Introduction
Since 2003, breast-conserving therapy (the
combination of breast-conserving surgery and post-operative
radiation therapy to the conserved breast) has been performed more
frequently than mastectomy for the treatment of stage I or II
invasive breast cancer (primarily tumors ≤3 cm) in Japan (1). Furthermore, it has been observed that
the combined usage of post-operative radiation not only prevents
recurrence within the preserved breast, but that it also
contributes to improved patient survival (1–3). Adverse
events, including radiation dermatitis and secondary cancer, have
been reported following the application of breast-conserving
therapy; however, the majority of radiation dermatitis cases
develop during the acute stages of the treatment and the mechanism
of late occurrence is unknown (1).
Radiation recall dermatitis has also been reported, which is a
condition triggered by drug administration following radiation
therapy, and is characterized by an inflammatory response that is
localized to the irradiated site of the body (4–9).
The present study describes a case of radiation
recall dermatitis that occurred 6 years and 4 months after
breast-conserving surgery, along with a review of the relevant
literature. Written informed consent was obtained for publication
of the present study.
Case report
A 37-year-old woman was referred to the Department
of Surgery II, Tokyo Women's Medical University (Tokyo, Japan) in
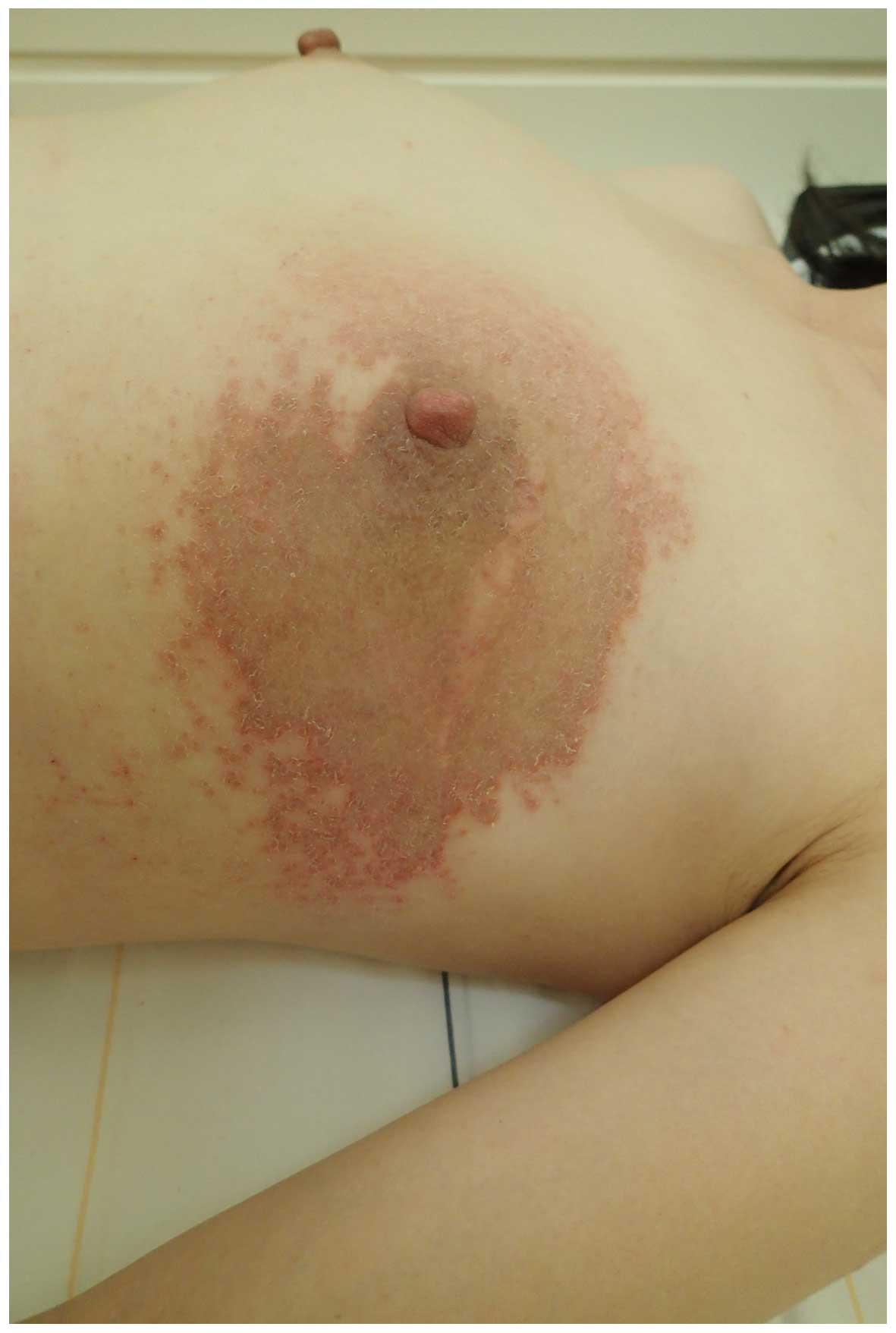
2014, presenting with scaly erythema and itching of the skin on the
left breast (Fig. 1). The patient had
undergone left breast-conserving surgery 6 years and 4 months
previously for the treatment of breast cancer. It was noted that a
relatively distinct border surrounded the erythema and was observed
at the irradiated site on the left breast (Fig. 1).
In 2007, the patient underwent left
breast-conserving surgery to treat breast cancer of the left upper
outer quadrant, with a sentinel lymph node biopsy removed for
analysis. The pathology showed invasive ductal carcinoma, T1b (7×6
mm); gland, lymphatic invasion, 0; venous invasion, 0; histological
grade, 2+1+1; lymph node, 0/1; estrogen receptor-positive,
progesterone receptor-positive, human epidermal growth factor
receptor 2-negative. Post-operatively, the patient was treated with
oral anti-estrogen (Nolvadex tablets, 20 mg/day) for 5 years and a
subcutaneous luteinizing hormone-releasing hormone agonist (Leuplin
SR 11.25 mg Injection kit) for 2 years. In addition, whole breast
radiation + additional radiation [4 MV X-ray at 46 Gy (23 times) +
9 Gy (3 times)] was applied to the remaining breast tissue.
Subsequently, follow-up was conducted on an outpatient basis. There
was no history of pregnancy and also no significant family history
noted.
During the present case, blood biochemistry
demonstrated that the white blood cell count was 5,300/µl (normal
range, 4,000–8,600/µl), indicating that there was no inflammatory
response, and all other measurements were within normal ranges.
Furthermore, the carcinoembryonic antigen level was 0.8 ng/ml
(normal range, ≤5.0 ng/ml), and the cancer antigen 15-3 level was
6.5 U/ml (normal range, ≤25.0 U/ml), indicating that tumor markers
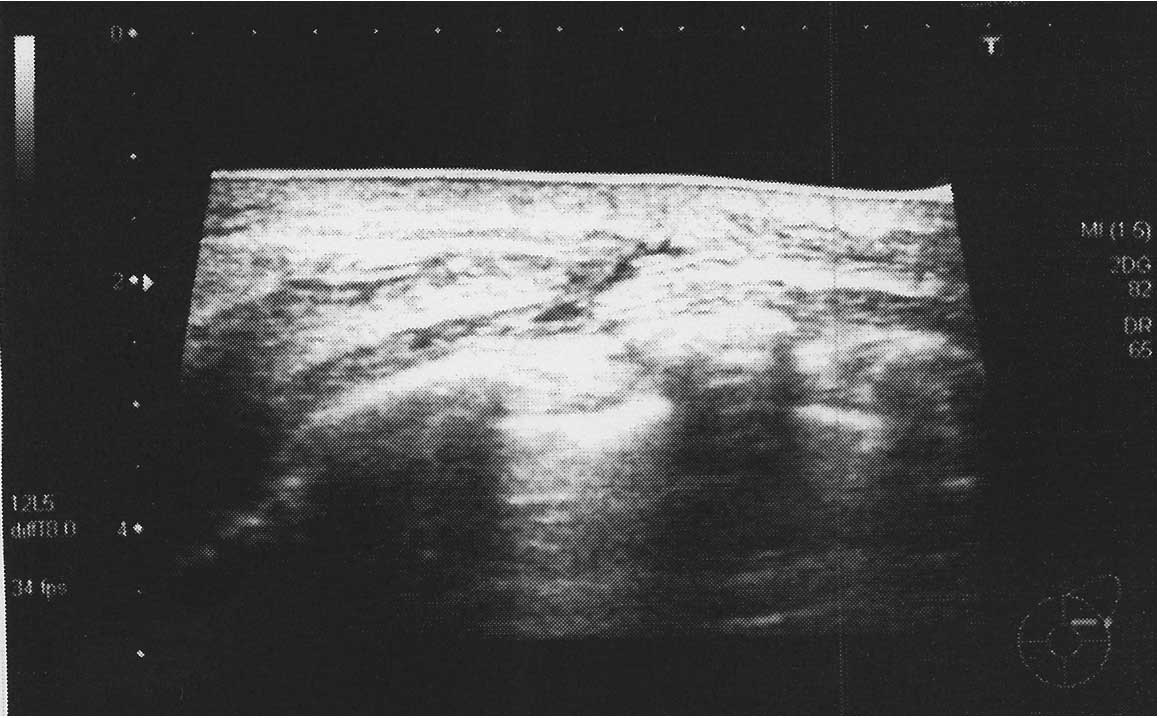
were not elevated. Ultrasonography of the breast detected skin
hypertrophy in the area of redness (Fig.
2). No marked lesions were observed in the mammary gland.
Despite erythema on the skin of the affected breast
initially suggesting the presence of inflammatory breast cancer,
the patient did not exhibit any signs of inflammation, including
burning sensations or pain. Breast ultrasonography only detected
skin hypertrophy. Since there was no dilation observed in the
lymphatic vessels, it was unlikely that the patient had
inflammatory breast cancer. Furthermore, a full body examination
was performed, including a chest X-ray, abdominal ultrasound and
bone scintigraphy, confirming that there was no breast cancer
recurrence.
Upon consultation with a dermatologist regarding the
development of the rash, the patient was diagnosed with eczema.
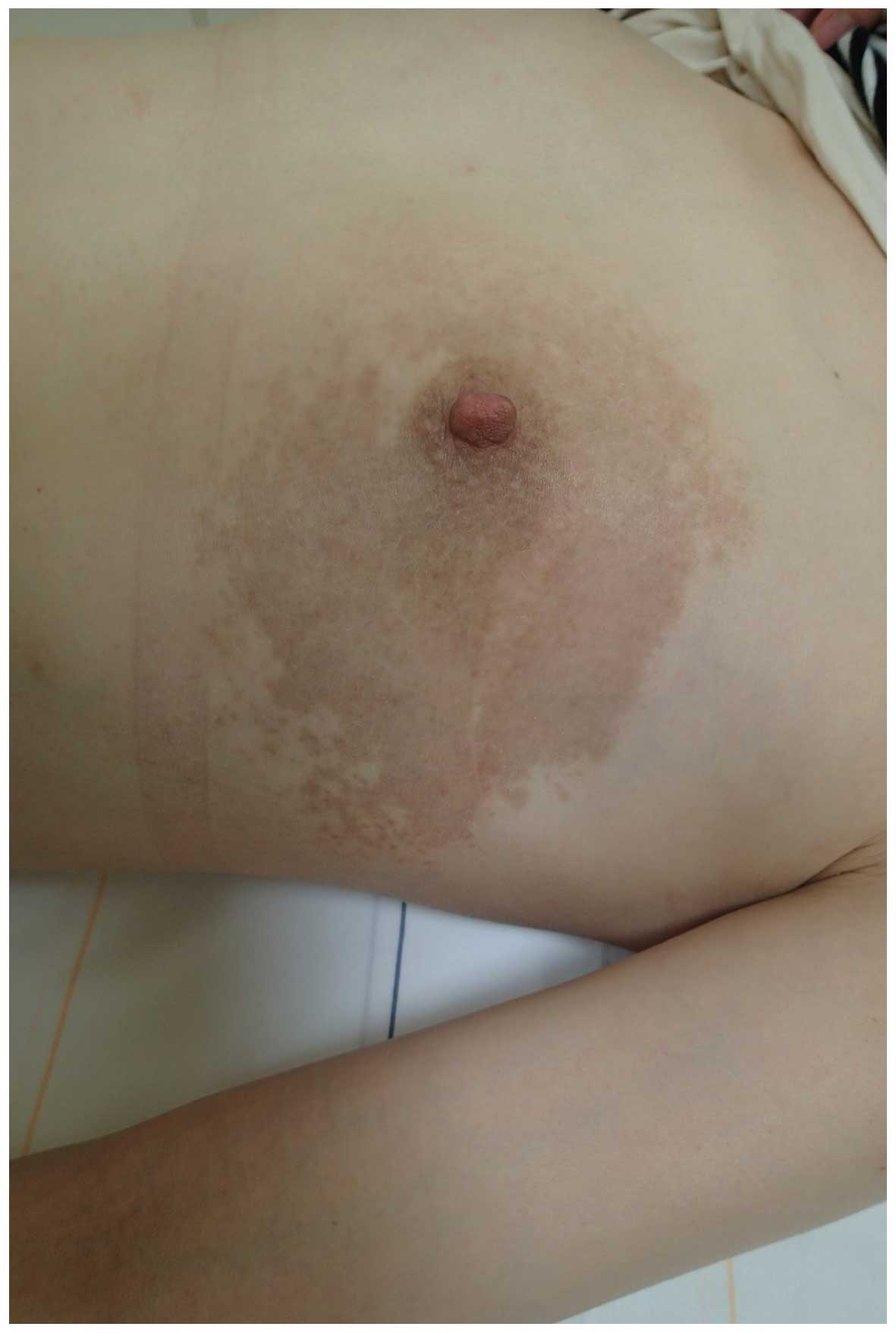
Following ~1 month of topical corticosteroid application (Antebate
ointment 0.05%) + oral second-generation antihistamine treatment
(Talion tablets, 10 mg/day), the erythema was alleviated and the
subjective symptoms, including the itching, also disappeared
(Fig. 3). Written informed consent
was obtained from the patient.
Discussion
Currently in Japan, breast-conserving therapy
(breast-conserving surgery and post-operative radiation therapy to
the conserved breast) is performed frequently for the treatment of
invasive breast cancer (primarily tumors ≤3 cm in size).
Furthermore, randomized, controlled studies have observed
significant decreases in cancer recurrence in the preserved breast
in all groups that received a combination of radiation therapy, and
it has been concluded, domestically and internationally, that
radiation treatments are necessary following breast-conserving
surgery (1,10). Additionally, it has been demonstrated
that radiation therapy not only prevents recurrence in the
preserved breast, but that it also contributes to improved patient
survival (1–3).
Radiation dermatitis is categorized into two groups:
Acute dermatitis and chronic dermatitis. Acute dermatitis develops
at sites that have received large doses of radiation over a short
period of time, whilst chronic dermatitis occurs at sites that have
repeatedly received small doses of radiation over a long period of
time. Radiation dermatitis and secondary cancer have been reported
as adverse events of combined treatment with post-operative
radiation, although the majority of radiation dermatitis cases are
considered to appear at the acute stage of treatment (1).
To the best of our knowledge, there are no previous
reports similar to the present case, where erythema with distinct
borders has rapidly appeared at the irradiated area 6 years and 4
months after breast-conserving surgery (6 years and 2 months after
radiation).
In the literature, a phenomenon called radiation
recall dermatitis has been reported; this is a condition triggered
by drug administration following radiation therapy, and is
characterized by an inflammatory response localized to the
irradiated body site (4–9). Although the precise mechanism underlying
radiation recall dermatitis is unknown, it has been proposed that
stem cell depletion, increased local vascular permeability,
overexpression of transforming growth factor β1 (TGF-β1) or the
expression of inflammatory cytokines may cause this condition
(4,5).
It has also been hypothesized that the condition is triggered by
specific drugs that are administered days to years after exposure
to ionizing radiation (6).
In general, it is considered to be difficult to
distinguish between a radiation-induced skin disorder and radiation
recall dermatitis. Nonetheless, in clinical practice, the two
conditions are often differentiated by focusing on the specific
clinical course of radiation recall dermatitis and on the fact that
the rash appears following the administration of certain drugs.
Drugs that initiate radiation recall dermatitis
include anticancer drugs (taxanes), antibiotics and
antituberculosis agents, with anticancer drugs accounting for
20–30% of cases (7).
In Western countries, four cases of tamoxifen
(TAM)-induced radiation recall dermatitis have been reported in
which the condition was triggered by the administration of
antiestrogen following radiation therapy for breast cancer
(Table I) (4,5,8,9). There
have been no reports thus far of any TAM-induced radiation recall
dermatitis cases located in Japan.
 | Table I.Cases of tamoxifen-induced radiation
recall dermatitis. |
Table I.
Cases of tamoxifen-induced radiation
recall dermatitis.
| Author, year | Gender/age,
years | Radiotherapy, Gy | Daily TAM dose,
mg | Skin biopsy | Appearance of
dermatitis | Treatment | Ref. |
|---|
| Kundranda and Daw,
2006 | F/48 | Post-operative,
50+14 | 20 | None | 6 daysa | Discontinuance of TAM
histamine antagonist | (4) |
| Singer et al,
2004 | F/88 | Pre-operative,
50.4+10 | 20 | Mild fibrosis and
chronic inflammation | 3 monthsa | Antibiotic
(levofloxacin) | (5) |
| Parry, 1992 | F/70 | Post-operative,
unknown | 20 | None | 5 daysa | Discontinuance of
TAM | (8) |
| Boström et al,
1999 | F/48 | Post-operative,
50+2 | 20 | Negative for
recurrence | 2 monthsa | Antibiotic
(clindamycin) steroid cream | (9) |
| Present case | F/37 | Post-operative,
46+9 | 20 | None | 1.25
yearsb | Histamine antagonist
steroid cream |
|
In all four of the aforementioned cases, erythema
appeared consistently at the site of irradiation within 3 months of
initiating oral TAM therapy, which was administered either
pre-operatively or post-operatively, and the rash promptly
disappeared with TAM discontinuation, or with the administration of
topical corticosteroids, oral antihistamines or oral antibiotics
(4,5,8,9).
Following careful examination of the rashes in the
studies regarding radiation recall dermatitis (4–9), it is
evident that, as in the present case, erythema with an itching
sensation localized to the area of irradiation was observed in all
cases. In addition, the patient in the present case had taken TAM
over a 5-year period post-operatively, and based on the evidence
that the rash appeared following the initiation of post-radiation
drug treatment, radiation recall dermatitis was suspected.
However, the patient in the present case had already
completed the oral TAM treatment, and therefore the timing of oral
TAM duration and rash development did not coincide, as observed in
the four cases that have been reported as TAM-induced radiation
recall dermatitis.
It has been suggested that a certain number of days
after the completion of radiation treatment, the irradiated site
becomes ‘prepared᾽ for radiation recall dermatitis, indicating that
the late development of the rash may depend on external stimuli or
the immune status of the patient, even in patients similar to the
present case where a year had passed since the completion of oral
medication (11).
In the present case, a skin biopsy was not
performed. Since there are few reports regarding biopsies performed
in cases of radiation recall dermatitis, information concerning the
typical pathological findings was not located. However, if a skin
biopsy had been performed, this would have permitted greater
examination of the pathology, possibly allowing confirmation of the
primary locus of inflammation in the skin tissue.
Radiation recall dermatitis has been described in a
limited number of cases in the radiological and dermatological
fields, but not in the surgical field. The incidence of this
disorder is predicted to increase, primarily due to the increasing
number of patients undergoing breast-conserving therapy, and also
due to the extended indications for various drugs (including
taxanes).
In conclusion, the present study reported a case of
radiation recall dermatitis that developed 6 years and 4 months
after breast-conserving surgery. It is predicted that the incidence
of this disease will increase in the future. Therefore, when
erythema appears during post-operative follow-up at an area that
coincides with the irradiated site, routine medical care should be
provided, keeping in mind the possibility of radiation recall
dermatitis, in addition to inflammatory breast cancer.
Acknowledgements
The authors would like to gratefully acknowledge the
assistance of Forte Science Communications (Tokyo, Japan) in the
preparation of this manuscript.
Glossary
Abbreviations
Abbreviations:
References
|
1
|
The Japanese Breast Cancer Society:
Clinical Practice Guidelines for Breast Cancer Treatment (2nd).
Kanehara & Co., Ltd. 2013.
|
|
2
|
Vinh-Hung V and Verschraegen C:
Breast-conserving surgery with or without radiotherapy:
Pooled-analysis for risks of ipsilateral breast tumor recurrence
and mortality. J Natl Cancer Inst. 96:115–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG). Darby S, McGale P, Correa C, Taylor
C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J,
et al: Effect of radiotherapy after breast-conserving surgery on
10-year recurrence and 15-year breast cancer death: Meta-analysis
of individual patient data for 10,801 women in 17 randomised
trials. Lancet. 378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kundranda MN and Daw HA: Tamoxifen-induced
radiation recall dermatitis. Am J Clin Oncol. 29:637–638. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singer EA, Warren RD, Pennanen MF, Collins
BT and Hayes DF: Tamoxifen-induced radiation recall dermatitis.
Breast J. 10:170–171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Camidge R and Price A: Characterizing the
phenomenon of radiation recall dermatitis. Radiother Oncol.
59:237–245. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hird AE, Wilson J, Symons S, Sinclair E,
Davis M and Chow E: Radiation recall dermatitis: Case report and
review of the literature. Curr Oncol. 15:53–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parry BR: Radiation recall induced by
tamoxifen. Lancet. 340:491992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boström A, Sjölin-Forsberg G, Wilking N
and Bergh J: Radiation recall - another call with tamoxifen. Acta
Oncol. 38:955–959. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al:
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG):
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chihiro N, Ken I, Kazuto H and Goichi T:
Radiation recall dermatitis induced by cetuximab in a patient with
advanced colon carcinoma. Japanese J Clin Dermatol. 66:14–18.
2012.(In Japanese).
|