Introduction
Resveratrol is a non-flavonoid polyphenolic compound
with a stilbene structure that has various biological activities
and pharmacological effects. Previous findings showed that
resveratrol induces apoptosis of various tumor cells and inhibits
their proliferation, thereby exerting antitumor effects (1). Additionally the antitumor effect of
resveratrol is associated with the inhibition of telomerase
(2,3).
Telomerase is a specific chromosome terminal transferase and
telomerase activity has been reported to be detected in many
malignant tumor cells but is undetectable in normal cells. The
transition process of normal cells to tumor cells is mostly
accompanied with telomerase activation (4). Telomerase activation is one of the key
links in malignant tumors, and the catalytic subunit of human
telomerase reverse transcriptase (hTERT) is closely associated with
the activation of telomerase, which exhibits a positive correlation
(5,6).
At present, little is known concerning resveratrol inducing
apoptosis of skin cancer cells and its effect on the activity of
telomerase. In the present study, resveratrol intervention was used
on cultured human A431 epidermoid carcinoma cells, and its
value-added influence on A431 cell apoptosis and telomerase
activity was observed. The findings provided a theoretical basis
for the use of resveratrol in the clinical treatment of skin
tumors.
Materials and methods
Materials
Resveratrol was purchased from Alexis Biochemicals
Corp. (San Diego, CA, USA). The A431 cell line was obtained from
the China Center for Type Culture Collection of Wuhan University
(Wuhan, China). The main reagents used in the study were, DMEM high
glucose medium (Gibco, Grand Island, NY, USA), fetal bovine serum
(FBS) (Hangzhou Sijiqing Biological Engineering Materials Co.,
Ltd., Hangzhou, China), trypsin and DMSO (Amresco, LLC, Solon, OH,
USA), as well as a telomeric repeat amplification protocol
(TRAP)-polymerase chain reaction (PCR)-ELISA telomerase activity
assay kit (Roche Diagnostics GmbH, Boehringer Mannheim, Germany).
The main instruments employed in the study were automatic enzyme
mark instrument, type ELx808 (BioTek Instruments, Inc., Winooski,
VT, USA), and for PCR amplification, an Applied Biosystems Veriti
96-Well Thermal Cycler (Thermo Fisher Scientific, Waltham, MA,
USA).
Cell culture
A431 cells (1×107 ml) were inoculated in
DMEM medium containing 10﹪ FBS, and cultured in an incubator at
37°C, 5% CO2 and saturated humidity. The medium was
changed every 3 days, and the cells were observed under an inverted
microscope (Olympus IX83, Olympus Corporation, Tokyo, Japan). At
100% cell confluence, 0.25% trypsin was used to digest the culture
cell lines. Cells of the logarithmic growth phase were chosen for
experiments.
Grouping and administration of
medications
A431 cells of the fourth generation in the
logarithmic growth phase were selected, digested with 0.25%
trypsin, and counted under a light microscope. At
2×104/ml concentration, the cells were inoculated into
96-well plates. After 24 h of pretreatment with serum-free culture
medium, the cells were randomly divided into the blank control
group (group A), and three drug concentration groups: group B (0.1
mg/ml concentration), group C (0.2 mg/ml concentration), and group
D (0.3 mg/ml concentration), each with 8 wells.
Cell morphology
After 24 h incubation with different doses (0.1, 0.2
and 0.3 mg/ml concentration) of resveratrol, the cells were fixed
with 4% paraformaldehyde in 96-well plates at room temperature (RT)
for 30 min. Formaldehyde was then washed with distilled water, and
hematoxylin and eosin staining was used to observe the
morphological changes of A431 cells under an optical
microscope.
Status of A431 cell proliferation at
different concentrations of resveratrol intervention by MTT
assay
Resveratrol was dissolved in DMSO to the desired
concentration, and was added to the three drug concentration groups
as mentioned above. In addition, 20 µl 1% DMSO/20 µl DMEM was added
into each well of the blank control group. After 24 h of drug
intervention, 5 mg/ml MTT [made by pH 7.4 phosphate-buffered saline
(PBS); NDP-1201, Ningbo Novatech Biotechnology Co., Ltd. Ningbo,
China] 20 µl, was added to each well and incubated at 37°C for 4 h.
The supernatant was carefully absorbed, 150 µl DMSO was added per
well with oscillation for 10 min, and the crystalline particles
were produced and dissolved into homogeneous blue purple, with zero
blank well. The absorbance (A) value of each well was determined by
the enzyme mark instrument at a wavelength of 490 nm.
Effect of resveratrol on telomerase
activity of A431 cells
The resveratrol was dissolved in DMSO to the desired
concentration, 20 µl was added into the 3 drug concentration groups
as described above in each well, and 20 µl of 1% DMSO/DMEM was
added into each well of the blank control group. The cells were
collected in accordance with the standard method after 24 h of
incubation at 37°C. Each reaction of 2×105 cells was
moved into a new Eppendorf tube (Shanghai Hao Ran Biological
Technology Co., Ltd. hanghai, China), assayed according to a
TRAP-PCR-ELISA telomerase activity assay kit (BMK018-1, Hao Ran
Biological Technology Co., Ltd., Shanghai, China), and the
absorbance (A) value was determined by the enzyme mark instrument
at 690 nm wavelength with the reference wavelength of 450 nm, and
calculated as A = A450nm-A690nm, where the
value of A indicated the activity of telomerase.
Effect of resveratrol on hTERT protein
level in A431 cells
Western blot analysis was used for detection.
According to the groupings and doses, after 24 h culture at 37°C,
the cells were collected, washed twice with pre-cold PBS, cell
lysate was added, and was kept on ice for 10–20 min. The cells were
removed with a cell scraper and homogenized, at 4°C, and
centrifuged for 10 min, at 300 × g. The supernatant was removed and
1/3 volume of 4X sample buffer was added, and the solution was
boiled for 10 min until protein denaturation. The sample proteins
were separated by gel electrophoresis, power was transferred to the
nitrocellulose membrane, after 1 h closed at the RT, and the hTERT
monoclonal antibody (1:500) was added and incubated overnight at
4°C. After rinsing, goat anti-rabbit immunoglobulin G (1:500)
labeled with 37% horseradish peroxidase was added, and incubated
for 1 h. Subsequent to rinsing, staining was shown by enhanced
chemiluminescence with exposure, and the film was washed. The ratio
of the density gray value of the hTERT protein band and internal
control β-actin protein band was considered as the relative level
of protein expression.
Statistical analysis
Experimental data were analyzed using the SPSS 12.0
software (SPSS, Inc., Chicago, IL, USA) package and expressed as
mean ± standard deviation. The mean comparison of multiple groups
by single factor variance analysis was tested by t-test.
Results
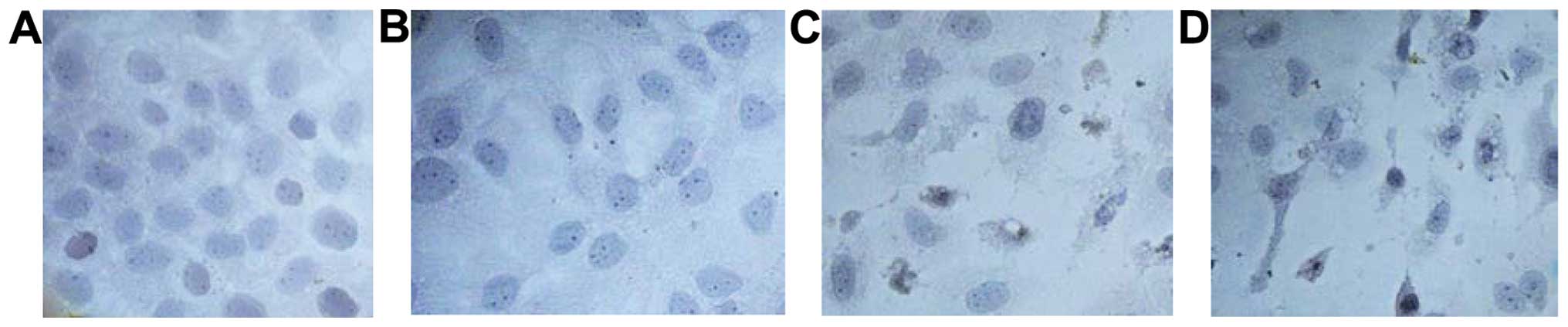
Resveratrol induces morphological
changes in A431 cell apoptosis
After 24 h of treatment with resveratrol, apoptotic
features at different degrees were observed in A431 cells (groups
B, C and D) under a contrast microscope. However, due to the poor
adherence of cells to the wall, the cell morphology was not
regular, cytoplasm was condensed, particles in the cytoplasm
increased significantly with karyopyknosis, and some cells showed
nuclear fragmentation, and apoptotic bodies. In addition, an
aliquot of the cells were floating in the culture medium. This
effect was enhanced with the increased resveratrol concentration,
whereas in the control group (A), the cells adhered firmly with
good growth, normal cell morphology, little cytoplasmic granules
and without apoptotic bodies (Fig.
1).
Effect of resveratrol on A431 cell
proliferation
A431 cells were treated with different
concentrations of resveratrol (0.1, 0.2 and 0.3 mg/ml). The MTT
assay was used to detect cell proliferation. The results showed
that compared to the blank control group, the resveratrol group
significantly inhibited the proliferation of A431 cells (P<0.01)
in a dose-dependent manner (Table
I).
 | Table I.Effect of different resveratrol
concentrations on the proliferation of A431 cells (mean ± SD,
n=6). |
Table I.
Effect of different resveratrol
concentrations on the proliferation of A431 cells (mean ± SD,
n=6).
| Groups | Concentration | A value |
|---|
| A | – | 0.485±0.050 |
| B | 0.1 mg/ml |
0.228±0.018a |
| C | 0.2 mg/ml |
0.177±0.016a |
| D | 0.3 mg/ml |
0.155±0.008a |
Effect of resveratrol on telomerase
activity of A431 cells
After 24 h of resveratrol treatment, the telomerase
activity of A431 cells was significantly inhibited compared to the
control group without treatment (P<0.01) (Table II). An increase in drug concentration
led to an increase in the inhibition of the telomerase
activity.
 | Table II.Effect of different resveratrol
concentrations on telomerase activity of A431 cells (mean ± SD,
n=6). |
Table II.
Effect of different resveratrol
concentrations on telomerase activity of A431 cells (mean ± SD,
n=6).
| Groups | Concentration | Telomerase
activity |
|---|
| A | – | 0.719±0.064 |
| B | 0.1 mg/ml |
0.623±0.068a |
| C | 0.2 mg/ml |
0.608±0.057b |
| D | 0.3 mg/ml |
0.506±0.043b |
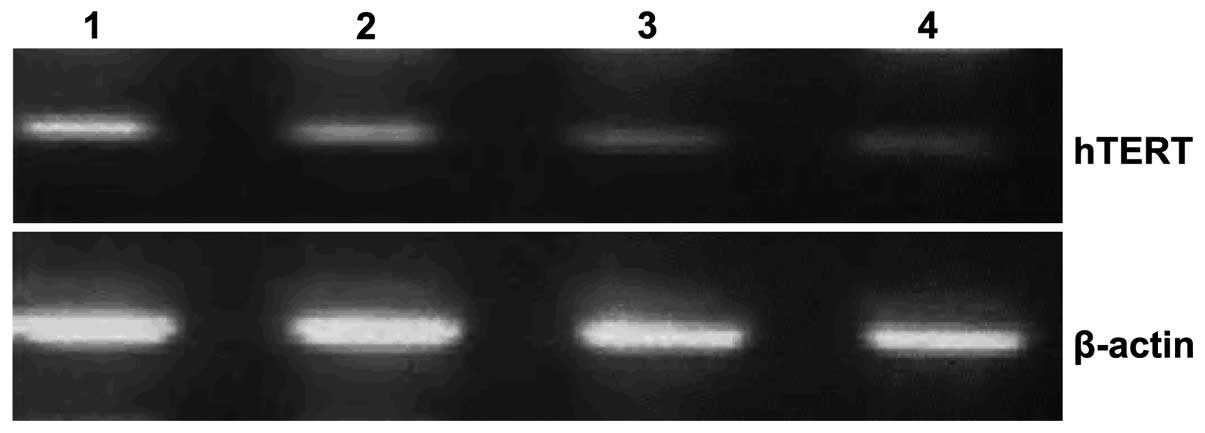
Effect of resveratrol on the hTERT
protein level in A431 cells
After 24-h treatment of A431 cells with different
concentrations of resveratrol, the hTERT protein level was
decreased significantly as detected by western blot analysis.
Matlab image analysis software [China Daheng (Group) Co., Ltd.
Beijing Image Vision Technology Branch, Beijing China] analyzed the
relative gray value for the blank control and resveratrol groups.
Gray values for the three concentration groups (0.1, 0.2 and 0.3
mg/ml) were lower than those for the blank control group and the
differences were statistically significant (P<0.05) (Fig. 2).
Discussion
Skin cancer is the malignant proliferation of
epidermal keratinocytes, including squamous cell and basal cell
carcinoma (7). In recent years, the
incidence of skin cancer has been on the increase. The disease is
associated with environmental pollution and other factors causing
the destruction of the ozone layer, resulting in an increase in
ultraviolet radiation reaching the earth, and leading to the
passive acceptance of irradiation intensity (8). At present, the specific pathogenesis of
skin cancer remains unclear, and clinical treatment of skin cancer
is also limited. Identification of a new and effective natural
medicine for the prevention and treatment of skin cancer has become
one of the issues to be solved. In recent years, the in-depth study
of resveratrol has revealed antagonistic activity on many types of
tumor cells, such as liver (9),
gastric (10), esophageal (11), breast (12), and cervical cancer (13), as well as leukemia (14), whose mechanism may be associated with
the change of telomerase activity (15). However, there are currently no studies
available regarding whether resveratrol affects skin cancer cell
proliferation, apoptosis and telomerase activity. Therefore, in the
present study, we provided a theoretical basis for the use of
resveratrol as a clinical treatment for skin cancer.
Telomere is a short section of DNA-protein complex
located at the end of the eukaryotic chromosome arm, whose function
is to protect the chromosome (16).
Telomerase is a specific chromosome terminal transferase, whose
main function is taking its RNA as a template. Catalytic synthesis
of the telomeric repeat sequence of the ends of chromosomes makes
up for the loss of telomeric DNA cell division and causes infinite
cell proliferation, and its expression level is closely associated
with the occurrence, development and prognosis of malignant tumors.
In the majority of tumor cells, there is an abnormal expression of
telomerase activity, whereas in normal human cells telomerase
activity cannot be detected. Activation of telomerase is one of the
key links of malignant tumors, whose activity can be achieved
through the regulation of telomerase RNA component and/or protein
components, of which catalytic subunit hTERT is the most important
(17).
In the present study, resveratrol intervention in
A431 cells, resulted in typical morphological features of
apoptosis. MTT assay results after different concentrations of
resveratrol intervention showed that A431 cell proliferation was
inhibited to some extent, and the inhibition increased with the
increase of resveratrol concentration, indicating that this
inhibition is dose-dependent. The results of the detection of
telomerase activity experiment showed that resveratrol inhibited
the telomerase activity of A431 cells and was independent of the
concentration. A431 cells treated with different concentrations of
resveratrol for 24 h showed that the expression of hTERT protein
was significantly lower, as detected by western blot analysis.
The experimental results showed that resveratrol
inhibit A431 proliferation of human epidermoid carcinoma cells,
inducing its apoptosis, the mechanism of which may be associated
with the inhibition of hTERT mRNA transcription, decreasing the
protein expression, thereby reducing the activity of
telomerase.
In conclusion, resveratrol is capable of
downregulating the expression of hTERT protein and inhibits the
ability of telomerase of A431, which is an important mechanism of
action of resveratrol with regard to inhibition of A431 cell
proliferation.
Acknowledgements
This study was funded by Xuzhou Science and
Technology Plan Project (no. XM08C048).
References
|
1
|
Chen W and Yang H: Progression of
anticancer mechanism of resveratrol. Int J Pathol Clin Med.
28:403–407. 2014.
|
|
2
|
Pearce VP, Sherrell J, Lou Z, Kopelovich
L, Wright WE and Shay JW: Immortalization of epithelial progenitor
cells mediated by resveratrol. Oncogene. 27:2365–2374. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fuggetta MP, Lanzilli G, Tricarico M, et
al: Effect of resveratrol on proliferation and telomerase activity
of human colon cancer cells in vitro. J Exp Clin Cancer Res.
25:189–193. 2006.PubMed/NCBI
|
|
4
|
Boukamp P, Popp S and Krunic D:
Telomere-dependent chromosomal instability. J Investig Dermatol
Symp Proc. 10:89–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cairney CJ and Keith WN: Telomerase
redefined: Integrated regulation of hTR and hTERT for telomere
maintenance and telomerase activity. Biochimie. 90:13–23. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kyo S, Takakura M, Fujiwara T and Inoue M:
Understanding and exploiting hTERT promoter regulation for
diagnosis and treatment of human cancers. Cancer Sci. 99:1528–1538.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ping W and Bi Z: Progress in the study of
molecular mechanism of skin cancer induced by UVB. Foreign Med Sci.
31:44–46. 2005.
|
|
8
|
Li L and Bai X: Effects of ultraviolet
radiation on human skin health. Hyg Sect Foreign Med Sci.
35:198–201. 2008.
|
|
9
|
Huang D, Shan H and Xie W: Molecular
mechanisms of metastasis in hepatoma carcinoma cells induced by
resveratrol. Acta Univ Med Anhui. 48:1071–1074. 2013.
|
|
10
|
Liu X, Zhao W and Qiu G: Effects of
resveratrol on proliferation and expression of matrix
metalloproteinase-9 in gastric carcinoma cells. J Xi'an Jiaotong
Univ Med Sci. 32:606–609. 2011.
|
|
11
|
Zhang L, Tan Z, Chen W, Yang G, Wu Y and
Xu J: Effect of resveratrol on growth and apoptosis of human
esophageal cancer cells. Med J Wuhan Univ. 28:54–57. 2007.
|
|
12
|
Guo H and Zhang X: The inhibitory action
of resveratrol on proliferation of MCF-7 breast cancer cells. Chin
J Clin Oncol. 38:1424–1426. 2011.
|
|
13
|
Zhang P, Sun Y, Yao Y and Liu J: Effects
of resveratrol on female reproductive system malignant tumors. J
Dalian Med Univ. 37:403–407. 2015.
|
|
14
|
Xiong X and Ann C: Research progress of
resveratrol on anti-tumor. Heilongjiang Med J. 23:59–61. 2010.
|
|
15
|
Lanzilli G, Fuggetta MP, Tricarico M,
Cottarelli A, Serafino A, Falchetti R, Ravagnan G, Turriziani M,
Adamo R, Franzese O, et al: Resveratrol down-regulates the growth
and telomerase activity of breast cancer cells in vitro. Int J
Oncol. 28:641–648. 2006.PubMed/NCBI
|
|
16
|
Wojtyla A, Gladych M and Rubis B: Human
telomerase activity regulation. Mol Biol Rep. 38:3339–3349. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujiwara T and Tanaka N:
Telomerase-specific oncolytic virotherapy for human cancer with the
hTERT promoter. Uirusu. 58:11–18. 2008. View Article : Google Scholar : PubMed/NCBI
|