master (Rox), Annexin V-FITC and propidium iodide were purchased
from Roche Diagnostics (Basel, Switzerland). First Strand cDNA
Synthesis Kit was purchased from Qiagen, Inc. (Valencia, CA, USA).
Lipofectamine 2000, pcDNA3.1 vector, and pGEM-T vector were
purchased from Invitrogen (Thermo Fisher Scientific, Inc.). Trypsin
and PBS were purchased from Sigma-Aldrich, Inc. (Shanghai, China).
Rabbit anti-SASH1 polyclonal antibody and mouse anti-human β-actin
monoclonal antibody were purchased from Abcam Corporation
(Cambridge, UK). ReadyPrep™ Protein Extraction kit and Quick Start™
Bradford Protein assay were purchased from Bio-Rad Laboratories
(Richmond, CA, USA). Restriction endonuclease BamHI and
XhoI and DNA marker were obtained from Takara Corporation
(Dalian, Liaoning, China). T4 DNA ligation was purchased from
Promega Corporation (Beijing, China). Taq DNA polymerase and the
prestaining protein ladder were purchased from Fermentas, Inc.
(Glen Burnie, MD, USA).
Transwell invasion chamber was purchased from
Corning Corporation (Midland, MI, USA). Matrigel was obtained from
Collaborative Biomedical Products (Bedford, MA, USA). IRDye 800
conjugated affinity purified goat anti-mouse IgG and IRDye 800
conjugated affinity purified goat anti-rabbit IgG were purchased
from Li-COR Biotechnology (Lincoln, NE, USA). Hoechst 33342 dye was
purchased from Beyotime Institute of Technology, Inc. (Haimen,
China).
RT-qPCR for detecting the expression
levels of SASH1
RT-qPCR for SASH1 was used to detect the expression
levels of SASH1 in 79 ovarian carcinoma tissues and adjacent normal
tissues. SASH1 (GenBank code: NM_015278.3) forward primer P1:
5′-ATACCTCGGCTTGACATT-3′, reverse primer P2:
5′-ATACCTCGGCTTGACATT-3′. Ki-67 (GenBank code: AJ567756.1) forward
primer P1: 5′-ACTTGCCTCCTAATACGC-3′, reverse primer P2:
5′-CAGGTTGCCACTCTTTCT-3′. Internal marker gene GAPDH (GenBank code:
NM_002046) forward primer P1: 5′-CCACAGTCCATGCCATCACT-3′, reverse
primer P2: 5′-TCCACCACCCTGTTGCTGTAG-3′. All of the above primers
were synthesized and provided by Shanghai Invitrogen Biotechnology
Co. Ltd (Shanghai, China).
Total RNA was extracted from tissue samples using
TRIzol reagent according to the manufacturer's protocol. Each
tissue sample (100 mg) was added, and the sample was homogenized
and the total RNA was extracted from the tissue samples. Next, the
reverse transcriptase (RT) reactions were performed with a First
Strand cDNA Synthesis kit and the first-strand cDNA was synthesized
and stored at −20°C in small aliquots.
The synthetic primers for SASH1, Ki-67 and
GAPDH from Shanghai Invitrogen Biotechnology Company were
dissolved with ddH2O and stored in small aliquots at
−20°C for later use. PCR amplification was initiated with one cycle
of 95°C for 10 min, followed by 40 cycles of 94°C for 15 sec and
60°C for 60 sec on a StepOne™ Real-Time PCR system (Thermo Fisher
Scientific, Inc.). The GAPDH was amplified as an internal
control. The relative quantification of SASH1 expression was
evaluated using the comparative quantification cycle (Cq) method.
The raw data were presented as the relative quantity of
SASH1, normalized with respect to GAPDH. Each sample
was examined in triplicate. Mean normalized gene expression ±
standard deviation (SD) were calculated from independent
experiments.
Western blot for detecting the
expression levels of SASH1
In each experiment, 100 mg of tissue sample
preserved in liquid nitrogen was homogenized with a protein
extraction kit. The homogenate was centrifuged at 16,000 × g for 30
min. The supernatant was saved, and its protein concentration was
determined with a protein quantification kit. A 6% stacking and 12%
separation SDS-PAGE gel were prepared, 50 µg of total protein was
applied to each lane, and electrophoresis was performed. The
proteins were transferred from the gel to a PVDF membrane and then
the PVDF membrane was blocked with 5% non-fat dry milk in TBST
buffer, and incubated with the rabbit anti-SASH1 polyclonal
antibody (1:500 dilution), and mouse anti-human monoclonal β-actin
antibody (1:1,000 dilution), respectively, followed by overnight
incubation at 4°C. The membrane was washed with TBST buffer, and
further incubated with secondary antibody: the secondary antibodies
with the corresponding IRDye 800 labeling (1:2,000 dilution in PBS)
at room temperature for 2 h. After TBST washing, film scanning was
performed with the Odyssey Infrared Imaging System (LI-COR
Biotechnology). The relative expression levels of SASH1 were
represented by the grayscale ratio of SASH1/β-actin. The grayscale
density was analyzed with QuantityOne version 4.62 software
(Bio-Rad Laboratories, Hercules, CA, USA).
Plasmid construction
Primer Premier 5 software (Premier Biosoft, Palo
Alto, CA, USA) was used to design for Primer on the flank of gene
SASH1 ORF and the restriction enzyme analysis. The forward primer,
5′-CGGGATCCATGGAGGACGCGGGAGCAGC-3′, contained the BamHI restriction
enzyme site; the reverse primer,
5′-CCCTCGAGCATGGCCTCAGGGCCTGGCG-3′, contained the XhoI restriction
enzyme site. All primers were synthesized by Shanghai Invitrogen
Corporation.
The fragment of gene SASH1 ORF was amplified
by PCR with the primers for the SASH1 gene. The products of
PCR were cloned into the pGEM-T vector. The correct recombinant
plasmid pGEM-SASH1 was identified with restriction
endonuclease, and sequenced. The vector pcDNA3.1 and the
recombinant plasmid pGEM-SASH1 were simultaneously digested
with restriction endonucleases BamHI and XhoI. The
targeted fragments were ligated by T4 DNA ligase, and the
recombinant plasmid pcDNA3.1- SASH1 were transformed into
DH5α competent cells (Sangon Biotech Co., Ltd., Shanghai,
China).
Cell culture and transfection
SKOV3 cells were cultured in RPMI-1640 medium,
containing 5% fetal bovine serum and penicillin (100
U/ml)/streptomycin (100 µg/ml) (Thermo Fisher Scientific, Inc.) at
37°C, 5% CO2. Cell growth was observed by an inverted
phase contrast microscope. When cell growth reached ~80%
confluence, the cells were digested with 0.25% trypsin and
passaged. The culture medium was changed each day, and the cells
were passaged every 2 to 3 days. Cells in the exponential growth
phase were selected for experiments.
SKOV3 cells that were cultured under normal
conditions were inoculated uniformly into 6-well culture plates at
a density of 3×105 cells/ml. According to the operating
instructions for Lipofectamine 2000, transfections were conducted
with 4 µg empty vector (pcDNA3.1) as a control or recombinant
expression plasmid pcDNA3.1-SASH1. The normal
(untransfected) control group was also established. RPMI-1640
medium without serum was used to dilute the plasmids, and 250 µl
Lipofectamine 2000 was added to the medium. After being mixed
mildly, the mixture was incubated under room temperature for 20
min, then added to the SKOV3 cell culture medium. After 5 h, the
culture medium was switched to RPMI-1640 medium containing 5% fetal
bovine serum, the mixture was incubated for another 48 h. Western
blot analysis was used to determine the expression of SASH1.
Determination of cell cycle by
FCM
The effect of SASH1 expression on the cell cycle of
SKOV3 cells was investigated with FCM. SKOV3 cells were cultured at
a density of 3×105 cells/ml in a 6-well plate in a
volume of 1,000 µl. The transfection methods and grouping were the
same as the above. A total of 48 h after transfection, the cells
were treated with trypsin, washed twice in PBS, and fixed in 70%
cold ethanol overnight at −20°C. The next day, after being washed
with PBS, the SKOV3 cells were incubated with RNase solution (100
µg/ml; Promega Corporation, Madison, WI, USA) for 30 min at 37°C.
Finally, the SKOV3 cells were incubated in propidium iodide (PI)
solution (100 µg/ml in PBS) in the dark at 4°C overnight. The PI
fluorescence of individual nuclei was measured with a FCM machine
(BD FACScalibur, BD Bioscience, San Jose, CA, USA).
Growth curve assay
After cell transfection for 24, 48, 72 or 96 h,
SKOV3 cells were stained with Hoechst 33342 dye (5 µg/ml). Stained
cells were observed under a fluorescence microscope (DM IL LED;
Leica Microsystems, Wetzlar, Germany), and cell numbers of the
total population were counted with the aid of an Image Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA). The cell
counting was performed using 10 visual fields in 3 wells.
FCM detection of the effects of SASH1
on the cellular apoptosis
The transfection methods and grouping were the same
as above. A total of 48 h after transfection, the cells were
digested by trypsin, washed twice in PBS, and resuspended in 195 µl
Annexin V-FITC binding buffer (Roche Diagnostics). A total of 5 µl
Annexin V-FITC was added and mixed gently, and the SKOV3 cells were
incubated at room temperature in the dark for 10 min. Then, the
SKOV3 cells were centrifuged at 1,000 × g for 5 min and gently
resuspended in 190 µl of Annexin V-FITC binding buffer, 10 µl PI
solution was added and mixed gently, and the cells were kept on ice
in the dark and immediately subjected to FCM using (BD FACSCalibur;
BD Biosciences, Franklin Lakes, NJ, USA). Cell Quest and Macquit
FCM software (BD Biosciences) were used to analyze the data. The
experiment was repeated three times.
Transwell detection of the effects of
SASH1 on the cellular migration
The number of cells that migrated through a
polycarbonate membrane was calculated to show the migration ability
of SKOV3 cells. Post-transfection, SKOV3 cells were plated on the
upper side of a polycarbonate membrane of Transwell chamber in
medium without serum. The cells were washed twice with PBS and
stained with Hoechst 33342 dye after being cultured under normal
conditions for 48 h. The number of cells migrating through the
Transwell polycarbonate membrane was calculated under a
fluorescence microscope (Leica DM IL LED): 10 randomly selected
fields were examined. The results are presented as the mean ± SD,
with three repeated experiments for each group.
Statistical analysis
Stata 7.0 (StataCorp LP, College Station, TX, USA)
software was used for statistical analysis using the χ2
test, t test and one-way analysis of variance. The threshold for
statistical significance was P<0.05.
Results
The expression of SASH1 in ovarian
carcinoma tissues
The mRNA and protein levels of ovarian carcinoma
tissues and adjacent normal tissues were evaluated and compared
using RT-qPCR and western blotting analysis, respectively. The
SASH1 mRNA expression rate was 60.8% in the ovarian carcinoma
tissues, which was significantly lower than that in adjacent normal
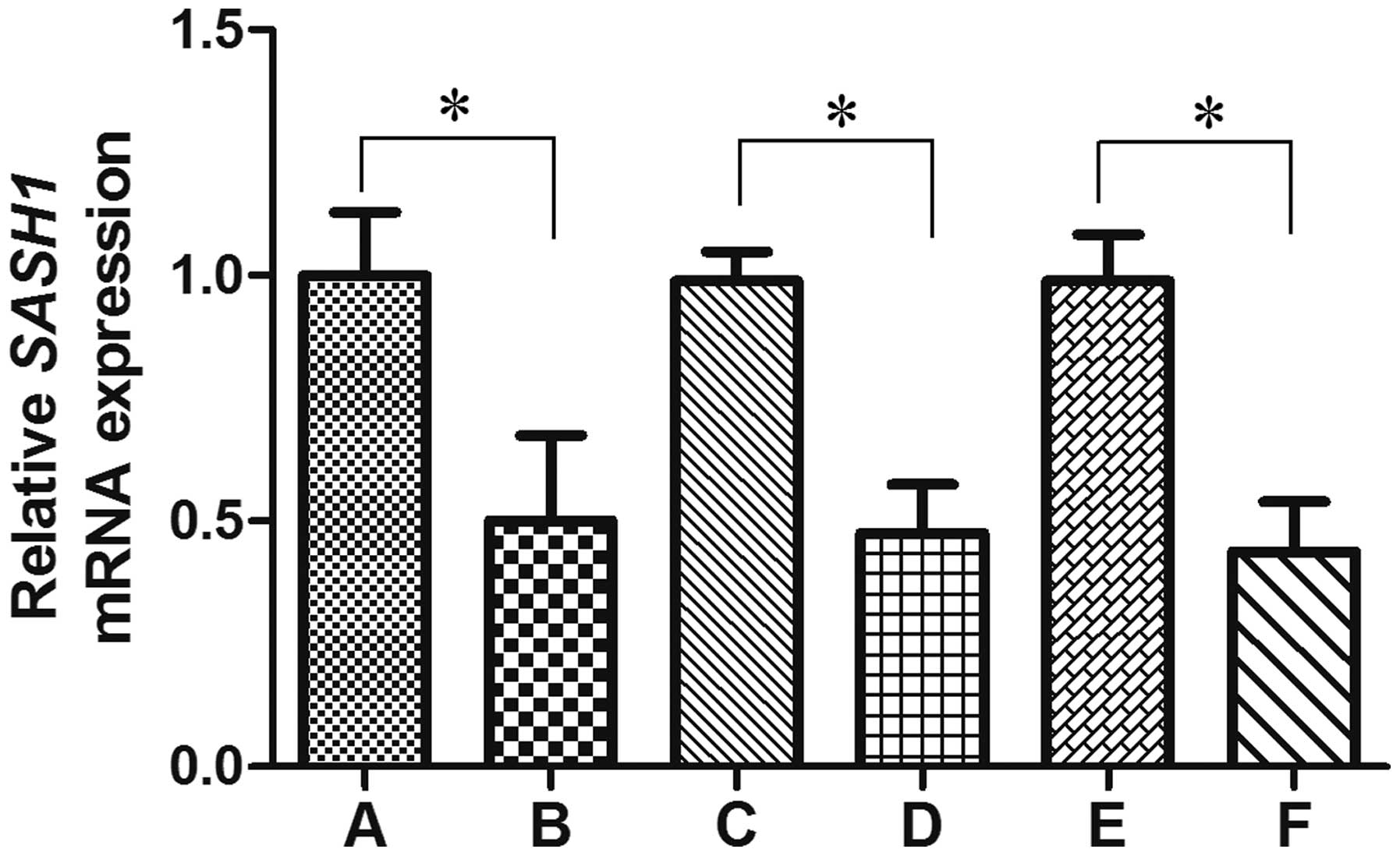
tissues (100.0%) (P=0.000) (Fig. 1).
The SASH1 mRNA expression level decreased in the ovarian carcinoma
tissues with increasing FIGO stage(P=0.016) (Fig. 1). The SASH1 mRNA expression level in
the ovarian carcinoma tissues from patients with lymph nodes
metastasis (46.0%) was significantly lower than that from patients
with negative lymph nodes metastasis (71.4%) (P=0.021) (Fig. 1). However, the expression of SASH1
mRNA in ovarian tissues was independent of the patient's age,
histological type or tumor size (P=0.953, 0.344, 0.127,
respectively), as shown in Table I.
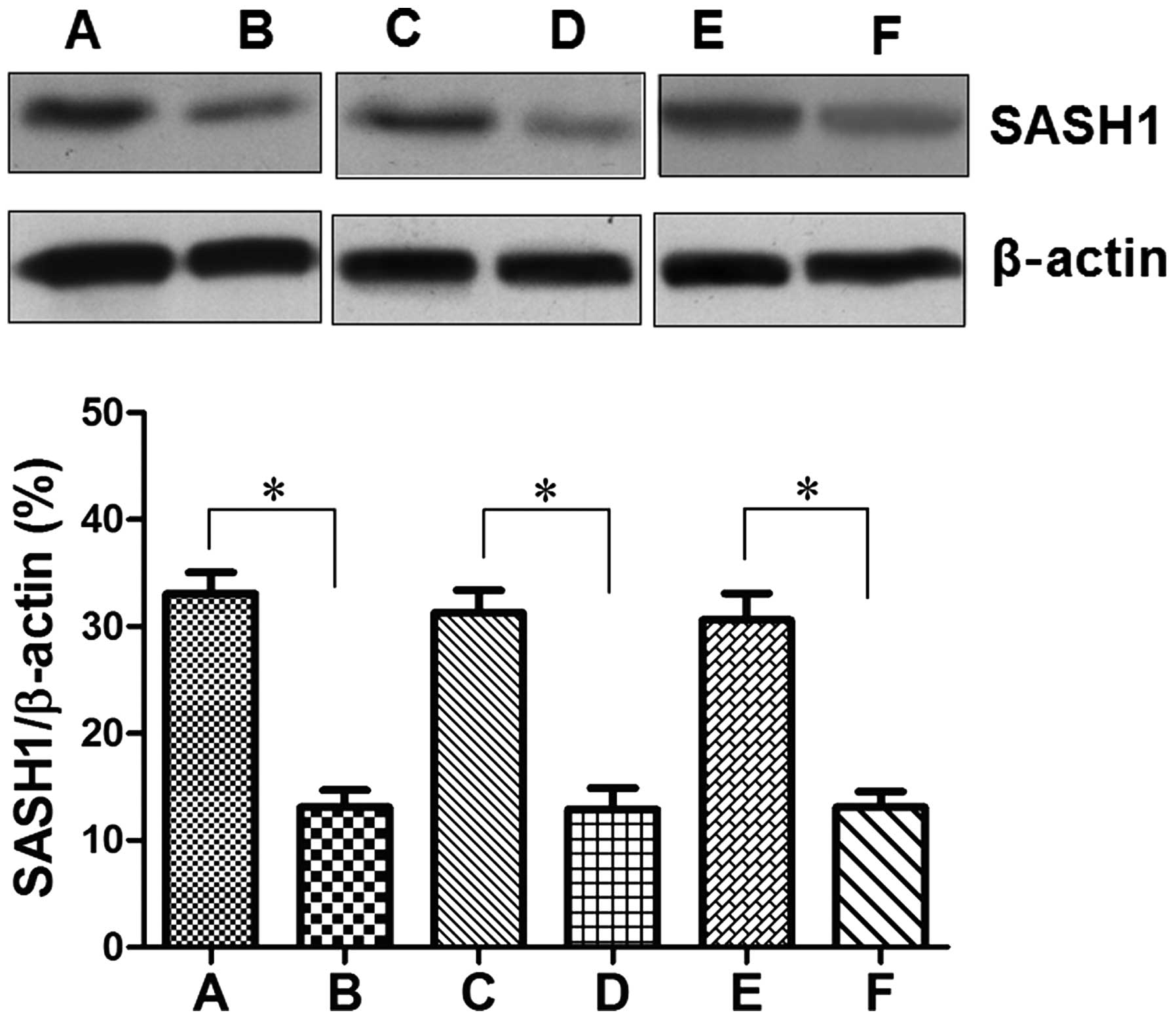
The protein levels of SASH1 in ovarian carcinoma tissues and
adjacent normal tissues were similar to the mRNA levels of SASH1
(Fig. 2).
Regarding the correlation of SASH1 mRNA
expression levels with Ki-67 mRNA expression, 20/48 patients with
SASH1 mRNA-positive expression were also positive for mRNA
expression of Ki-67. The mRNA expression levels of SASH1 and
Ki-67 in ovarian carcinoma were negatively correlated (r=−0.3189,
P=0.005) (Table II).
 | Table II.Correlation between SASH1 and
Ki-67 mRNA expression levels in ovarian cancer tissues. |
Table II.
Correlation between SASH1 and
Ki-67 mRNA expression levels in ovarian cancer tissues.
|
| SASH1 mRNA
expression |
|
|
|---|
|
|
|
|
|
|---|
| Ki-67 mRNA | Negative | Positive | r | P |
|---|
| Negative | 8 | 28 | −0.3189 | 0.005a |
| Positive | 23 | 20 |
|
|
The effect of SASH1 on SKOV3 cell
proliferation
To analyze the effect of SASH1 expression on the
biological characteristics of SKOV3 cells, recombined expression
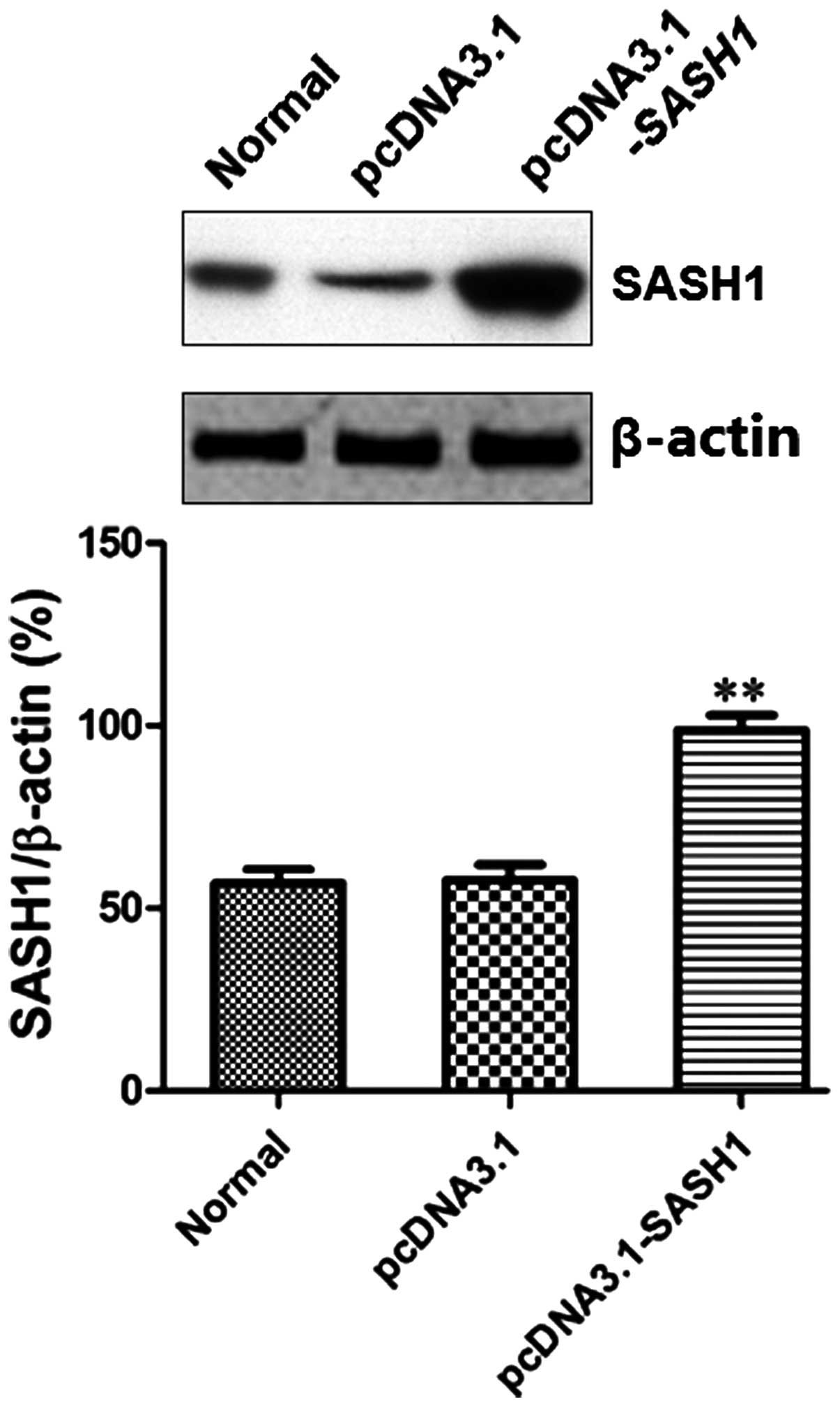
plasmid pcDNA3.1-SASH1 was constructed. After SKOV3 cells were
transfected with recombinant plasmid pcDNA3.1-SASH1 or empty vector
48 h, western blot was used to detect the expression of SASH1. The
result showed that the SASH1 protein level in the pcDNA3.1-SASH1
transfection group was significantly higher than that observed in
the normal control group (P<0.01) or the empty vector (pcDNA3.1)
control group (P<0.01) (Fig.
3).
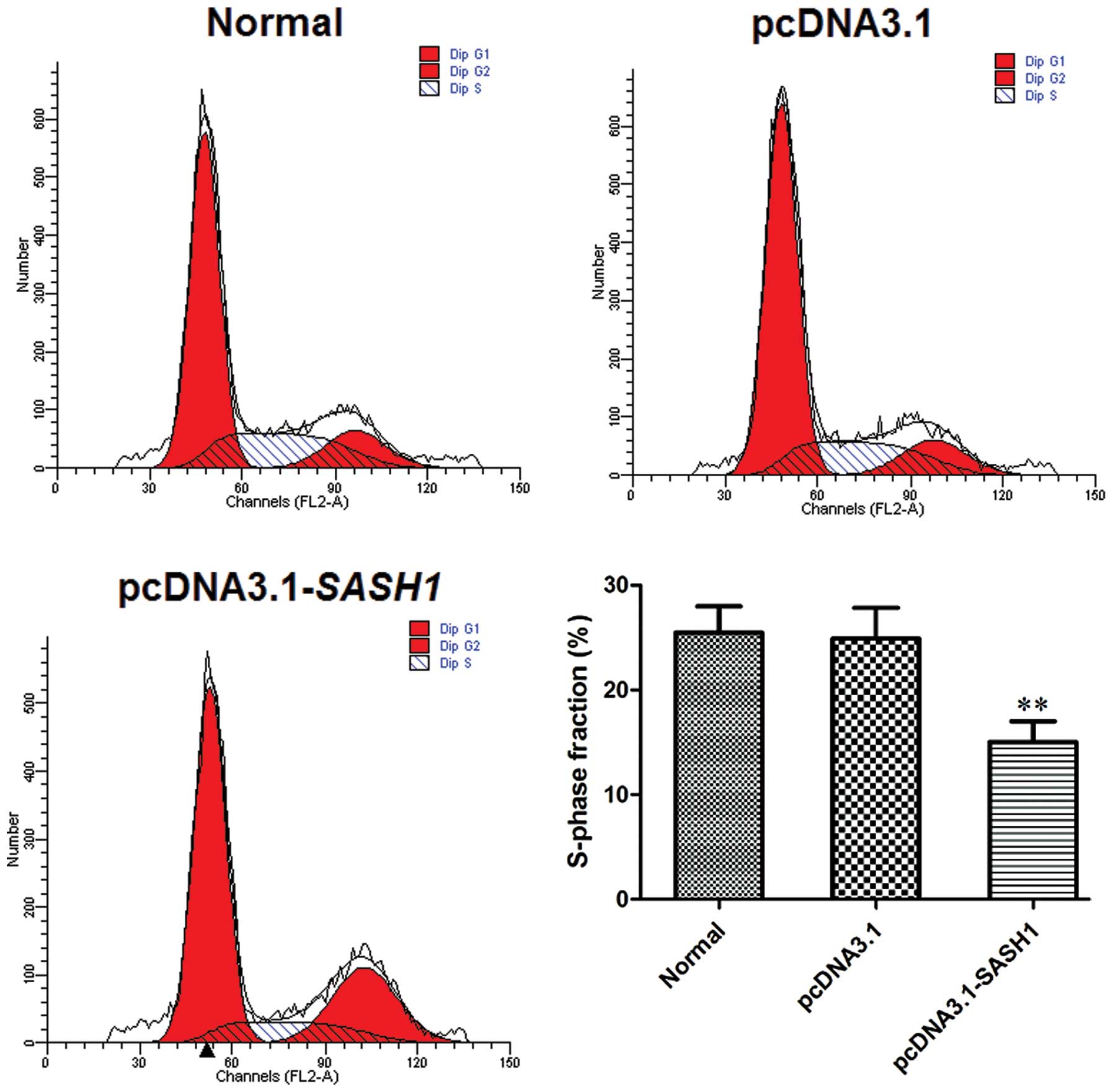
The effect of SASH1 on SKOV3 cell cycle was analyzed
by FCM. FCM analysis showed that the percentage of S-phase in the
pcDNA3.1-SASH1 transfected group was lower compared to the
normal control group (P<0.01) or the empty vector control group
(P<0.01) (Fig. 4). The S-phase
fraction (%) did not differ between the normal control group and
the empty vector control group.
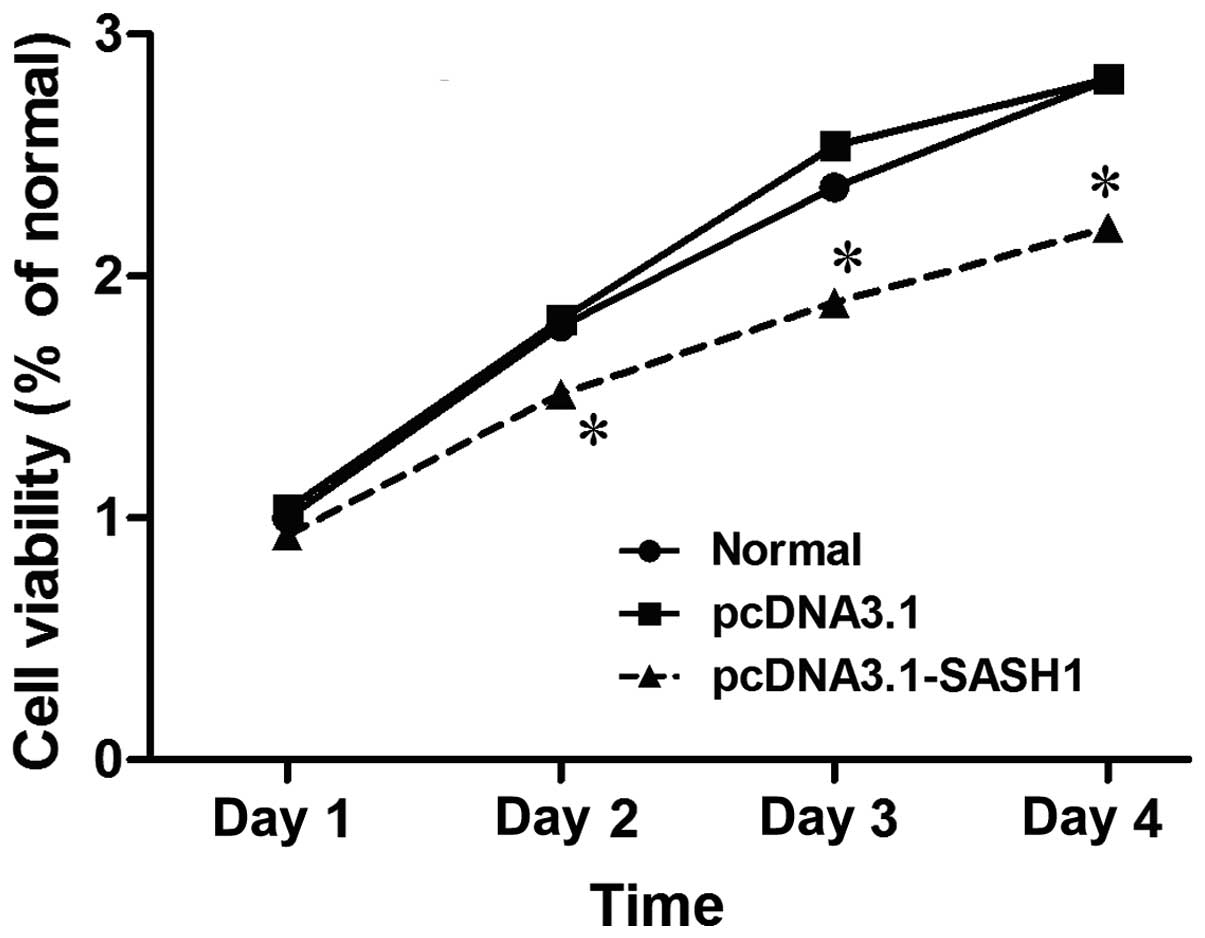
A cell growth curve was used to observe cell growth
of SKOV3 cells transfected with pcDNA3.1-SASH1. Cell growth
was reduced in SKOV3 cells transfected with pcDNA3.1-SASH1
for 48 h, 72 h or 96 h, compared with cells transfected with empty
vector or normal (control)(P<0.05)(Fig. 5). These results indicated that SASH1
may inhibit SKOV3 cell proliferation.
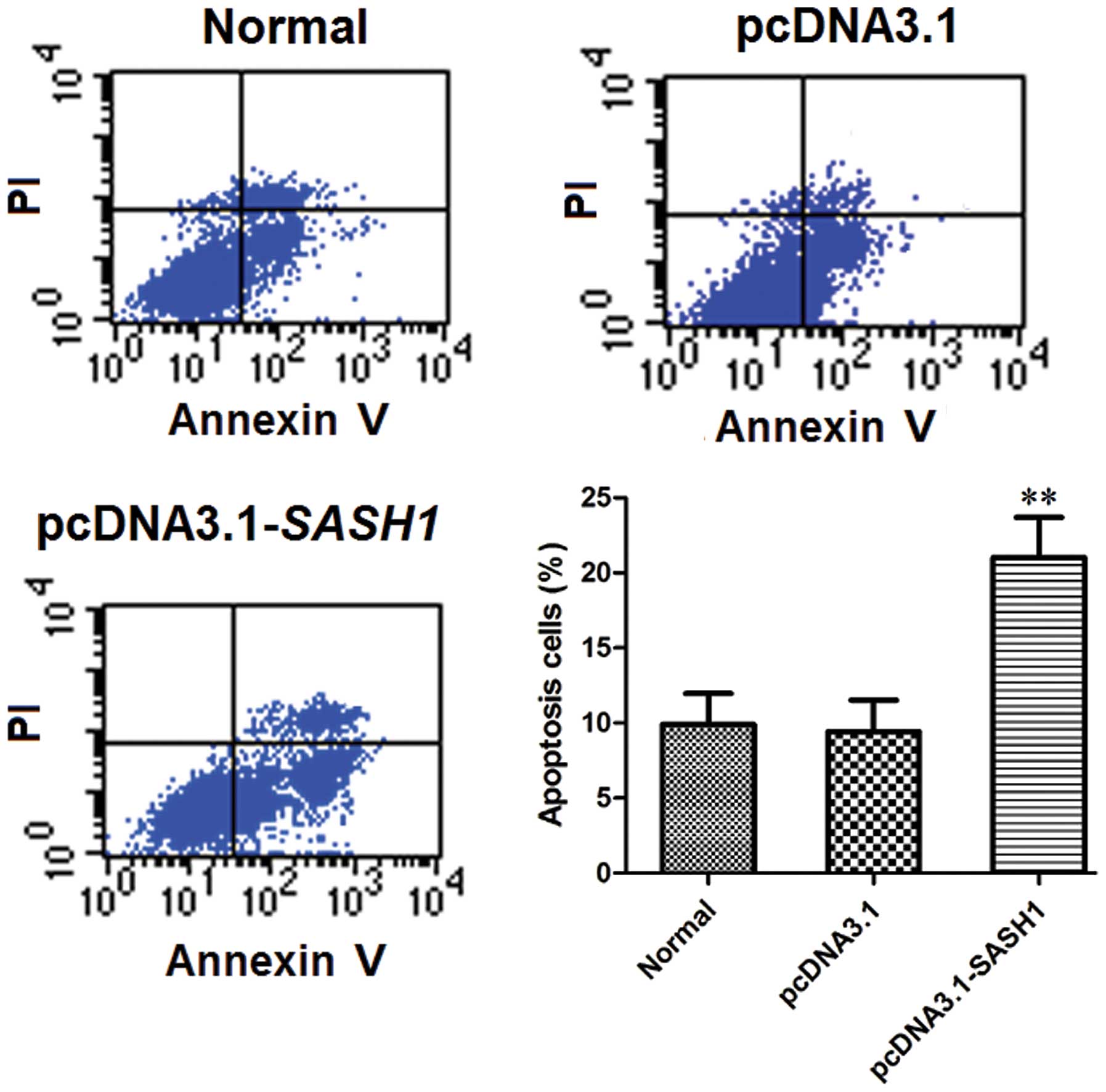
The effect of SASH1 on SKOV3 cell
apoptosis
FCM analysis of cell apoptosis levels showed that
the percentage of apoptotic cells in the normal control group and
the empty vector control group was significantly lower than that in
the pcDNA3.1-SASH1 transfection group (P<0.01) (Fig. 6). The percentage of apoptotic cells
did not differ significantly between the normal control group and
the empty vector control group. These data indicated that the
overexpression of SASH1 may enhance SKOV3 cell apoptosis.
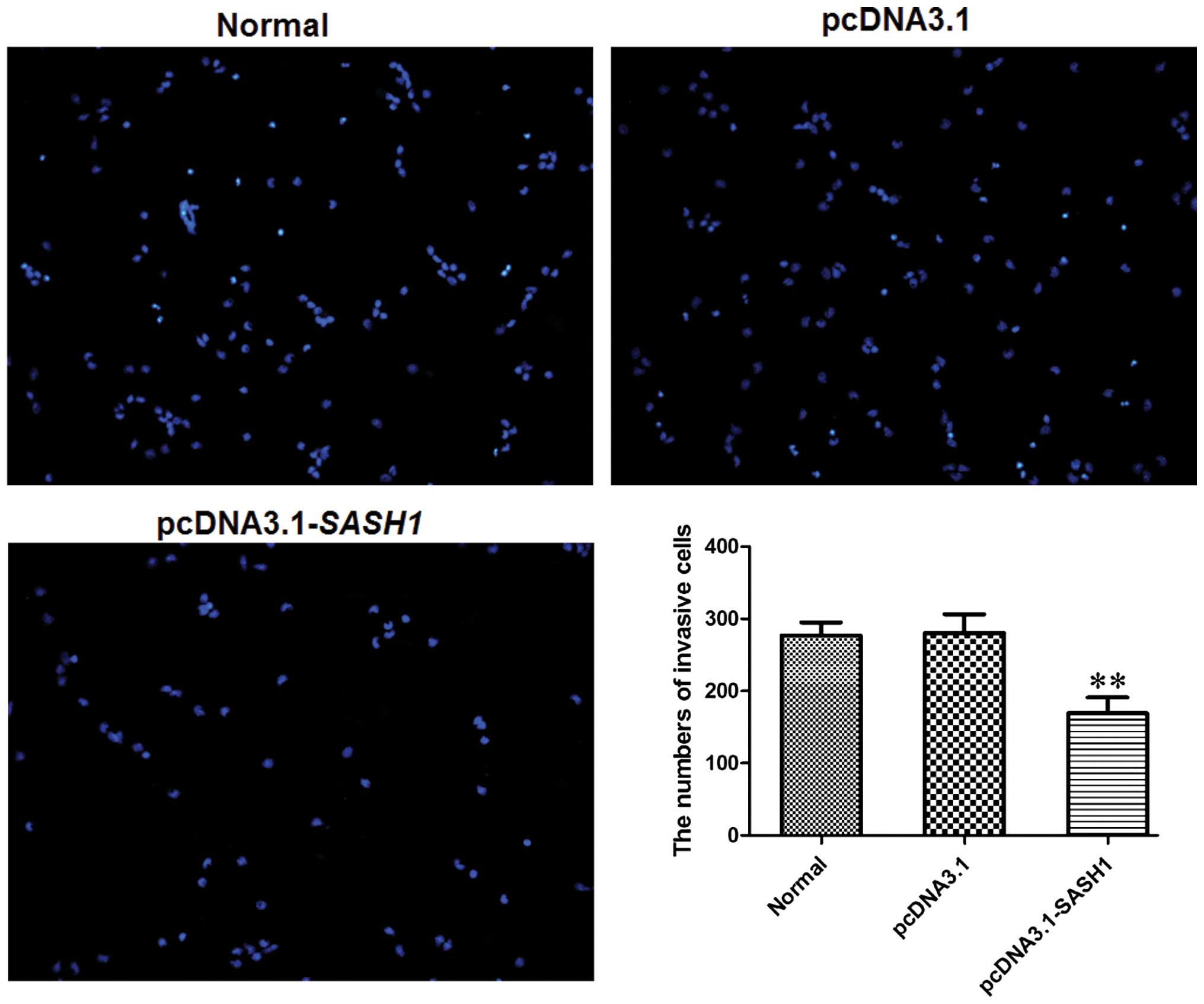
The effect of SASH1 on SKOV3 cell
migration
The Transwell invasion chamber system was used to
evaluate the invasive ability of transfected cells. The number of
cells in the pcDNA3.1-SASH1 transfection group that passed through
the polycarbonate membrane was decreased significantly (P<0.01)
compared to the normal control group and the empty vector control
group (Fig. 7). These results
suggested that SASH1 overexpression could suppress SKOV3 cell
migration.
Discussion
Ovarian carcinoma is one of the most common
gynecological tumors, with reported ~14,000 deaths in 2009
(8,27). Due to the late detection of this
disease, ~30% of patients with peritoneal metastasis at the time of
diagnosis had a five-year survival rate of ~26.9% (28,29). The
most common staging criteria, an ovarian carcinoma staging system,
was developed by FIGO (30,31). Although previous research has
attempted to explain the specific causes for ovarian carcinoma, the
molecular mechanism of occurrence and development of this disease
remains unclear. Therefore, to clarify the molecular mechanism of
metastasis of ovarian carcinoma cells is a serious challenge for
the clinical treatment and research of ovarian tumor in the
future.
SASH1 is located on 6q24.3, and includes 22
exons and 21 introns, and has two important structural domains:
SH3- and SAM-domains. Both domains can mediate protein-protein
interactions (13). However, the SAM
domains can serve a more complex function in the way that they
mediate protein-protein interactions through homologous and
heterologous oligomerization with the SAM regions on other proteins
(18). Meanwhile, the SAM domains can
mediate binding between Smaug proteins and mRNAs to regulate
transcription (13). SASH1 has been
suggested as a candidate tumor suppressor. The reduction or absence
SASH1 is closely related to tumor growth, invasion, metastasis, and
poor prognosis (13,14,23,24).
Zeller et al (13) reported
that SASH1 mRNA expression was significantly reduced or
completely absent in 6 breast cancer cell lines, and that it was
also significantly decreased in primary thyroid cancers. They also
showed that the down-regulation of SASH1 expression was
correlated with the degree of malignancy (13). These data suggested that the
down-regulation of SASH1 may serve an important role in tumor
occurrence, development, and evolution. However, the role of SASH1
in ovarian carcinoma remains unclear.
Ovarian carcinomas originate from the ovary, and can
be divided into various histopathological subtypes (32,33). They
differ in their biological behavior and response to current
treatment modalities (34). Despite
improvements in the application of aggressive cytoreductive surgery
and combination chemotherapy, and that the five year survival rate
of ovarian carcinoma has not improved, ovarian carcinoma has the
most unfavorable total prognosis and tendency to develop
chemotherapy resistance (27,35,36).
Therefore, investigation of novel genetic or molecular biomarkers
for early diagnosis, survival prediction, or therapeutic targets is
needed. In the present study, the expression of SASH1 in
ovarian carcinoma was investigated by RT-qPCR and western blotting
and the correlation between its expression with clinical
pathological features and clinical significance. The results
indicated that the expression of SASH1 in ovarian carcinoma tissue
was significantly lower than that in adjacent normal tissue. So, we
hypothesize that SASH1 may serve an important role in inhibiting
ovarian carcinoma cell proliferation, which may be an explanation
for the fact that SASH1 demonstrated down-regulation in ovarian
carcinoma tissues.
In order to analyze effects of SASH1 on SKOV3 cell
biological characteristics, a recombinant expression vector of
SASH1 was constructed. SASH1 was overexpressed in SKOV3
cells transfected with pcDNA3.1-SASH1, and a low expression
level was observed in the empty vector group and normal control
group. So, an overexpression cell model of SASH1 was successfully
established. The effects of SASH1 on SKOV3 cell growth or
proliferation by using cell growth curve or FCM. FCM analysis
showed that the percentage of cells in S-phase in the
pcDNA3.1-SASH1 transfection group was lower than that in the
normal group or empty vector group. The number of SKOV3
pcDNA3.1-SASH1 transfected cells decreased at all time
intervals. These results indicated that SASH1 may inhibit SKOV3
growth and proliferation. These results demonstrated that
SASH1 may be identified as a tumor suppressor gene by
inhibiting tumor cell growth and proliferation.
However, the exact mechanism remains to be
determined. FCM was used to detect the effect of SASH1 on SKOV3
apoptosis by Annexin V and PI double staining. FCM analysis of cell
apoptosis levels showed that the percentage of apoptotic cells in
the pcDNA3.1-SASH1 transfection group was significantly
higher than that in the normal group and the empty vector group.
The result suggested that SASH1 also enhanced apoptosis and
may inhibit tumor cell growth and proliferation through its role as
a tumor inhibitor gene.
Lymph nodes are involved in ~50–70% of cases of
advanced ovarian carcinoma (37–39).
Lymphatic metastasis is an important malignant progression factor
in ovarian carcinoma (40,41). In advanced disease (FIGO stages
III–IV) particularly, nodal metastases to the pelvic and
para-aortic lymph nodes are common (25). SASH1 expression in ovarian carcinoma
tissues from patients with lymph nodes metastases was significantly
lower than that in ovarian carcinoma tissues with negative lymph
nodes metastases. Moreover, SASH1 expression decreased as the FIGO
stages increased. These data suggested that SASH1 may be involved
in the invasion and metastasis of ovarian carcinoma, and SASH1 may
also serve an important role in suppressing metastasis processes.
Therefore, the present study investigated the effect of SASH1
expression on the invasion ability of SKOV3 cells by Transwell
migration assays. The number of cells in pcDNA3.1-SASH1
transfection group that passed through the polycarbonate membrane
was significantly reduced compared to the normal group and the
empty vector control group. SASH1 overexpression suppressed the
cell migration of SKOV3 cells The results indicated that SASH1 may
be involved in invasion and metastasis-associated molecular
pathway.
In conclusion, the results of the present study
indicated that the loss or down-regulation of SASH1 expression may
serve an important role in tumor occurrence, invasion and
metastasis of ovarian carcinoma. The specific mechanisms underlying
the effects of SASH1 on the tumor occurrence, progression, and
invasion of ovarian carcinoma require further research. These
studies may provide novel strategies and targets for the treatment
of ovarian carcinoma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402226).
References
|
1
|
Schwab CL, English DP, Roque DM, Pasternak
M and Santin AD: Past, present and future targets for immunotherapy
in ovarian cancer. Immunotherapy. 6:1279–1293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seidman JD, Vang R, Ronnett BM,
Yemelyanova A and Cosin JA: Distribution and case-fatality ratios
by cell-type for ovarian carcinomas: A 22-year series of 562
patients with uniform current histological classification. Gynecol
Oncol. 136:336–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thériault BL, Cybulska P, Shaw PA, Gallie
BL and Bernardini MQ: The role of KIF14 in patient-derived primary
cultures of high-grade serous ovarian cancer cells. J Ovarian Res.
7:1232014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ottolina J, Ferrandina G, Gadducci A,
Scollo P, Lorusso D, Giorda G, Breda E, Savarese A, Candiani M,
Zullo F and Mangili G: Is the endometrial evaluation routinely
required in patients with adult granulosa cell tumors of the ovary?
Gynecol Oncol. 136:230–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pham E, Birrer MJ, Eliasof S, Garmey E,
Lazarus D, Lee CR, Man S, Matulonis UA, Peters CG, Xu P, et al:
Translational impact of nanoparticle-drug conjugate CRLX101 with or
without bevacizumab in advanced ovarian cancer. Clin Cancer Res.
21:808–818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang X, Shen F, Hu W, Coleman RL and Sood
AK: New ways to successfully target tumor vasculature in ovarian
cancer. Curr Opin Obstet Gynecol. 27:58–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bian Z, Yu Y, Quan C, Guan R, Jin Y, Wu J,
Xu L, Chen F, Bai J, Sun W and Fu S: RPL13A as a reference gene for
normalizing mRNA transcription of ovarian cancer cells with
paclitaxel and 10-hydroxycamptothecin treatments. Mol Med Rep.
11:3188–3194. 2015.PubMed/NCBI
|
|
8
|
Ye H, Karim AA and Loh XJ: Current
treatment options and drug delivery systems as potential
therapeutic agents for ovarian cancer: A review. Mater Sci Eng C
Mater Biol Appl. 45:609–619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun X, Lou LG, Sui DH and Wu XH:
Preclinical activity of lobaplatin as a single agent and in
combination with taxanes for ovarian carcinoma cells. Asian Pac J
Cancer Prev. 15:9939–9943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen H, Cai M, Zhao S, Wang H, Li M, Yao S
and Jiang N: CYR61 overexpression associated with the development
and poor prognosis of ovarian carcinoma. Med Oncol. 31:1172014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vermeersch KA, Wang L, McDonald JF and
Styczynski MP: Distinct metabolic responses of an ovarian cancer
stem cell line. BMC Syst Biol. 8:1342014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen K, Ma H, Li L, Zang R, Wang C, Song
F, Shi T, Yu D, Yang M, Xue W, et al: Genome-wide association study
identifies new susceptibility loci for epithelial ovarian cancer in
Han Chinese women. Nat Commun. 5:46822014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeller C, Hinzmann B, Seitz S, Prokoph H,
Burkhard-Goettges E, Fischer J, Jandrig B, Schwarz LE, Rosenthal A
and Scherneck S: SASH1: A candidate tumor suppressor gene on
chromosome 6q24.3 is downregulated in breast cancer. Oncogene.
22:2972–2983. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rimkus C, Martini M, Friederichs J,
Rosenberg R, Doll D, Siewert JR, Holzmann B and Janssen KP:
Prognostic significance of downregulated expression of the
candidate tumour suppressor gene SASH1 in colon cancer. Br J
Cancer. 95:1419–1423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gambetta MC and Muller J: O-GlcNAcylation
prevents aggregation of the polycomb group repressor polyhomeotic.
Dev Cell. 31:629–639. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McCorvie TJ, Kopec J, Hyung SJ,
Fitzpatrick F, Feng X, Termine D, Strain-Damerell C, Vollmar M,
Fleming J, Janz JM, et al: Inter-domain communication of human
cystathionine beta synthase: Structural basis of
S-adenosyl-L-methionine activation. J Biol Chem. 289:36018–36030.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Courcet JB, Elalaoui SC, Duplomb L, Tajir
M, Riviere JB, Thevenon J, Gigot N, Marle N, Aral B, Duffourd Y, et
al: Autosomal-recessive SASH1 variants associated with a new
genodermatosis with pigmentation defects, palmoplantar keratoderma
and skin carcinoma. Eur J Hum Genet. 23:957–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dauphinee SM, Clayton A, Hussainkhel A,
Yang C, Park YJ, Fuller ME, Blonder J, Veenstra TD and Karsan A:
SASH1 is a scaffold molecule in endothelial TLR4 signaling. J
Immunol. 191:892–901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tolcher AW, Khan K, Ong M, Banerji U,
Papadimitrakopoulou V, Gandara DR, Patnaik A, Baird RD, Olmos D,
Garrett CR, et al: Anti-tumour activity in RAS-driven tumours by
blocking AKT and MEK. Clin Cancer Res. 21:739–748. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takata A, Terauchi M, Hiramitsu S, Uno M,
Wakana K and Kubota T: Dkk-3 induces apoptosis through
mitochondrial and Fas death receptor pathways in human mucinous
ovarian cancer cells. Int J Gynecol Cancer. 25:372–379. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao X, Zhou Y, Nie M, Xian S, Chen H, Wen
Y, Zhang L, Huang Y, Chen M and Wang S: EMSY promoted the growth
and migration of ovarian cancer cells. Tumour Biol. 36:3085–3092.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng Q, Zheng M, Liu H, Song C, Zhang W,
Yan J, Qin L and Liu X: SASH1 regulates proliferation, apoptosis
and invasion of osteosarcoma cell. Mol Cell Biochem. 373:201–210.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin S, Zhang J, Xu J, Wang H, Sang Q, Xing
Q and He L: Effects of SASH1 on melanoma cell proliferation and
apoptosis in vitro. Mol Med Rep. 6:1243–1248. 2012.PubMed/NCBI
|
|
24
|
Yang L, Liu M, Gu Z, Chen J, Yan Y and Li
J: Overexpression of SASH1 related to the decreased invasion
ability of human glioma U251 cells. Tumour Biol. 33:2255–2263.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeppernick F and Meinhold-Heerlein I: The
new FIGO staging system for ovarian, fallopian tube and primary
peritoneal cancer. Arch Gynecol Obstet. 290:839–842. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang S, Zhao X, Wang J, Wen Y, Zhang L,
Wang D, Chen H, Chen Q and Xiang W: Upregulation of microRNA-203 is
associated with advanced tumor progression and poor prognosis in
epithelial ovarian cancer. Med Oncol. 30:6812013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sasano T, Mabuchi S, Kuroda H, Kawano M,
Matsumoto Y, Takahashi R, Hisamatsu T, Sawada K, Hashimoto K, Isobe
A, et al: Preclinical efficacy for AKT targeting in clear cell
carcinoma of the ovary. Mol Cancer Res. 13:795–806. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Colombo PE, Boustta M, Poujol S, Jarlier
M, Bressolle F, Teulon I, Ladjemi MZ, Pinguet F, Rouanet P and Vert
M: Intraperitoneal administration of novel doxorubicin loaded
polymeric delivery systems against peritoneal carcinomatosis:
Experimental study in a murine model of ovarian cancer. Gynecol
Oncol. 122:632–640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pereira A, Pérez-Medina T, Magrina JF,
Magtibay PM, Rodríguez-Tapia A, Peregrin I, Mendizabal E and
Ortiz-Quintana L: International federation of gynecology and
obstetrics staging classification for cancer of the ovary,
fallopian tube and peritoneum: Estimation of survival in patients
with node-positive epithelial ovarian cancer. Int J Gynecol Cancer.
25:49–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grigoriadis C, Eleftheriades M,
Panoskaltsis T, Bacanu AM, Vitoratos N, Kondi-Pafiti A, Tsangkas A,
Tympa A and Hassiakos D: Ovarian cancer diagnosed during pregnancy:
Clinicopathological characteristics and management. G Chir.
35:69–72. 2014.PubMed/NCBI
|
|
32
|
Baratta MG, Schinzel AC, Zwang Y,
Bandopadhayay P, Bowman-Colin C, Kutt J, Curtis J, Piao H, Wong LC,
Kung AL, et al: An in-tumor genetic screen reveals that the BET
bromodomain protein, BRD4, is a potential therapeutic target in
ovarian carcinoma. Proc Natl Acad Sci USA. 112:232–237. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song Y, Xin X, Zhai X, Xia Z and Shen K:
Sequential combination therapy with flavopiridol and autocatalytic
caspase-3 driven by amplified hTERT promoter synergistically
suppresses human ovarian carcinoma growth in vitro and in mice. J
Ovarian Res. 7:1212014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Taube ET, Denkert C, Pietzner K, Dietel M,
Sehouli J and Darb-Esfahani S: Prognostic impact of neuroendocrine
differentiation in high-grade serous ovarian carcinoma. Virchows
Arch. 466:333–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chao A, Lai CH, Chen HC, Lin CY, Tsai CL,
Tang YH, Huang HJ, Lin CT, Chen MY, Huang KG, et al: Serum
microRNAs in clear cell carcinoma of the ovary. Taiwan J Obstet
Gynecol. 53:536–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu L, Hu Z, Liu J, Gao J and Lin B: Gene
expression profile analysis identifies metastasis and
chemoresistance-associated genes in epithelial ovarian carcinoma
cells. Med Oncol. 32:4262015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeong HJ, Kim HJ, Lee EH, Lee HW and Kim
MK: Perimenopausal ovarian carcinoma patient with subclavian node
metastasis proven by immunohistochemistry. J Menopausal Med.
20:43–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nafisi H, Cesari M, Karamchandani J,
Balasubramaniam G and Keith JL: Metastatic ovarian carcinoma to the
brain: An approach to identification and classification for
neuropathologists. Neuropathology. 35:122–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Resta G, Vedana L, Marino S, Scagliarini
L, Bandi M and Anania G: Isolated splenic metastasis of ovaric
cancer. Case report and literature review. G Chir. 35:181–184.
2014.PubMed/NCBI
|
|
40
|
Kumar PM and Manisha M: Epidural hematoma
secondary to solitary skull metastasis from an ovarian carcinoma.
Asian J Neurosurg. 9:112–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Longo R, Platini C, Eid N, Elias-Matta C,
Buda T, Nguyen D and Quétin P: A late, solitary brain metastasis of
epithelial ovarian carcinoma. BMC Cancer. 14:5432014. View Article : Google Scholar : PubMed/NCBI
|