Introduction
The most common brain tumours are intracranial
metastases (1). Over 20% of patients
with solid malignant tumours develop brain metastases (BM);
patients with small-cell lung cancer (45%), melanomas (45%),
non-small-cell lung cancer (30%), breast cancer and renal cancer
(20%) have the highest risk for the occurrence of intracranial
metastases (2). The incidence of
clinically detected BM in patients with metastatic breast cancer is
16–20%, and autopsy studies suggest a prevalence of BM of up to 34%
(3–6).
A number of risk factors for cerebral metastases in breast cancer
patients have been reported in the literature; in addition to young
age (<35 years), large primary tumour size (>2 cm)and
negative estrogen receptor (ER) status, the overexpression of human
epidermal growth factor receptor 2 (HER2) has been demonstrated to
be associated with a higher risk of BM (7–10).
The management of BM includes radiotherapeutic,
surgical and medical options. Consideration of a local treatment
modality depends on the number of intracranial metastases and
potential local complications resulting from their location and
size. Patients with multiple BM typically receive whole-brain
radiotherapy (WBRT), whilst neurosurgery or stereotactic
radiosurgery (SRS) are possible treatment options for patients with
a single BM. The application of intrathecal chemotherapy may be
selected as the therapeutic regime in cases with meningeal
involvement (11).
The aim of the current study was to characterise the
role of radiotherapy in patients with BM from metastatic breast
cancer in the context of modern local therapy modalities, current
systemic treatment options and prognostic factors.
Patients and methods
Patients
The current retrospective analysis was conducted on
female patients with BM from breast cancer who were treated at the
Department of Radiation Oncology of Martin Luther University
Halle-Wittenberg [Halle (Saale), Germany] between the January 2000
and December 2010. A total of 86 consecutive patients were
identified. Patient charts were reviewed to obtain data regarding
the primary tumour, including the date of diagnosis, ER status and
HER2 status, in addition to data on the diagnosis of metastatic
disease, including BM, as well as treatment and follow-up
information. Survival status was determined via local registry
offices for each patient. This retrospective analysis was performed
in concordance with the Helsinki declaration.
Statistical analysis
Overall survival was defined as the time from the
start of radiotherapy of BM until the date of mortality or last
follow-up. Patients were censored if alive at last follow-up.
Survival analyses were performed using SPSS software version 19
(IBM SPSS, Armonk, NY, USA). Survival times were calculated by the
Kaplan-Meier method, and survival of subgroups was compared by
log-rank test. P<0.050 was considered to indicate a
statistically significant difference. In order to identify
confounding factors, the multivariate Cox regression method was
used.
Results
Baseline characteristics
Patient characteristics are presented in Table I. The age at initial diagnosis ranged
from 26 to 81 years (median, 57.7 years) in the present cohort. In
38 cases (44.2%), there was detection of ER or progesterone
receptor (PR) or both; 39 patients (45.4%) had hormone receptor
(HR)-negative breast cancer at diagnosis. The HER2-overexpressing
group, including those with HER2 expression detected at any time,
contained 35 patients (40.7%). BM were diagnosed via computed
tomography (29%) or magnetic resonance imaging (64%), whilst the
type of imaging was not documented in 7%. The median time from
diagnosis of primary tumour until detection of BM was 33.4 months
(range, 0–391.03 months). More than 3 BM were found in 55 patients
(64.0%). There were 34 patients (39.5%) in whom the diameter of the
largest BM was ≤20 mm.
 | Table I.Patient characteristics (n=86). |
Table I.
Patient characteristics (n=86).
| Parameter | Value |
|---|
| Age at diagnosis of
BM, years |
|
|
Median | 58 |
|
Range | 26–81 |
| T stage of primary
tumour |
|
| T1 | 20 (23.3%) |
| T2 | 32 (37.2%) |
| T3 | 9
(10.5%) |
| T4 | 19 (22.1%) |
|
Tx/n.a. | 6 (6.9%) |
| N stage of primary
tumour |
|
| N0 | 28 (32.5%) |
| N1 | 40 (46.5%) |
| N2 | 4 (4.7%) |
| N3 | 5 (5.8%) |
|
Nx/n.a. | 9
(10.5%) |
| M stage of primary
tumour |
|
| M0 | 50 (58.1%) |
| M1 | 21 (24.4%) |
|
Mx/n.a. | 15 (17.5%) |
| Grade of primary
tumour |
|
| G1 | 2 (2.3%) |
| G2 | 33 (38.4%) |
| G3 | 40 (46.5%) |
|
Gx/n.a. | 11 (12.8%) |
| HR status of
primary tumour |
|
|
ER-positive | 9
(10.5%) |
|
PR-positive | 2 (2.3%) |
|
ER+PR-positive | 27 (31.4%) |
|
HR-negative | 39 (45.4%) |
|
n.a. | 9
(10.4%) |
|
HER2-statusa |
|
|
Positive | 35 (40.7%) |
|
Negative | 39 (45.3%) |
|
n.a. | 12 (14.0%) |
| Extracranial
disease |
|
| Bone
only/no visceral metastases | 13 (15.1%) |
|
Bone+visceral or only visceral
metastases | 70 (81.4%) |
|
n.a. | 3 (3.5%) |
| BM: number of
lesions |
|
| ≤3 | 20 (23.3%) |
|
>3 | 55 (63.9%) |
|
n.a. | 11 (12.8%) |
| BM: diameter of
largest lesion |
|
| ≤20
mm | 34 (39.5%) |
| >20
mm | 22 (25.6%) |
|
n.a. | 30 (34.9%) |
| BM: type of
therapy |
|
|
Surgery | 6 (6.9%) |
|
WBRT | 86 (100%) |
|
Boost | 19 (22.1%) |
| WBRT, total
dose |
|
| 30
Gy | 25 (29.1%) |
| 36
Gy | 40 (46.5%) |
| 40
Gy | 5 (5.8%) |
|
Other | 16 (18.6%) |
| Boost
treatment |
|
|
Single | 1 (1.2%) |
|
Fractionated | 15 (17.4%) |
|
Single+fractionated | 3 (3.5%) |
| No
boost | 67 (77.9%) |
Therapy for BM included WBRT for all patients. The
dose ranged from 3 to 46 Gy (median dose, 36 Gy; early termination
after 3 Gy in two cases). In addition a boost treatment was applied
in 19 patients (22.1%): 1 patient received a single-fraction boost,
15 patients were treated with a fractionated scheme and 3 patients
received both types of boost. The median total dose of fractionated
boost treatment was 18 Gy (range, 6–30 Gy). The doses for
single-fraction boost were 5 Gy (1 case) and 17 Gy (3 cases). In
53% of patients, systemic therapy (chemotherapy, hormonotherapy or
therapy with the monoclonal antibody against HER2, trastuzumab) was
applied following the commencement of brain irradiation. Taxanes
and capecitabine were the most frequently used chemotherapeutic
agents, and 17 patients (19.8%) were treated with trastuzumab.
Survival
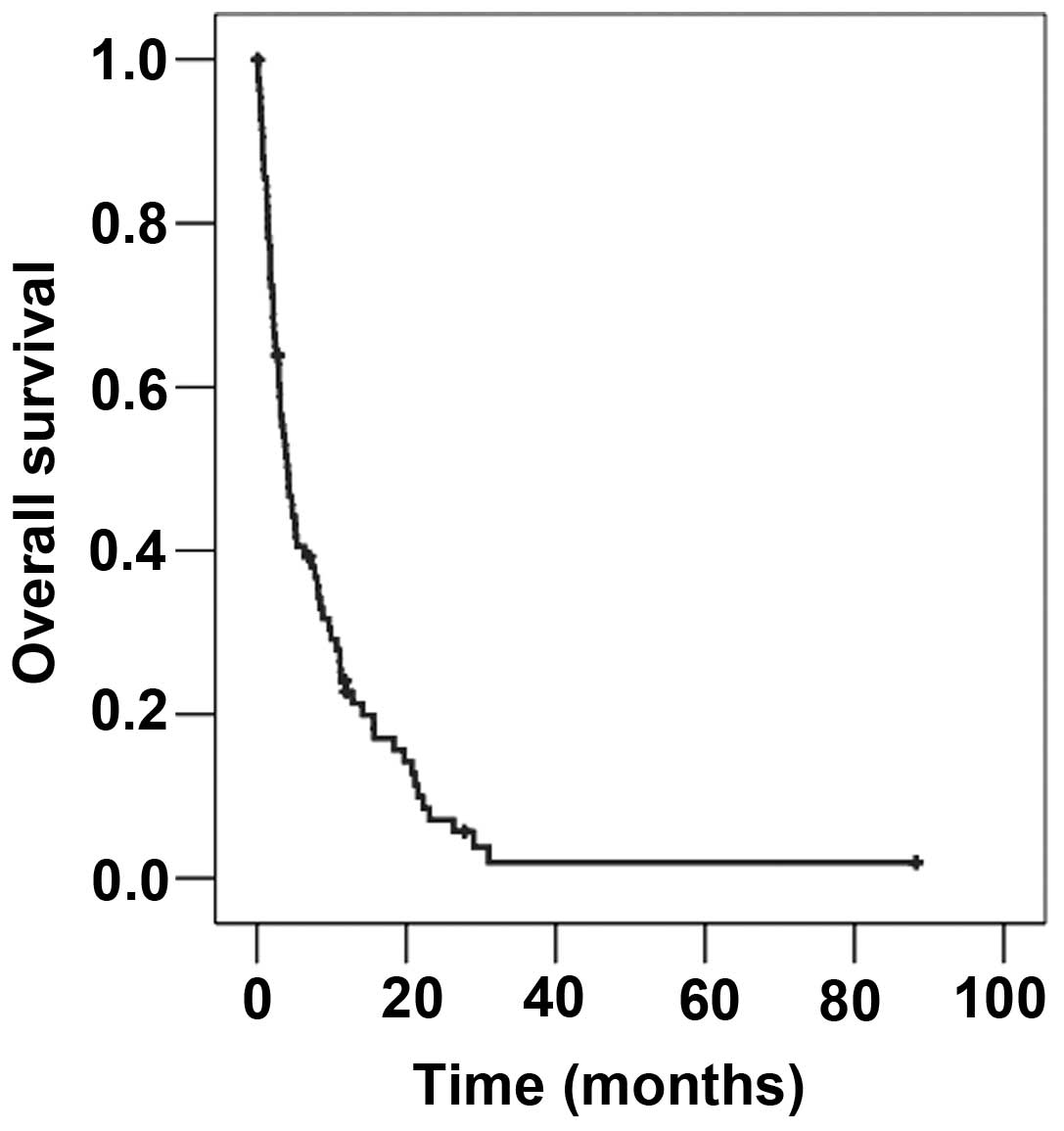
The median survival time from the beginning of
radiotherapy of BM in the entire cohort was 4.14 months (Fig. 1). The median age at the beginning of
radiotherapy was 58.0 years. Patients younger than 58 years
survived a median of 4.0 months, whilst patients older than this
survived 4.14 months (P=0.592).
There was no statistical evidence for the effect of
initial tumour stage or grade on survival. The median survival
times according to stage and grade were as follows: T1, 3.8 months;
T2, 3.1 months; T3, 2.5 months; T4, 4.7 months (T-stage, P=0.642);
N1, 3.7 months; N2, 2.9 months; N3, 4.3 months (N-stage, P=0.468);
M0, 3.8 months; M1 7.7 months (M-stage, P=0.265); G1/G2, 6.3
months; G3, 3.5 months (grade, P=0.250). Similarly, HR-status did
not influence survival in this study. Patients with HR-positive
breast cancer survived 4.2 months vs. 4.1 months for women with
HR-negative disease (P=0.683). The median survival time in the
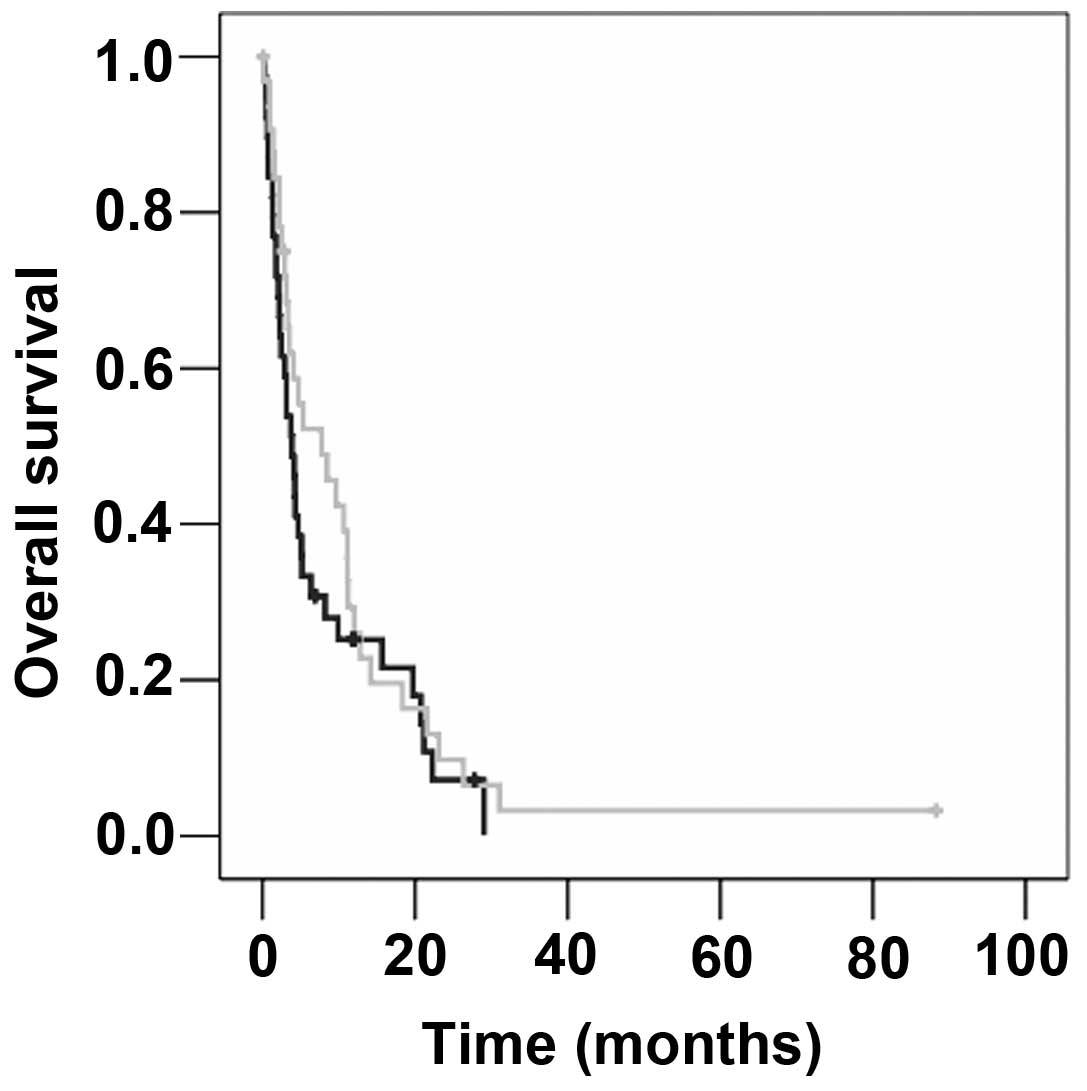
group with HER2-overexpressing metastatic breast cancer was longer
than that of patients with HER2 non-overexpressing disease (7.7 vs.
3.8 months), but this difference was not significant (P=0.317;
Fig. 2). Localisation of distant
metastases, histology of primary tumour, diameter of largest BM and
treatment with trastuzumab were also factors that did not
significantly influence survival.
On univariate analysis, the median survival time of
patients who underwent surgery was significantly longer than those
who did not undergo surgery with (26.4 months vs. 4.0 months;
P=0.003). The investigation of WBRT-dose revealed a median survival
time of 2.2 months for patients treated with ≤35 Gy vs. 8.5 months
for those treated with >35 Gy (P=0.001). Another significant
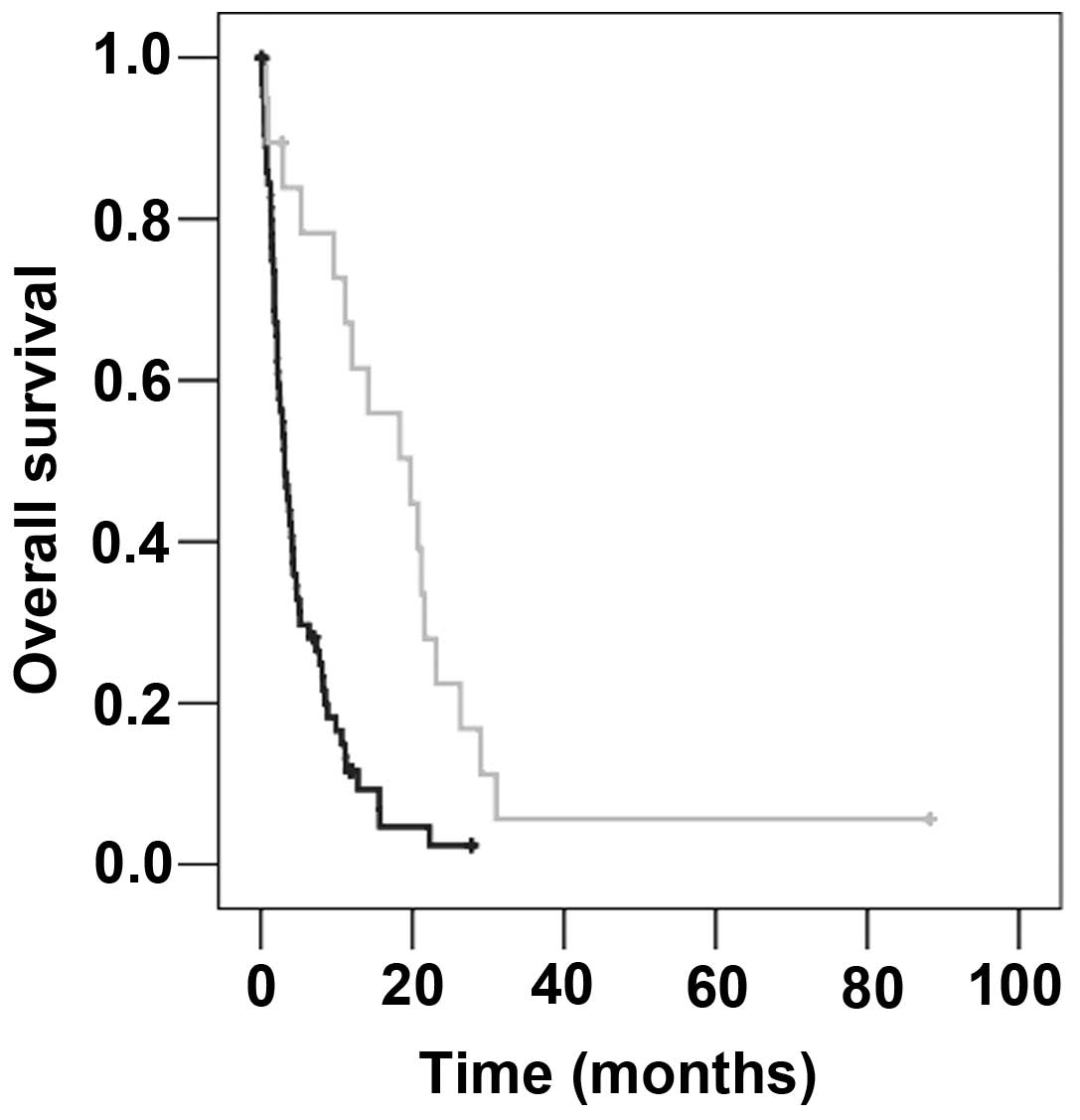
factor was the use of boost irradiation subsequent to WBRT (boost
vs. no boost, 19.7 vs. 3.1 months; P<0.001; Fig. 3; Table
II).
 | Table II.Results of univariate and
multivariate analyses. |
Table II.
Results of univariate and
multivariate analyses.
|
| P-value |
|---|
|
|
|
|---|
| Variable | Log-rank test | Cox regression |
|---|
| Number of brain
metastases | 0.050 | 0.200 |
| Surgery | 0.003 | 0.164 |
| Whole-brain
radiotherapy dose | 0.001 | 0.063 |
| Boost
treatment | <0.001 | 0.030 |
| Chemotherapy | 0.050 | 0.433 |
The number of BM was slightly associated with
survival time: Patients with ≤3 BM survived 7.3 months compared
with 4.0 months in patients with >3 BM (P=0.050). Equally,
marginally prolonged survival was observed in patients receiving
chemotherapy following the commencement of radiation therapy
compared with those who did not receive chemotherapy (2.1 vs. 1.3
months; P=0.050).
All factors that appeared to exhibit a slight trend
(P<0.100) on univariate analysis were included in a multivariate
Cox regression analysis (Table II).
According to this analysis, only boost treatment following WBRT was
a statistically significant factor affecting survival time
(P=0.030).
Discussion
In the present study of patient with BM from breast
cancer, the median overall survival time for the entire group of
patients was 4.14 months. In comparison to other data in the
literature, this interval is slightly shorter; median survival
times between 4.5 and 10 months have been reported (12–16).
A number of studies have reported a significantly
longer survival time for patients with BM in HER2-overexpressing
breast cancer (17–19). In the present analysis, a
non-significantly prolonged median survival time of patients with
HER2-positive disease was observed. These findings may be explained
by the so-called HER2-paradigm (20).
Thereby, specific therapy against HER2 with trastuzumab, typically
combined with other chemotherapeutic agents, leads to improved
extracranial tumour control compared to HER-2-negative patients,
potentially increasing the risk of intracranial metastasis, since
the brain may become a sanctuary site for tumor cells (21). Le Scodan et al (15) determined survival times in the groups
HER2 non-overexpressing patients, HER2-overexpressing patients
without trastuzumab treatment and HER2-overexpressing patients with
trastuzumab treatment of 5.9, 5.6 and 19.53 months, respectively
(P<0.004). The corresponding survival times in a study by Church
et al (22) were 3.8, 3.0 and
11.9 months. In the present cohort, a similar trend was observed
(3.8 vs. 3.5 vs. 9.7 months, respectively).
Besides medical treatment of metastatic breast
cancer, surgical and radiotherapeutic options are available. In the
present cohort, surgical resection was typically performed in
patients with a solitary brain metastasis. It is well established
that a smaller number of BM is associated with longer survival
(7,14,15,23). In
the present cohort, the group of patients that underwent surgical
resection was very small. Several reports in the literature focused
on the comparison of local therapy (surgery/radiosurgery) of BM in
combination with WBRT vs. WBRT alone; a survival benefit was
observed for selected patients treated with combinations of local
therapy and WBRT (24,25). In addition, the application of local
therapy alone may be justified. A randomised trial reported by
Kocher et al (16) compared
patients with ≤3 BM of distinct solid tumours who were treated with
surgery/radiosurgery with or without WBRT. The published data
indicated that there is a reduction of intracranial relapse and
neurological deaths in patients with a limited number of BM if
treatment with local therapy plus WBRT is administered. SRS and
surgery also seemed to produce similar results (26).
WBRT alone is used in cases with multiple BM or in
patients with a limited number of BM when surgery or SRS is not
possible (2). A dose of 30–36 Gy is
commonly applied. The present analysis revealed a survival
advantage for patients treated with a higher dose of WBRT (>35
Gy). This topic is controversial in the literature. Certain authors
evaluated various radiation schemes and were unable to detect
improved survival if patients were treated with a total dose >30
Gy (27–29). By contrast, two retrospective studies
by Mahmoud-Ahmed et al (30)
and Meyer et al (31) observed
a survival benefit in patients who were treated with a WBRT dose of
>30 Gy. It is unclear whether these findings are associated with
a selection bias. If a dose of >30 Gy is used, it may be given
preferentially to patients with a better prognosis due to younger
age, limited number of BM and higher Karnofsky performance status
(2). On the other hand, higher WBRT
doses may be used for patients who are not candidates for a boost
due to higher number of metastases.
Multivariate analysis conducted in the current study
identified only boost irradiation following WBRT as having an
significant effect on survival. In comparison to other trials, the
median survival time of 19.7 months for this subgroup is relatively
long. For example, Hsu et al (32) reported a median survival time in
patients with metastatic cancer from different tumour entities of
12.1 months if they were treated with stereotactic boost
irradiation following WBRT. Another analysis by Kondziolka et
al (33) revealed a survival time
of 11 months following a combination of WBRT and SRS treatment. In
a study by Andrews et al (34), survival subsequent to WBRT plus boost
irradiation was 6.5 months in patients with a single BM.
Furthermore, Assouline et al (35) reported that patients with ≤3 BM from
lung cancer, breast cancer or melanoma survived significantly
longer if treated with WBRT plus boost treatment compared with WBRT
alone (8.9 vs. 4.0 months; P=0.002). Despite the significant
association of boost treatment with improved survival in the
present study, the findings must be interpreted with caution.
Patients who received boost treatment subsequent to WBRT were those
with a limited number of BM. Considering the studies discussed,
survival is improved in breast cancer patients with a limited
number of BM who are treated with boost following WBRT.
Recently, the application of WBRT in patients with
single BM has been discussed controversially in the literature. In
summary, local control is improved, but there is no effect on
overall survival time if patients are treated with WBRT following
surgery or SRS (16,36). In addition, SRS plus WBRT affects
learning and memory function more than SRS alone (37). Patients with multiple cerebral
metastases are seldom treated with boost irradiation. Whether a
local enhancement of dose is considered reasonable depends on the
number, localisation and size of the BM. To date, no randomised
clinical trials have examined the effects of SRS on survival in
patients with multiple BM. However, a prospective observational
study by Yamamoto et al (38)
revealed no inferiority for the management of ≤10 BM with SRS; the
median overall survival time was similar in the groups with 2–4 BM
vs. 5–10 BM (10.8 months for both), as was the documented
toxicity.
In conclusion, within the constraints of a
retrospective analysis, the results of the present study inform
about the current efficacy of brain irradiation in patients with BM
from metastatic breast cancer. In particular boost irradiation
following WBRT improved survival times in selected patients.
Although there was no statistical evidence for the effect of HER2
in this data set, it appears to be a relevant variable affecting
survival in larger cohorts.
Glossary
Abbreviations
Abbreviations:
|
BM
|
brain metastasis/metastases
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HR
|
hormone receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
WBRT
|
whole-brain radiotherapy
|
|
SRS
|
stereotactic radiosurgery
|
References
|
1
|
Gavrilovic IT and Posner JB: Brain
metastases: Epidemiology and pathophysiology. J Neurooncol.
75:5–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
German Society for Neurology: Brain
metastases and meningeosis neoplastica. Leitlinien für Diagnostik
und Therapie in der Neurologie: Registry Number 030/060. 2011.(In
German).
|
|
3
|
DiStefano A, Yap Yong Y, Hortobagyi GN and
Blumenschein GR: The natural history of breast cancer patients with
brain metastases. Cancer. 44:1913–1918. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho SY and Choi HY: Causes of death and
metastatic patterns in patients with mammary cancer. Ten-year
autopsy study. Am J Clin Pathol. 73:232–234. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee YT: Breast carcinoma: Pattern of
metastasis at autopsy. J Surg Oncol. 23:175–180. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsukada Y, Fouad A, Pickren JW and Lane
WW: Central nervous system metastasis from breast carcinoma.
Autopsy study. Cancer. 52:2349–2354. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evans AJ, James JJ, Cornford EJ, Chan SY,
Burrell HC, Pinder SE, Gutteridge E, Robertson JF, Hornbuckle J and
Cheung KL: Brain metastases from breast cancer: Identification of a
high-risk group. Clin Oncol (R Coll Radiol). 16:345–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeichner SB, Cavalcante L, Suciu GP, Ruiz
AL, Hirzel A and Krill-Jackson E: Long-term survival of women with
locally advanced breast cancer with ≥10 involved lymph nodes at
diagnosis. Asian Pac J Cancer Prev. 15:3435–3441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pestalozzi BC, Zahrieh D, Price KN,
Holmberg SB, Lindtner J, Collins J, Crivellari D, Fey MF, Murray E,
Pagani O, et al: Identifying breast cancer patients at risk for
central nervous system (CNS) metastases in trials of the
international breast cancer study group (IBCSG). Ann Oncol.
17:935–944. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gabos Z, Sinha R, Hanson J, Chauhan N,
Hugh J, Mackey JR and Abdulkarim B: Prognostic significance of
human epidermal growth factor receptor positivity for the
development of brain metastasis after newly diagnosed breast
cancer. J Clin Oncol. 24:5658–5663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kreienberg R, Albert US, Follmann M, Kopp
IB, Kühn T and Wöckel A: Interdisciplinary GoR level III Guidelines
for the Diagnosis, Therapy and Follow up Care of Breast Cancer:
Short version AWMF Registry No.: 032 045OL AWMF Register Nummer:
032 045OL Kurzversion 3.0, Juli 2012. Geburtshilfe Frauenheilkd.
73:556–583. 2013.PubMed/NCBI
|
|
12
|
Karam I, Nichol A, Woods R and Tyldesley
S: Population-based outcomes after whole brain radiotherapy and
re-irradiation in patients with metastatic breast cancer in the
trastuzumab era. Radiat Oncol. 6:1812011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogawa K, Yoshii Y, Nishimaki T, Tamaki N,
Miyaguni T, Tsuchida Y, Kamada Y, Toita T, Kakinohana Y, Tamaki W,
et al: Treatment and prognosis of brain metastases from breast
cancer. J Neurooncol. 86:231–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dawood S, Gonzalez-Angulo AM, Albarracin
C, Yu TK, Hortobagyi GN, Buchholz TA and Woodward WA: Prognostic
factors of survival in the trastuzumab era among women with breast
cancer and brain metastases who receive whole brain radiotherapy: A
single-institution review. Cancer. 116:3084–3092. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le Scodan R, Jouanneau L, Massard C,
Gutierrez M, Kirova Y, Cherel P, Gachet J, Labib A and
Mouret-Fourme E: Brain metastases from breast cancer: Prognostic
significance of HER-2 overexpression, effect of trastuzumab and
cause of death. BMC Cancer. 11:3952011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kocher M, Soffietti R, Abacioglu U, Villà
S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD,
Carrie C, et al: Adjuvant whole-brain radiotherapy versus
observation after radiosurgery or surgical resection of one to
three cerebral metastases: Results of the EORTC 22952–26001 study.
J Clin Oncol. 29:134–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nam BH, Kim SY, Han HS, Kwon Y, Lee KS,
Kim TH and Ro J: Breast cancer subtypes and survival in patients
with brain metastases. Breast Cancer Res. 10:R202008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anders CK, Deal AM, Miller CR, Khorram C,
Meng H, Burrows E, Livasy C, Fritchie K, Ewend MG, Perou CM and
Carey LA: The prognostic contribution of clinical breast cancer
subtype, age, and race among patients with breast cancer brain
metastases. Cancer. 117:1602–1611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sperduto PW, Kased N, Roberge D, Chao ST,
Shanley R, Luo X, Sneed PK, Suh J, Weil RJ, Jensen AW, et al: The
effect of tumor subtype on the time from primary diagnosis to
development of brain metastases and survival in patients with
breast cancer. J Neurooncol. 112:467–472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin NU and Winer EP: Brain metastases: The
HER2 paradigm. Clin Cancer Res. 13:1648–1655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gabos Z, Sinha R, Hanson J, Chauhan N,
Hugh J, Mackey JR and Abdulkarim B: Prognostic significance of
human epidermal growth factor receptor positivity for the
development of brain metastasis after newly diagnosed breast
cancer. J Clin Oncol. 24:5658–5663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Church DN, Modgil R, Guglani S, Bahl A,
Hopkins K, Braybrooke JP, Blair P and Price CG: Extended survival
in women with brain metastases from HER2 overexpressing breast
cancer. Am J Clin Oncol. 31:250–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu MT, Hsieh CY, Wang AY, Chang TH, Pi
CP, Huang CC, Huang CY and Liou CH: Prognostic factors affecting
the outcome of brain metastases from breast cancer. Support Care
Cancer. 14:936–942. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patchell RA, Tibbs PA, Walsh JW, Dempsey
RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS and Young
B: A randomized trial of surgery in the treatment of single
metastases to the brain. N Engl J Med. 322:494–500. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rades D, Kieckebusch S, Haatanen T,
Lohynska R, Dunst J and Schild SE: Surgical resection followed by
whole brain radiotherapy versus whole brain radiotherapy alone for
single brain metastasis. Int J Radiat Oncol Biol Phys.
70:1319–1324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'Neill BP, Iturria NJ, Link MJ, Pollock
BE, Ballman KV and O'Fallon JR: A comparison of surgical resection
and stereotactic radiosurgery in the treatment of solitary brain
metastases. Int J Radiat Oncol Biol Phys. 55:1169–1176. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murray KJ, Scott C, Greenberg HM, Emami B,
Seider M, Vora NL, Olson C, Whitton A, Movsas B and Curran W: A
randomized phase III study of accelerated hyperfractionation versus
standard in patients with unresected brain metastases: A report of
the radiation therapy oncology group (RTOG) 9104. Int J Radiat
Oncol Biol Phys. 39:571–574. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rades D, Haatanen T, Schild SE and Dunst
J: Dose escalation beyond 30 grays in 10 fractions for patients
with multiple brain metastases. Cancer. 110:1345–1350. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davey P, Hoegler D, Ennis M and Smith J: A
phase III study of accelerated versus conventional hypofractionated
whole brain irradiation in patients of good performance status with
brain metastases not suitable for surgical excision. Radiother
Oncol. 88:173–176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mahmoud-Ahmed AS, Suh JH, Lee SY,
Crownover RL and Barnett GH: Results of whole brain radiotherapy in
patients with brain metastases from breast cancer: A retrospective
study. Int J Radiat Oncol Biol Phys. 54:810–817. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meyer A, Steinmann D, Malaimare L,
Karstens JH and Bremer M: Prediction of prognosis regarding
fractionation schedule and survival in patients with whole-brain
radiotherapy for metastatic disease. Anticancer Res. 28:3965–3969.
2008.PubMed/NCBI
|
|
32
|
Hsu F, Kouhestani P, Nguyen S, Cheung A,
McKenzie M, Ma R, Toyota B and Nichol A: Population-based outcomes
of boost versus salvage radiosurgery for brain metastases after
whole brain radiotherapy. Radiother Oncol. 108:128–131. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kondziolka D, Patel A, Lunsford LD, Kassam
A and Flickinger JC: Stereotactic radiosurgery plus whole brain
radiotherapy versus radiotherapy alone for patients with multiple
brain metastases. Int J Radiat Oncol Biol Phys. 45:427–434. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Andrews DW, Scott CB, Sperduto PW,
Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J,
Bahary JP, et al: Whole brain radiation therapy with or without
stereotactic radiosurgery boost for patients with one to three
brain metastases: Phase III results of the RTOG 9508 randomised
trial. Lancet. 363:1665–1672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Assouline A, Levy A, Chargari C,
Lamproglou I, Mazeron JJ and Krzisch C: Whole brain radiotherapy:
Prognostic factors and results of a radiation boost delivered
through a conventional linear accelerator. Radiother Oncol.
99:214–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aoyama H, Shirato H, Tago M, Nakagawa K,
Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, et al:
Stereotactic radiosurgery plus whole-brain radiation therapy vs
stereotactic radiosurgery alone for treatment of brain metastases:
A randomized controlled trial. JAMA. 295:2483–2491. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang EL, Wefel JS, Hess KR, Allen PK,
Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH and
Meyers CA: Neurocognition in patients with brain metastases treated
with radiosurgery or radiosurgery plus whole-brain irradiation: A
randomised controlled trial. Lancet Oncol. 10:1037–1044. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamamoto M, Serizawa T, Shuto T, Akabane
A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S, et
al: Stereotactic radiosurgery for patients with multiple brain
metastases (JLGK0901): A multi-institutional prospective
observational study. Lancet Oncol. 15:387–395. 2014. View Article : Google Scholar : PubMed/NCBI
|